Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.112
Peer-review started: August 12, 2014
First decision: August 27, 2014
Revised: September 19, 2014
Accepted: October 20, 2014
Article in press: October 21, 2014
Published online: January 7, 2015
Processing time: 147 Days and 21 Hours
Gastric cancer (GC) is one of the leading causes of cancer-related mortality worldwide. Cancer stem cells (CSCs), which were first identified in acute myeloid leukemia and subsequently in a large array of solid tumors, play important roles in cancer initiation, dissemination and recurrence. CSCs are often transformed tissue-specific stem cells or de-differentiated transit amplifying progenitor cells. Several populations of multipotent gastric stem cells (GSCs) that reside in the stomach have been determined to regulate physiological tissue renewal and injury repair. These populations include the Villin+ and Lgr5+ GSCs in the antrum, the Troy+ chief cells in the corpus, and the Sox2+ GSCs that are found in both the antrum and the corpus. The disruption of tumor suppressors in Villin+ or Lgr5+ GSCs leads to GC in mouse models. In addition to residing GSCs, bone marrow-derived cells can initiate GC in a mouse model of chronic Helicobacter infection. Furthermore, expression of the cell surface markers CD133 or CD44 defines gastric CSCs in mouse models and in human primary GC tissues and cell lines. Targeted elimination of CSCs effectively reduces tumor size and grade in mouse models. In summary, the recent identification of normal GSCs and gastric CSCs has greatly improved our understanding of the molecular and cellular etiology of GC and will aid in the development of effective therapies to treat patients.
Core tip: Cancer stem cells (CSCs) play important roles in cancer initiation, dissemination and recurrence. The recent identification of normal gastric stem cells and gastric CSCs has greatly improved our understanding of the molecular and cellular etiology of gastric cancer and will help with the design of effective treatments. In this article, we review the literature on the recent progress in the identification and characterization of normal gastric stem cells and gastric CSCs and discuss the implications for the treatment of gastric cancer.
- Citation: Zhao Y, Feng F, Zhou YN. Stem cells in gastric cancer. World J Gastroenterol 2015; 21(1): 112-123
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.112
Gastric cancer (GC) is the fourth most common cancer and the second most deadly cancer worldwide, with one million newly diagnosed patients and 600000 deaths each year[1-3]. Approximately 70% of all GC cases occur in East Asia, Central and Eastern Europe, South Africa, and Central and South America[3,4]. GCs are grouped into two major histological types: the well-differentiated intestinal-type (IGC) and the undifferentiated diffuse-type (DGC)[5]. The IGC type occurs more frequently (60%-80%) in the distal part of the stomach (antrum) and in aged patients, while the DGC type is more common in younger patients[6]. The mechanism that underlies GC initiation and progression is not well understood, but both genetic and environmental factors contribute to GC development. Epidemiologic studies have demonstrated that an excessive intake of salt, a low intake of vegetables and fruits, and smoking are risk factors for GC. Notably, infection with the gram-negative bacterium Helicobacter pylori (H. pylori), a type I carcinogen according to the WHO classification, is strongly associated with both GC subtypes[7,8]. The detailed molecular mechanisms on how H. pylori infection leads to GC are under intense investigation and have been reviewed elsewhere[2,9]. In this article, we focus on recent progress in the identification of normal and cancer stem cells (CSCs) in the stomach and discuss the implications for the treatment of GC.
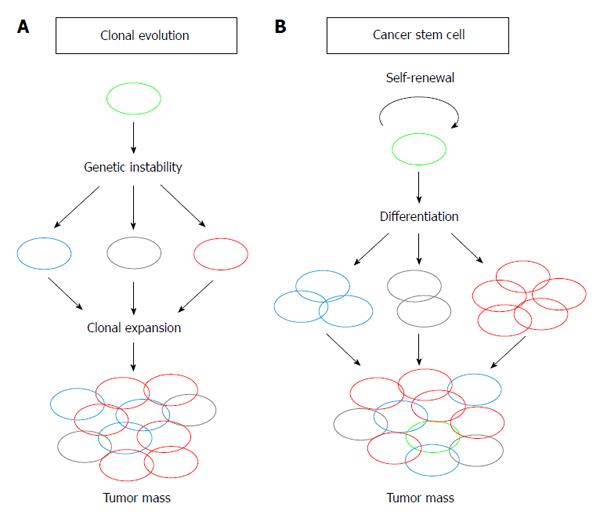
Human primary tumors often contain phenotypically heterogeneous cells. Two hypotheses, the clonal evolution hypothesis and the CSC hypothesis, have been proposed to explain the observed cellular heterogeneity, initiation, progression and metastasis of tumors[10,11] (Figure 1). In the clonal evolution hypothesis, cellular heterogeneity is generated by genetic instability, such as changes in chromosomal number or mutations in the tumor cell genome. Cells with genetic compositions that confer growth advantages are selected and clonally expanded[10] (Figure 1A). In contrast, the CSC hypothesis proposes that only a small fraction of cancer cells, namely CSCs, resides at the top of the cellular hierarchy and govern tumor heterogeneity; these cells divide to generate identical CSCs (self-renewal) and differentiate into phenotypically heterogeneous, but typically less proliferative, tumor cells (Figure 1B). The presence of CSCs was first demonstrated in human acute myeloid leukemia as a CD34+CD38- population. Interestingly, normal hematopoietic stem cells also express identical cell surface markers, which led to the hypothesis that CSCs are transformed tissue-specific stem cells or de-differentiated transit amplifying progenitor cells[11,12]. The existence of CSCs was soon demonstrated in solid tumors from several organs, including brain, breast, colon, prostate, liver, pancreatic, skin, and in areas of the head and neck[13-23].
Experimentally, CSCs are characterized by their capacity for tumor propagation, which is the generation of tumors that are full phenocopies of the primary tumors after they are serially transplanted into immunocompromised recipient mice. The tumor-propagating capacity can also be evaluated by in vitro clonogenic assays, such as the spheroid colony-forming or co-culture assays. These surrogate assays allow for the measurement of self-renewal and differentiation of cells of interest at the single-cell level and therefore serve as good complementary strategies to the mouse xenograft approach[24].
CSCs are responsible for cancer metastasis because of their tumor-propagating capacity. In human pancreatic cancer, only the CXCR4-expressing fraction of CD133+ CSCs is able to metastasize. The depletion of these cells from the CSC pool abrogates the metastatic phenotype, but does not affect tumorigenic potential[22]. In colorectal cancer, metastatic capacity is restricted to the CD26+ subpopulation of CSCs, and the presence of this subpopulation predicts subsequent liver metastasis in patients with primary colon cancer[25].
CSCs are more resistant to chemo- and radiotherapies, and therefore likely contribute to cancer recurrence. It is believed that, similar to normal tissue-specific stem cells, a quiescent subpopulation of CSCs exists[26,27]. These CSCs are more resistant to chemo- and radiotherapies because of their quiescent nature. In addition, CSCs express high levels of cellular efflux pumps and anti-apoptotic proteins, low levels of reactive oxygen species, and are more efficient in the repair of DNA damage[28-31]. Consequently, CSCs are often enriched after chemotherapy or radiotherapy[22,25,29,32,33] and cause cancer recurrence[26].
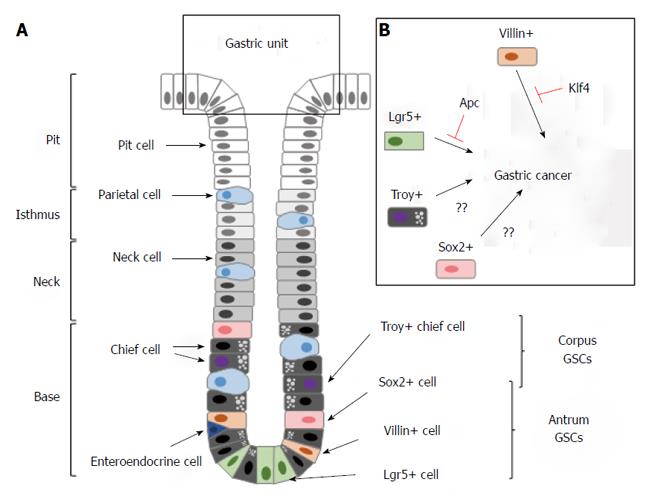
The stomach can be divided into three distinct anatomic regions: the cardiac region; the corpus; and the pyloric antrum. The basic structural elements of the gastric epithelium are gastric units, each of which is composed of a planar surface epithelium, tubular invaginations of the surface epithelium called pits, and tubular extensions of the pits called glands. The glands can be further divided into the isthmus, neck and base[34] (Figure 2A). The pits occupy the apical portion of the invaginations and contain mucus-producing pit cells. The isthmus connects the pit and the associated gland and contains cells that are morphologically undifferentiated, secretory, granule-free and highly proliferative. The neck is below the isthmus and contains mucus-producing neck cells. The base region is located at the very bottom of the gland and contains slowly dividing, digestive enzyme-producing chief cells[35-39] (Figure 2A). Gastric units of the corpus and antrum are similarly organized, except that the units of the corpus consist of more acid-producing parietal cells and gastric hormone-producing enteroendocrine cells.
In a series of classical electron microscopic autoradiographic experiments, Karam and Leblond suggested that the morphologically immature, granule-free isthmus cells are actively dividing multipotent gastric stem cells (GSCs) in the mouse and human stomach[35-40]. However, the potential of these putative stem cells to differentiate into fully committed pit, neck and zymogenic cells was not rigorously demonstrated. The existence of GSCs that replenish all of the populations of the gastric units was not convincingly demonstrated until lineage-tracing experiments were performed. These experiments used either X-chromosome inactivation as a clonality marker or transgenic mice that carry a β-galactosidase (lacZ) reporter[41-43].
The molecular markers that define multipotent GSCs were only discovered very recently. The first biomarker that labels GSCs is Villin, an epithelial cell-specific, calcium-regulated actin-binding protein that modulates the reorganization of microvillar actin filaments[44]. Qiao et al[45] discovered that transgenic mice that express a Villin promoter-driven LacZ or GFP reporter labeled a rare population of cells in the antrum that are long-lived and capable of multi-lineage replenishment (Figure 2A). Compared with the highly proliferative, putative GSCs in the isthmus[35], the Villin promoter-marked gastric stem cells (V-GSCs) likely represent a different cell population because they are quiescent and located in the lower third of the antral glands[45]. Recently, Clevers and colleagues identified another population of GSCs that expresses the G protein-coupled receptor Lgr5 (also known as Gpr49)[46]. The Lgr5+ GSCs (L-GSCs) are found at the base of the corpus and antral glands in the neonatal stomach, but become restricted to the antral glands in adults (Figure 2A). Similar to the V-GSCs, L-GSCs also give rise to all cells that comprise the gastric units, but are highly proliferative. Intriguingly, purified L-GSCs can form long-lived gastric organoids that highly resemble gastric units in cell culture, which demonstrate a remarkable proliferative and differentiation potential[46]. The co-existence of active and quiescent stem cells has been demonstrated in several tissue types[27]. It has been hypothesized that the active stem cells are responsible for physiological tissue renewal, while the quiescent stem cells serve as a reserve population primarily for injury repair[27]. Whether the V-GSCs and L-GSCs exhibit distinct capacities with respect to tissue renewal and injury repair of the antral epithelium has not been investigated.
Because both V-GSCs and L-GSCs are primarily found in the antrum, but not in the corpus, the main body of the stomach[45,46], it is intriguing as to the identity of the corpus stem cells. Recently, Clevers and colleagues demonstrated that a subpopulation of fully committed zymogenic chief cells, which reside at the base of the gastric glands of the corpus, are multipotent and can generate all cell lineages of the stomach epithelium[47] (Figure 2A). This population of chief cells expresses Troy, a member of the tumor necrosis factor receptor superfamily[48]. Troy+ cells divide slowly and become more active after cytotoxic drug-induced tissue injury[47].
In addition to the Troy+ cells, Sox2+ cells also represent GSCs in the corpus as well as the antrum. During embryonic development, Sox2 is highly expressed in the foregut region and plays essential roles in the patterning of the stomach[49]. Genetic tracing experiments have demonstrated that these Sox2+ cells are capable of generating all cell types that comprise the gastric units. Selective ablation of Sox2+ cells leads to the disruption of the physiological renewal of the gastric epithelium[50].
Evidence that supports the existence of CSCs in GC has emerged in recent years. Because CSCs are often transformed from tissue-specific stem cells[51-53], whether GC originates from cancerous GSCs is an intriguing question (Figure 2B). Two general approaches were used to identify the putative gastric CSCs. One approach involved genetic manipulation and tracing of specific cell populations in mouse models of GC. Another approach involved the identification of cells with tumor-propagating capacities within human primary gastric tumors or tumor-derived cell lines using mouse xenograft models.
Because V-GSCs are enriched within the lesser curvature of the antrum[45], the frequent anatomical site of human GC[54], it has been hypothesized that the transformation of V-GSCs could lead to GC. Klf4 is a Kruppel-like, zinc finger transcription factor that is highly expressed in the gut and plays critical roles in the reprogramming of terminally differentiated somatic cells into induced pluripotent stem cells[55]. Klf4 down-regulation is associated with human GC initiation and progression, which alludes to its role as a tumor suppressor[56]. The deletion of Klf4 in mouse stomach using a Foxa3-Cre transgene, which expresses Cre recombinase in all cells of the glandular stomach, leads to widespread hypertrophy and premalignant metaplasia in the antrum and corpus within 6 mo[57]. Interestingly, Klf4 deletion specifically in the V-GSCs with a Villin-Cre transgene leads to pronounced hypertrophy between 35 to 50 wk and spontaneous gastric adenomas by 80 wk[58]. Furthermore, these lesions were only observed in the antrum of the stomach where most V-GSCs reside. In this model, tumor initiation is greatly accelerated by the administration of the chemical mutagen N-nitroso-N-methylurea (NMU). It is noteworthy that in this mouse model, the gastric adenomas do not progress into adenocarcinomas even in the presence of NMU, which suggests that additional genetic mutations are required for cancer progression. Nonetheless, these data demonstrated that the transformation of the V-GSCs can initiate GC and that Klf4 plays a critical role in the suppression of tumorous growth of normal GSCs[58] (Figure 2B).
The mechanism by which Klf4 suppresses the conversion of normal stem cells to CSCs could provide important insight into the molecular etiology of GC. Klf4 can suppress cell proliferation by activating the expression of cyclin-dependent kinase-inhibitors such as p21 and p27[57,59,60]. Klf4 can suppress the expression of Klf5, a pro-proliferation transcription factor from the Kruppel-like family in esophageal tissues[61]. Klf4 deletion also leads to increased expression of the pro-proliferative factor FoxM1 in gastric tissue[58]. Together, these mechanisms can contribute to the transformation of V-GSCs upon Klf4 deletion.
Similarly, whether the rapidly dividing L-GSCs can transform into CSCs was examined. Inactivating mutations in Apc are frequently found in human colorectal cancer and IGC[62-64]. Mice with germline Apc mutations develop multiple adenomas in the small intestine[65]. Apc deletion in Lgr5+ crypt stem cells using an Lgr5 promoter-driven CreER recombinase leads to rapid stem cell transformation and the appearance of macroscopic adenomas in the intestine within 3 to 5 wk[52]. In the same mouse model, Barker et al[46] specifically disrupted Apc in L-GSCs. Upon the administration of tamoxifen, L-GSCs were rapidly transformed, and microscopic adenomas were detected in the antrum within 2-3 wk. However, it is unclear whether these adenomas would eventually progress into adenocarcinomas as it was necessary to sacrifice the animals used in the study due to the tumor load in the intestine[46]. These studies demonstrated that the Lgr5+ stem cells in the stomach and intestine could be tumor-initiating cells (Figure 2B).
Because inflammatory signals, which are often caused by infection with the bacterium H. pylori and may predispose individuals to GC, can recruit bone marrow-derived cells (BMDCs), Wang and colleagues hypothesized that GC originates from BMDCs[66]. Using a mouse model, these authors demonstrated that chronic infection with H. felis resulted in intense bone marrow-derived inflammation and repopulation of the stomach with BMDCs, which subsequently progressed through metaplasia and dysplasia to intra-epithelial cancer. In contrast, BMDCs are not recruited to the stomach under circumstances of acute injury or acute inflammation. Thus, this study demonstrated that gastric CSCs can also have a bone-marrow origin[66].
CD133: CD133 (also known as prominin-1) belongs to the prominin family of pentaspan membrane proteins and resides within plasma membrane protrusions, such as epithelial microvilli[67]. A glycosylated form of CD133, the AC133 antigen, was first identified as a marker that is strictly expressed by CD34+ hematopoietic stem cells[68,69] and later by normal and cancer stem cells of several solid organs[14,15,17,18,70-72]. In the mouse intestine, CD133+ cells located at the base of the crypts co-express Lgr5 and are capable of generating all cells of the intestinal epithelium; therefore, they represent the multipotent stem cell population[51]. The activation of canonical Wnt signaling in CD133+ cells in the crypt by the forced expression of a stable form of β-catenin led to intestinal cancer[51]. Recently, CD133+ cells were identified in the stem cell zone of normal human gastric glands[73]. However, it is still unclear whether these gastric CD133+ cells perform stem cell functions[73]. CD133+ cells can be found in over half of human gastric tumors and in both diffuse and intestinal subtypes; additionally, CD133 expression is associated with a poor prognosis[74-76].
CD44: CD44 was first described as a lymphocyte homing receptor and is expressed in many cell types[77]. It is the major cell surface receptor for hyaluronate[78], the most abundant component of the extracellular matrix[79]. CD44 is encoded by a single gene with 20 exons and can generate a variety of structurally distinct molecules because of alternative splicing of the primary transcripts, N- and O-linked glycosylation, and glycosaminoglycan modification[80-82]. The most commonly expressed isoform, CD44s, has seven extracellular domains, one transmembrane domain and one cytoplasmic domain[83]. Alternatively spliced exons can be incorporated into the extracellular domains to generate variant isoforms[82]. CD44 became a valid cancer marker when one variant isoform, CD44v, which is only expressed by a fraction of embryonic epithelial cells[84], was found to be associated with the metastatic potential of tumor cells[85]. CD44 was subsequently demonstrated to be specifically expressed by tumor-propagating cells that were isolated from human cancer cell lines and solid tumors[13,21,86]. It has been suggested that CD44 has key functions in CSCs, including the following: the mediation of adhesion and homing to the stem cell niche; the indirect enhancement of the expression of anti-apoptotic proteins and surface efflux pumps; the regulation of the cellular redox status; and the response to the activation of the canonical Wnt pathway[87-89].
CD44+ cells were found in 65 out of 100 human primary gastric adenocarcinomas, but were absent in normal human gastric tissues[90]. In addition, CD44+ tumors are more common in the intestinal subtype and are associated with a worse outcome[90]. Consistently, a CD44+ subpopulation was identified in multiple established human GC cell lines. Compared with CD44- cells, purified CD44+ cells are superior in the generation of spheroid colonies in culture, are more resistant to radiation and DNA damage-inducing drugs, and are more tumorigenic when they are injected into the stomachs of immunocompromised mice; they can also give rise to CD44- cells, and therefore represent putative gastric CSCs[91]. In cases of sporadic and hereditary DGC, a CD44v6 variant was expressed at levels that inversely correlated with the expression of E-cadherin[92]. Another variant isoform, CD44v8-10, has been demonstrated to be a CSC marker in primary GC tumors. Purified CD44v8-10+ cells possess a tumor-propagating capacity when they are serially transplanted into immunocompromised mice. Importantly, the knock-down of total CD44 by shRNA dramatically reduced the tumor-propagating capacity of these cells, and only the re-introduction of the CD44v8-10 variant, but not the CD44s isoform, rescued the tumor-propagating capacity[93]. Recently, the CD44 variant v9 was reported to be a predictive marker for cancer recurrence in patients with GC who received curative endoscopic submucosal dissection[94]. In a mouse model of gastric neoplasia, where the canonical Wnt and prostaglandin E2 pathways were co-activated in the gastric epithelium, disruption of the CD44 gene significantly reduced tumor size and grade. This result suggests that CD44 is not simply a marker of gastric CSCs, but it is also actively involved in tumor growth and progression[89].
H. pylori is classified by WHO as a type I carcinogen, and its infection is strongly associated with the incidence of both IGC and DGC[7,8]. Whether gastric CSCs are the primary targets of H. pylori infection has been investigated. In addition to the recruitment of BMDCs to the stomach to initiate GC[66], infection with H. pylori also transforms GSCs. H. pylori infection of gastric epithelial cells disrupts the epithelial apical junctional complex and induces the transition to a mesenchymal phenotype[95], which is often associated with tumor invasion, metastasis and drug resistance[96]. Bessède et al[97] demonstrated that only the CD44+ cells of cultured human gastric epithelial cells can be induced to assume a mesenchymal phenotype by H. pylori infection. Compared with CD44- and uninfected CD44+ cells, the infected CD44+ cells are able to form spheroid colonies more readily in culture and are more tumorigenic in a mouse xenograft model, which suggests that H. pylori preferentially transforms GSCs. There is also evidence to suggest that H. pylori bacteria can evolve to establish symbiosis with gastric stem or progenitor cells. Giannakis et al[98] isolated H. pylori strains before and after a single human host progressed from chronic atrophic gastritis (ChAG) to gastric adenocarcinoma over a 4-year interval. The cancer-associated strain was less fit in a mouse model of human ChAG, but readily established itself within a mouse gastric epithelial progenitor-derived cell line (mGEP). Transcriptional profiling that compared control and infected mGEP cells revealed that infection of the cancer-associated H. pylori strain regulates cell signaling, metabolism and tumor suppressor genes in a very different way from the ChAG-associated strain. This observation suggests that adaptation of H. pylori to GSCs is critical for the progression of ChAG to GC.
The identification of genetic mutations in patients with GC has greatly advanced our understanding of the disease etiology. Several studies have generated a comprehensive list of mutations in IGC and DGC through cancer genome/exome sequencing[63,99,100]. Here, we only focus on CDH1 mutations, which are strongly associated with DGC and likely contribute to the acquisition of stem cell-like features through the mediation of epithelial-mesenchymal transition (EMT).
CDH1 encodes the epithelial cell-expressed, calcium-dependent, cell adhesion membrane protein E-cadherin. E-cadherin regulates the architecture of the epithelium via the mediation of cell-cell adhesion and the regulation of biological processes, such as signal transduction and cytoskeletal remodeling. E-cadherin consists of five extracellular cadherin repeats, a single transmembrane domain and a well-conserved cytoplasmic domain. The extracellular domain is responsible for the adhesive recognition and interaction with extracellular domains from adjacent cells; the cytoplasmic domain contains the docking site for β-catenin and a variety of other effectors, such as tyrosine kinase receptors, phosphatases and cytoskeleton regulators[101,102].
Loss-of-function mutations of CDH1 have been frequently found in sporadic DGC but not in IGC[103,104]. Immunohistochemistry for E-cadherin in DGC samples further confirmed that E-cadherin levels are usually low or undetectable, which suggests that the loss of E-cadherin leads to DGC. Germline mutations in CDH1 were found in three Maori kindred with hereditary diffuse gastric cancer (HDGC), which provides genetic evidence that a deficiency in CDH1 increases the susceptibility to DGC[105]. Germline inactivating mutations in CDH1 were subsequently verified in patients with HDGC from other ethnic groups and were determined to account for 30% of all HDGC cases[106-108]. The penetrance of CDH1 germline mutations is 67%-83%[109]. In patients with HDGC, the germline mutation only affects one CDH1 allele. The second CDH1 allele is inactivated by mechanisms that include methylation-mediated transcriptional silencing, somatic mutations or loss of heterozygosity[109-114]. The extracellular fragment of E-cadherin, which results from the cleavage of the functional membrane-bound form, is soluble and serves as an important prognostic marker for GC[115].
How E-cadherin deficiency causes DGC is not fully understood. Based on the known properties of E-cadherin, several mutually non-exclusive mechanisms have been proposed. One mechanism is that the loss of E-cadherin enhances canonical Wnt signaling. Because a fraction of β-catenin is normally anchored to the membrane via the cytoplasmic tail of E-cadherin, the down-regulation of E-cadherin may release this fraction into the cytoplasm, which likely enhances the nuclear transcriptional activity of β-catenin/TCF. This model is supported by the observation that the levels of E-cadherin inversely correlate with the transcriptional activity of β-catenin during mouse embryonic development[116]. However, the model is challenged by the observations that the loss of E-cadherin is insufficient to modulate Wnt signaling in cultured cell lines[117], and tumors are initiated in a β-catenin-independent manner in a mouse pancreatic cancer model[118]. Most importantly, APC mutations, which lead to enhanced Wnt signaling, are associated with IGC and colorectal cancer, but are rarely found in DGC[63]. This fact suggests that a deficiency in E-cadherin initiates DGC in a manner independent of the canonical Wnt pathway. Another proposed mechanism is that a deficiency of E-cadherin leads to the loss of contact inhibition and unchecked proliferation of epithelial cells. This hypothesis is supported by the findings that E-cadherin inhibits EGF signaling by direct binding to the kinase receptor EGFR[119-122], which lies upstream of the proliferation-promoting Ras-Erk pathway. Consistent with this idea, mouse xenografts derived from poorly differentiated human GC samples were often amplified at the EGFR locus and were responsive to the EGFR monoclonal antibody Cetuximab[123]. Another potential mechanism is that the loss of E-cadherin induces the acquisition of mesenchymal features by epithelial cells, which are more stem cell-like[124,125], more invasive and resistant to genotoxic insult[96]. The functional loss of E-cadherin is a hallmark of EMT. It has been demonstrated that pre-cancerous mammary epithelial cells acquire stem cell-like features after they transition into mesenchymal cells, at which time they express stem cell markers and are capable of forming spheroid colonies in culture[124]. However, it is currently unclear whether the EMT that results from a CDH1 deficiency contributes to DGC initiation and progression.
Because CSCs can propagate tumors that are a phenocopy of the primary lesion, the residual CSCs that survive standard chemotherapy and surgery are sufficient for cancer recurrence. This situation is exacerbated by the increased resistance of CSCs to cytotoxic drugs and irradiation. Due to their tumor-propagating capacity, CSCs are also considered the source of metastasis. Therefore, the development of strategies that specifically eliminate CSCs could be more effective, but less toxic than standard therapeutic strategies. For example, CSCs may be induced to differentiate by reagents such as retinoic acid (RA) and its derivatives. RA can induce the differentiation of the CD133+ CSCs isolated from human glioblastoma cell lines and reduce their in vitro spheroid colony-forming capacity and tumorigenicity in a mouse xenograft model[126-128]. It has also been reported that RA induced the differentiation of CD44+ breast CSCs and reduced their in vitro sphere-forming capacity[129]. Because CD133 and CD44 are expressed by CSCs of many cancers, strategies for the elimination of CD133+ and/or CD44+ cells have been tested in cell culture and animal models. The knock-down of CD133 in human melanoma cells led to a decreased in vitro spheroid colony-forming capacity and metastatic potential[130]. The elimination of CD44+ cells in cultured GC cells and in transgenic mice reduced tumor-propagating capacity, tumor size and tumor grade[89,93]. Furthermore, cytotoxic antibodies against CD133 effectively inhibited the proliferation of cultured hepatocellular carcinoma cells and GC cells. Intriguingly, these antibodies significantly reduced the tumor mass of hepatocellular carcinoma in a mouse xenograft model[131]. Paclitaxel-loaded nanoparticles conjugated to CD133 antibodies effectively eliminated liver CSCs in cultured liver cancer cells and induced tumor regression in mouse xenografts[132]. Although the efficacy of these approaches requires further validation, a combinatorial approach of specific elimination of CSCs together with standard chemotherapy and surgical methods to remove the tumor mass will likely have a profound impact on the future treatment of patients with GC.
In summary, several populations of GSCs have been identified that regulate physiological tissue renewal and injury repair of the gastric epithelium. In the antrum, both the quiescent Villin-expressing GSCs and the actively proliferating Lgr5+ GSCs can differentiate into all types of fully committed gastric somatic cells[45,46] (Figure 2A). The transformation of either stem cell population leads to GC in mouse models[46,58] (Figure 2B). In the corpus, Troy+ chief cells and Sox2+ cells can replenish the entire cellular population of the gastric units (Figure 2A). However, whether GC originates from these cells remains unclear[47,50] (Figure 2B). Bone marrow-derived cells (BMDCs) can also cause GC in a mouse model of chronic Helicobacter infection[66]. In addition, cells that express CD133 or CD44, markers that define CSCs of solid tumors in many tissues, are frequently identified in human GC[74-76,89,90]. The targeted elimination of the CD133+ or CD44+ cells in mouse models of GC or in human GC-derived cell lines often reduces tumor formation[89,93].
The recent progress in the identification of normal and CSCs in the stomach has provided critical insights into the molecular and cellular etiology of GC. However, many unanswered questions remain. First, all of the GSC populations (Villin+, Lgr5+ Troy+ and Sox2+ cells) have been identified in mouse models. Whether similar GSCs are present in the human stomach and whether the transformation of these cells leads to GC need to be experimentally verified. Second, it remains unclear whether Troy+ cells, Sox2+ cells or a yet unidentified cell population comprise the CSCs in the corpus. The current data have only demonstrated that V-GSCs and L-GSCs in the antrum can convert to CSCs in mouse models. Third, it remains unclear whether tumors that originate from distinct GSC populations are identical. Does the transformation of a certain population of GSCs preferentially give rise to IGC or DGC? Do different tumor suppressors, such as Klf4 in V-GSCs and Apc in L-GSCs, prevent GC via the suppression of common or distinct molecular pathways? In addition, whether H. pylori infection plays a role in the transformation of human GSCs remains to be determined. If so, what is the molecular pathway that is affected by H. pylori? Answers to these questions will greatly improve our understanding of the molecular and cellular etiology of GC and will help with the development of more effective therapeutic strategies.
| 1. | Marx J. Medicine. Bone marrow cells: the source of gastric cancer? Science. 2004;306:1455-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 382] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 5. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 7. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1240] [Article Influence: 35.4] [Reference Citation Analysis (2)] |
| 8. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2761] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 9. | Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 11. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4928] [Article Influence: 169.9] [Reference Citation Analysis (1)] |
| 12. | Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3316] [Cited by in RCA: 3436] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 13. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7800] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 14. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5627] [Article Influence: 255.8] [Reference Citation Analysis (0)] |
| 15. | Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946-10951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1998] [Cited by in RCA: 2045] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 16. | Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328-9337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 939] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 17. | O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3068] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 18. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2952] [Cited by in RCA: 3053] [Article Influence: 152.7] [Reference Citation Analysis (0)] |
| 19. | Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158-10163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1679] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 20. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 934] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 21. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2455] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 22. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2163] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 23. | Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26:2871-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 25. | Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 429] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87-98. [PubMed] |
| 27. | Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 966] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 28. | Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4467] [Cited by in RCA: 4849] [Article Influence: 242.5] [Reference Citation Analysis (0)] |
| 29. | Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2102] [Cited by in RCA: 1961] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 30. | Moitra K, Lou H, Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin Pharmacol Ther. 2011;89:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (5)] |
| 31. | Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 826] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 32. | Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820-13825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1108] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 33. | Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1518] [Cited by in RCA: 1793] [Article Influence: 128.1] [Reference Citation Analysis (0)] |
| 34. | Karam SM, Leblond CP. Identifying and counting epithelial cell types in the “corpus” of the mouse stomach. Anat Rec. 1992;232:231-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 286] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 139] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec. 1993;236:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767-G777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Nomura S, Kaminishi M, Sugiyama K, Oohara T, Esumi H. Clonal analysis of isolated single fundic and pyloric gland of stomach using X-linked polymorphism. Biochem Biophys Res Commun. 1996;226:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Nomura S, Esumi H, Job C, Tan SS. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Braunstein EM, Qiao XT, Madison B, Pinson K, Dunbar L, Gumucio DL. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC, Gumucio DL. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1222] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 47. | Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 48. | Hashimoto T, Schlessinger D, Cui CY. Troy binding to lymphotoxin-alpha activates NF kappa B mediated transcription. Cell Cycle. 2008;7:106-111. [PubMed] |
| 49. | Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 50. | Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 642] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 51. | Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 52. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1695] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 53. | Cozzio A, Passegué E, Ayton PM, Karsunky H, Cleary ML, Weissman IL. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 483] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 54. | Odze RD. Unraveling the mystery of the gastroesophageal junction: a pathologist’s perspective. Am J Gastroenterol. 2005;100:1853-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18576] [Article Influence: 928.8] [Reference Citation Analysis (1)] |
| 56. | Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, Wu TT, Huang S, Xie K. Drastic down-regulation of Krüppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746-2754. [PubMed] |
| 57. | Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, Furth EE, Kaestner KH. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 58. | Li Q, Jia Z, Wang L, Kong X, Li Q, Guo K, Tan D, Le X, Wei D, Huang S. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology. 2012;142:531-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Wei D, Kanai M, Jia Z, Le X, Xie K. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res. 2008;68:4631-4639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 61. | Tetreault MP, Yang Y, Travis J, Yu QC, Klein-Szanto A, Tobias JW, Katz JP. Esophageal squamous cell dysplasia and delayed differentiation with deletion of krüppel-like factor 4 in murine esophagus. Gastroenterology. 2010;139:171-81.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y, Horii A. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 169] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 432] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 64. | Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159-170. [PubMed] |
| 65. | Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668-670. [PubMed] |
| 66. | Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 844] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 67. | Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 406] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 68. | Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002-5012. [PubMed] |
| 69. | Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013-5021. [PubMed] |
| 70. | Corti S, Nizzardo M, Nardini M, Donadoni C, Locatelli F, Papadimitriou D, Salani S, Del Bo R, Ghezzi S, Strazzer S. Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp Neurol. 2007;205:547-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92-97. [PubMed] |
| 72. | Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 452] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 73. | Wu C, Xie Y, Gao F, Wang Y, Guo Y, Tian H, Li Y, Fan W. Lgr5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markers. Gene. 2013;525:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 75. | Hashimoto K, Aoyagi K, Isobe T, Kouhuji K, Shirouzu K. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014;17:97-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, Chen JP, Zhou ZG, Mo XM, Hu JK. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS One. 2013;8:e59154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30-34. [PubMed] |
| 78. | Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303-1313. [PubMed] |
| 79. | Almond A. Hyaluronan. Cell Mol Life Sci. 2007;64:1591-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 80. | Stamenkovic I, Amiot M, Pesando JM, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989;56:1057-1062. [PubMed] |
| 81. | Goldstein LA, Zhou DF, Picker LJ, Minty CN, Bargatze RF, Ding JF, Butcher EC. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989;56:1063-1072. [PubMed] |
| 82. | Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160-12164. [PubMed] |
| 83. | Idzerda RL, Carter WG, Nottenburg C, Wayner EA, Gallatin WM, St John T. Isolation and DNA sequence of a cDNA clone encoding a lymphocyte adhesion receptor for high endothelium. Proc Natl Acad Sci USA. 1989;86:4659-4663. [PubMed] |
| 84. | Ruiz P, Schwärzler C, Günthert U. CD44 isoforms during differentiation and development. Bioessays. 1995;17:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13-24. [PubMed] |
| 86. | Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, Li ML, Tam KH, Lam CT, Poon RT. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 273] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 87. | Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 407] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 88. | Hao J, Chen H, Madigan MC, Cozzi PJ, Beretov J, Xiao W, Delprado WJ, Russell PJ, Li Y. Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br J Cancer. 2010;103:1008-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 89. | Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 963] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 90. | Ghaffarzadehgan K, Jafarzadeh M, Raziee HR, Sima HR, Esmaili-Shandiz E, Hosseinnezhad H, Taghizadeh-Kermani A, Moaven O, Bahrani M. Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World J Gastroenterol. 2008;14:6376-6381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 823] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 92. | da Cunha CB, Oliveira C, Wen X, Gomes B, Sousa S, Suriano G, Grellier M, Huntsman DG, Carneiro F, Granja PL. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab Invest. 2010;90:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 93. | Lau WM, Teng E, Chong HS, Lopez KA, Tay AY, Salto-Tellez M, Shabbir A, So JB, Chan SL. CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 2014;74:2630-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 94. | Hirata K, Suzuki H, Imaeda H, Matsuzaki J, Tsugawa H, Nagano O, Asakura K, Saya H, Hibi T. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 95. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 590] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 96. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7900] [Article Influence: 464.7] [Reference Citation Analysis (1)] |
| 97. | Bessède E, Staedel C, Acuña Amador LA, Nguyen PH, Chambonnier L, Hatakeyama M, Belleannée G, Mégraud F, Varon C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene. 2014;33:4123-4131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 98. | Giannakis M, Chen SL, Karam SM, Engstrand L, Gordon JI. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci USA. 2008;105:4358-4363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 99. | Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 640] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 100. | Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 514] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 101. | McLachlan RW, Yap AS. Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med (Berl). 2007;85:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Leckband D, Prakasam A. Mechanism and dynamics of cadherin adhesion. Annu Rev Biomed Eng. 2006;8:259-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 103. | Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845-3852. [PubMed] |
| 104. | Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, Sugimura T, Hirohashi S. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA. 1994;91:1858-1862. [PubMed] |
| 105. | Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1149] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 106. | Shinmura K, Kohno T, Takahashi M, Sasaki A, Ochiai A, Guilford P, Hunter A, Reeve AE, Sugimura H, Yamaguchi N. Familial gastric cancer: clinicopathological characteristics, RER phenotype and germline p53 and E-cadherin mutations. Carcinogenesis. 1999;20:1127-1131. [PubMed] |
| 107. | Yoon KA, Ku JL, Yang HK, Kim WH, Park SY, Park JG. Germline mutations of E-cadherin gene in Korean familial gastric cancer patients. J Hum Genet. 1999;44:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Gayther SA, Gorringe KL, Ramus SJ, Huntsman D, Roviello F, Grehan N, Machado JC, Pinto E, Seruca R, Halling K. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res. 1998;58:4086-4089. [PubMed] |
| 109. | Barber M, Murrell A, Ito Y, Maia AT, Hyland S, Oliveira C, Save V, Carneiro F, Paterson AL, Grehan N. Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer. J Pathol. 2008;216:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 329] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 111. | Machado JC, Oliveira C, Carvalho R, Soares P, Berx G, Caldas C, Seruca R, Carneiro F, Sobrinho-Simöes M. E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene. 2001;20:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 112. | Oliveira C, de Bruin J, Nabais S, Ligtenberg M, Moutinho C, Nagengast FM, Seruca R, van Krieken H, Carneiro F. Intragenic deletion of CDH1 as the inactivating mechanism of the wild-type allele in an HDGC tumour. Oncogene. 2004;23:2236-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 113. | Becker KF, Höfler H. Frequent somatic allelic inactivation of the E-cadherin gene in gastric carcinomas. J Natl Cancer Inst. 1995;87:1082-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Corso G, Roviello F, Paredes J, Pedrazzani C, Novais M, Correia J, Marrelli D, Cirnes L, Seruca R, Oliveira C. Characterization of the P373L E-cadherin germline missense mutation and implication for clinical management. Eur J Surg Oncol. 2007;33:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Chan AO, Lam SK, Chu KM, Lam CM, Kwok E, Leung SY, Yuen ST, Law SY, Hui WM, Lai KC. Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut. 2001;48:808-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 544] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 117. | Caca K, Kolligs FT, Ji X, Hayes M, Qian J, Yahanda A, Rimm DL, Costa J, Fearon ER. Beta- and gamma-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ. 1999;10:369-376. [PubMed] |
| 118. | Herzig M, Savarese F, Novatchkova M, Semb H, Christofori G. Tumor progression induced by the loss of E-cadherin independent of beta-catenin/Tcf-mediated Wnt signaling. Oncogene. 2007;26:2290-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 119. | Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375-1380. [PubMed] |
| 120. | Fedor-Chaiken M, Hein PW, Stewart JC, Brackenbury R, Kinch MS. E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes. 2003;10:105-118. [PubMed] |
| 121. | Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 122. | Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18:2013-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 123. | Zhang L, Yang J, Cai J, Song X, Deng J, Huang X, Chen D, Yang M, Wery JP, Li S. A subset of gastric cancers with EGFR amplification and overexpression respond to cetuximab therapy. Sci Rep. 2013;3:2992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 124. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6972] [Cited by in RCA: 6910] [Article Influence: 383.9] [Reference Citation Analysis (0)] |
| 125. | Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1116] [Cited by in RCA: 1255] [Article Influence: 69.7] [Reference Citation Analysis (1)] |
| 126. | Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res. 2010;16:2715-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 127. | Karsy M, Albert L, Tobias ME, Murali R, Jhanwar-Uniyal M. All-trans retinoic acid modulates cancer stem cells of glioblastoma multiforme in an MAPK-dependent manner. Anticancer Res. 2010;30:4915-4920. [PubMed] |
| 128. | Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, Guerrero-Cazares H, Quinones-Hinojosa A, Laterra J, Xia S. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene. 2011;30:3454-3467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 129. | Ginestier C, Wicinski J, Cervera N, Monville F, Finetti P, Bertucci F, Wicha MS, Birnbaum D, Charafe-Jauffret E. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle. 2009;8:3297-3302. [PubMed] |
| 130. | Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26:3008-3017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 131. | Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Van Orden KL. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008;99:100-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 132. | Jin C, Yang Z, Yang J, Li H, He Y, An J, Bai L, Dou K. Paclitaxel-loaded nanoparticles decorated with anti-CD133 antibody: a targeted therapy for liver cancer stem cells. J Nanoparticle Res. 2013;16:2157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Deans C S- Editor: Qi Y L- Editor: Webster JR E- Editor: Wang CH