Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.2091
Revised: December 7, 2013
Accepted: January 8, 2014
Published online: February 28, 2014
Processing time: 190 Days and 18.6 Hours
AIM: To investigate the effects of ZD 7288, a hyperpolarization-activated cyclic nucleotide-gated (HCN) channel blocker, on rats with chronic visceral pain.
METHODS: Rats with visceral hypersensitivity were generated using neonatal colon irritation during postnatal days 8-15 as described previously. Visceral hypersensitivity was evaluated using electromyographic (EMG) responses of abdominal external oblique muscles to 20-80 mmHg colorectal distentions (CRD). Abdominal withdrawal reflex (AWR) scores and pain thresholds were also detected in adult rats. Different doses of ZD 7288 (25, 50, and 100 nmol/L) were intrathecally administered in rats to study the role of spinal HCN channel in chronic visceral hypersensitivity.
RESULTS: EMG responses to 20-80 mmHg CRD and AWR scores under 20-60 mmHg CRD significantly increased in rats with visceral hypersensitivity compared to control rats (P < 0.05). The pain threshold in rats with visceral hypersensitivity significantly decreased compared to control rats (P < 0.05). Treatment with 50-100 nmol/L ZD 7288 significantly inhibited EMG responses (16%-62%, 80-20 mmHg CRD, P < 0.05) and AWR scores (24%-37%, 40-20 mmHg CRD, P < 0.05; 12%-61%, 80-20 mmHg CRD, P < 0.05, respectively), and significantly increased pain thresholds (32%-77%, P < 0.05).
CONCLUSION: Spinal HCN channels may play an important role in chronic visceral hypersensitivity.
Core tip: Intrathecal administration of ZD 7288, a hyperpolarization-activated cyclic nucleotide-gated channel blocker, significantly inhibited electromyographic responses and abdominal withdrawal reflex scores and significantly increased pain thresholds in rats with chronic visceral hypersensitivity. These results are important for clinicians and the fundamental scientific community and provide scientific evidence for ZD 7288 as a novel treatment for visceral pain in irritable bowel syndrome.
- Citation: Chen Y, Lin C, Tang Y, Chen AQ, Liu CY, Lu DL. ZD 7288, an HCN channel blocker, attenuates chronic visceral pain in irritable bowel syndrome-like rats. World J Gastroenterol 2014; 20(8): 2091-2097
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/2091.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.2091
Irritable bowel syndrome (IBS) is a common disorder characterized by abdominal pain, bloating and altered bowel habit[1]. No effective drug treatment is currently available for IBS[1-4]. The persistent colon hypersensitivity in IBS is associated with neuronal sensitization, which manifests as an increase in neuronal excitability[5]. Some ion channels may be the primary determinants of neuronal excitability[6,7]. Emery et al[8] stated “The rate of action potential firing in nociceptors is a major determinant of the intensity of pain. Possible modulators of action potential firing include the hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channels”. The HCN channel was first identified in the sinoatrial node (SAN) in rats[9]. These channels are permeable to both Na+ and K+. The HCN current, hyperpolarization-activated cation current (Ih), was found in neurons under hyperpolarization of the cell membrane[10]. Ih typically contributes to the pacemaker activity of cardiac SAN cells and a variety of spontaneously firing neurons. Ih is crucial in determining the frequency of firing of action potentials[11]. Much attention has focused on HCN channels and somatic pain in the past decade[8,12], and Ih is an important contributor of neuropathic pain. The HCN channel might be a valuable target for the treatment of neuropathic pain[10].
ZD 7288 [4-(N-Ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamino) pyrimidinium chloride] completely blocked Ih in a previous study, and eliminated the depolarizing sag of transmembrane potential[13]. ZD 7288 may reduce neuropathic pain and provide significant analgesic effects[14-16]. Ectopic discharges may be involved in the development of neuropathic pain[10]. A low concentration of ZD 7288 significantly suppressed ectopic discharges generated from DRG neurons[10]. HCN ion channels are called pacemakers of pain[11], but all conclusions about the relationship between HCN channels and pain have come from somatic pain research. No studies on the role of HCN channels in visceral pain have been conducted. The contribution of HCN channels to the genesis of visceral hypersensitivity must be explored.
Most studies on chronic visceral pain mechanisms focused on inflammatory mediators, neuron-active compounds, neurotransmitters and receptors in peripheral and central sensitization[17]. However, ion channels may be the primary determinants of neuronal excitability[18]. The HCN channel current creates a more positive resting membrane potential and up-regulates neuronal excitability[10]. ZD 7288 provides significant analgesic effects against neuropathic pain[10], and it may attenuate visceral pain through a similar mechanism. However, the role of ZD 7288 in chronic visceral pain has not been investigated.
An IBS-like rat model was established in the present study using neonatal colon irritation. Abdominal withdrawal reflex (AWR) scores and electromyographic (EMG) amplitudes were recorded. The effects of ZD 7288 on visceral sensitivity in IBS-like rats were evaluated.
Male Sprague-Dawley rats were obtained as preweanling neonates (5 d old) from the Shanghai SLAC Laboratory Animal Co., Ltd. (Animal approval number: SCXK 2007-0005). Twelve neonates were housed in a cage with 1 adult female rat until they were 28 d old. The adult female rat had access to food and water ad libitum. After separation, 4 young rats were housed in a plastic cage with sawdust bedding, given access to food and water ad libitum and maintained on a 12-h light/dark cycle. The irritation procedure and experimental testing were conducted during the light cycle. Experiments were performed when the rats were 8 wk old.
Neonatal colon irritation was applied once daily using colorectal distention (CRD) during postnatal days 8-15. The CRD procedure was modified from Lin and Al-Chaer[19]. The distention was applied using angioplasty balloons (length, 20.0 mm; diameter, 2.5 mm) inserted rectally into the descending colon. The balloon was distended with air exerting a pressure of 60 mmHg for 1 min, deflated and withdrawn. The control rats were handled similarly to the model group except that no CRD was applied. The experiments were approved by the Animal Care and Use Committee of Fujian Medical University.
EMG recordings were performed as described previously[20]. Rats were anesthetized with ether, and distention balloons (5 cm in length; made of the finger of a latex glove attached to polyethylene tubing) were inserted through the anus into the rectum and descending colon of adult rats. The tubing was taped to the tail to hold the balloon in place. Two silver bipolar electrodes were inserted into the external oblique muscle of the abdomen (EOMA). The rats were maintained in a supine position in a self-made restrainer and allowed at least 30 min to recover from inhalational anesthesia. The tubing was attached through a T-connector to a sphygmomanometer pump and a pressure gauge. Distention was produced by rapid inflation of the balloon to the desired pressure (20, 40, 60 or 80 mmHg) for 10 s followed by a 4-min rest. The magnitude of EMG was measured using an RM6240BD multi-channel physiological signal acquisition and processing system (Chengdu, China; high-frequency filter: 3 KHz; time constant: 0.001 s; sampling frequency: 40 KHz; sensitivity: 500 μV; scanning speed: 200 ms/div). Twenty amplitudes were measured during each 10 s distention period, and the mean amplitude represented the magnitude of EMG. EMG data were derived by subtracting the mean baseline amplitude (10-s pre-distention period) from the mean EMG amplitude of each pressure.
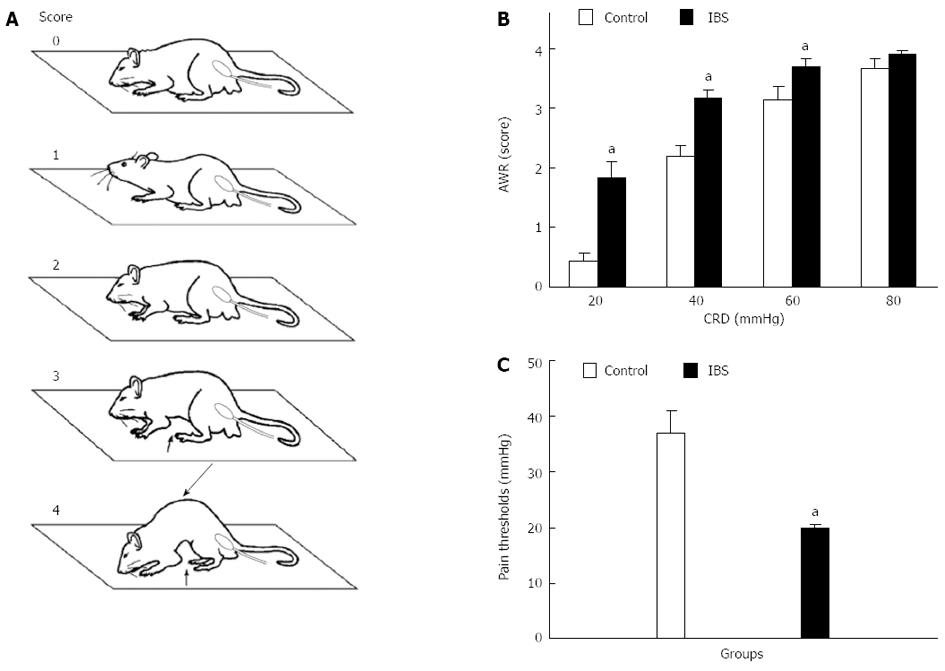
Behavioral responses to CRD were assessed via AWR measurement using a semi-quantitative score[21]. AWR is an involuntary motor reflex that is similar to the visceromotor reflex[22]. AWR measurement consisted of visual observation of animal responses to graded CRD (20, 40, 60, and 80 mmHg) by blinded observers who assigned AWR scores (Figure 1A, Table 1).
| Score | |
| 0 | No behavioral response to colorectal distention |
| 1 | Immobile during colorectal distention and occasional head clinching at stimulus onset |
| 2 | Mild contraction of the abdominal muscles but absence of abdomen lifting from the platform |
| 3 | Observed strong contraction of the abdominal muscles and lifting of the abdomen off the platform |
| 4 | Arching of the body and lifting of the pelvic structures and scrotum |
Visceral pain thresholds were evaluated via measurement of the pressure thresholds (increments of 5 mmHg, 3-min duration, 1-min rest) required to evoke abdominal contractions that caused the rat to lift its abdomen off the platform. The recordings were repeated three times for each animal, and the arithmetic mean value was calculated.
Rats were anesthetized with barbanylum (8%, 0.1 mL/100 g). A sterile polyethylene catheter (PE10 tubing, Becton Dickinson) was introduced at the L6/S1 interspace and threaded to the lumbar enlargement. Rats recovered for 1 wk after intrathecal cannulation. Various doses (25, 50, and 100 nmol/L) of ZD 7288 (molecular weight 292.81, Tocris Cookson, Ellisville, MI) diluted in sterile 0.9% saline were administered at a volume of 10 µL followed by a 10-µL saline flush. Visceral pain measurements were performed 30 min after intrathecal administration.
Data are expressed as means ± SEM (standard error). A two-way repeated measures analysis of variance (ANOVA) with Bonferroni post hoc analysis was used to assess changes in AWR or EMG in different groups across pressures. Comparisons of pain thresholds between IBS-like rats and control rats were analyzed using Student’s t test. One-way ANOVA followed by the Student-Newman-Keuls post-hoc test was used to compare differences in pain thresholds of IBS-like rats before and after different ZD 7288 doses. Data analysis was performed using SPSS 13.0. A P value < 0.05 was considered statistically significant.
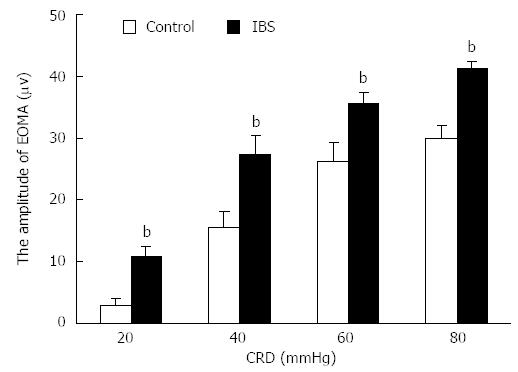
The AWR scores at 20-60 mmHg CRD were significantly higher in IBS-like rats than those in control rats (P < 0.05, Figure 1B). The pain threshold of IBS-like rats significantly decreased compared to control rats (P < 0.05, Figure 1C). EMG amplitudes in IBS-like rats significantly increased at 20-80 mmHg CRD compared to control rats (P < 0.05, Figure 2).
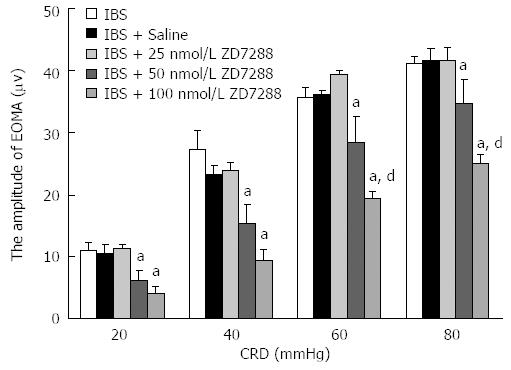
No differences in EMG amplitudes were observed between the IBS-like, IBS-like + saline and IBS-like + 25 nmol/L ZD 7288 groups. IBS-like rats given 50 and 100 nmol/L ZD 7288 demonstrated a significant CRD-dependent (20-80 mmHg) decrease in amplitudes compared to the IBS-like + saline group (50 nmol/L: P < 0.05; 100 nmol/L: P < 0.01, Figure 3). IBS-like rats given 50 nmol/L ZD 7288 showed a significant reduction in EMG amplitudes compared to IBS-like rats given normal saline (41%, 35%, 22%, and 16% at 20, 40, 60, and 80 mmHg CRD, respectively). Treatment with 100 nmol/L ZD 7288 significantly inhibited EMG amplitudes (62%, 59%, 46%, and 40% at 20, 40, 60, and 80 mmHg CRD, respectively) compared to IBS-like rats given normal saline.
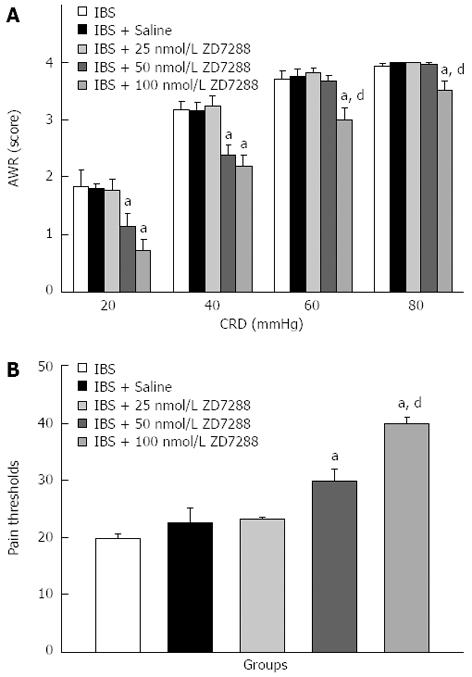
Furthermore, no differences in AWR scores were observed between the IBS-like, IBS-like + saline and IBS-like + 25 nmol/L ZD 7288 groups. IBS-like rats given 50 nmol/L (20-40 mmHg) and 100 nmol/L (20-80 mmHg) ZD 7288 showed a significant CRD-dependent decrease in AWR scores compared to the IBS-like + saline group. IBS-like rats given 50 nmol/L ZD 7288 exhibited significantly reduced AWR scores compared to IBS-like rats given normal saline (37% at 20 mmHg CRD and 24% at 40 mmHg CRD). ZD 7288 at 100 nmol/L significantly inhibited AWR scores (61%, 30%, 20%, and 12% at 20, 40, 60, and 80 mmHg CRD, respectively) (Figure 4A).
The pain thresholds of rats treated with 50 nmol/L ZD 7288 increased by 32% compared to rats given normal saline, and the pain thresholds of rats treated with 100 nmol/L ZD 7288 increased by 77% (Figure 4B).
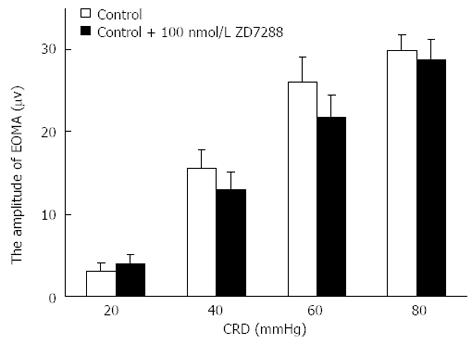
No significant differences in EMG amplitudes were observed between the control and control + 100 nmol/L ZD 7288 groups (Figure 5).
This study tested the effect of intrathecal ZD 7288, an HCN channel blocker, on the visceral hypersensitivity of IBS-like rats. The results of EMG, AWR and pain thresholds clearly demonstrate that 50-100 nmol/L ZD 7288 significantly attenuated chronic visceral hypersensitivity in IBS-like rats. These data indicate that spinal HCN channels contribute to visceral hypersensitivity in IBS-like rats.
Emery et al[8] reported that HCN channels play a central role in neuropathic pain. ZD 7288 effectively attenuates neuropathic pain[16]. Most of the drugs that are specifically approved for the treatment of visceral pain syndromes are effective treatments for chronic neuropathic pain states[23], which suggests that chronic visceral pain and chronic neuropathic pain share a common mechanism. Treatment with 50 nmol/L and 100 nmol/L ZD 7288 significantly inhibited EMG amplitudes in IBS-like rats in our study (16%-41% and 40%-62%, 80-20 mmHg CRD, respectively). Intrathecal administration of 100 nmol/L ZD 7288 significantly relieved mechanical allodynia in neuropathic pain rats with spinal nerve ligation, but 50 nmol/L ZD 7288 had no effect[16]. These results indicated that HCN channels may be the common mechanism in visceral and neuropathic pain, and these channels may play a more important role in visceral hypersensitivity.
Neuropathic pain is characterized by ectopic discharges, which are similar to the discharges observed in IBS-like rats[19]. The spontaneous activity of lumbosacral afferents and the number of dorsal roots activated by CRD are significantly enhanced in IBS-like rats compared to controls[19,21]. Spinal HCN channels contribute to the maintenance of neuropathic pain, most likely at the primary afferent terminals[6]. Ih amplitude is augmented in the ventral-lateral periaqueductal gray neurons in neuropathic pain models, and an increase in the frequency of ZD 7288-attenuated action potential firing is observed[24]. Therefore, we inferred that the hyperexcitability of spinal ascending neurons due to an up-regulation of HCN channels might underlie spinal sensitization in chronic visceral pain. However, more electrophysiological studies, such as whole-cell patch clamp recordings, are required.
Several treatments, such as anti-spasm medications, antidepressants, probiotics and acupuncture, are efficacious for IBS, but patients and clinicians question their efficacy due to the recurrence of abdominal pain, diarrhea and other symptoms[1-4]. For example, acupuncture is clinically effective for visceral pain due to bowel obstruction, inflammation or ulcer, but controversies exist due to the high recurrence rate[25,26]. Scientific evidence of acupuncture treatment efficacy is lacking, and its mechanisms require to be investigated[25]. Antagonists to NMDA receptors, such as MK-801[27] and AP-7[5], inhibit visceral hypersensitivity. However, the use of these agents in the treatment of chronic pain is restricted due to their serious side effects, including hallucinations, learning and memory impairments, and sensorimotor disturbances[28,29].
In this study, 100 nmol/L ZD 7288 exhibited stronger analgesic effects without apparent side effects, which is consistent with the results of Wan’s study on neuropathic pain[16]. Intrathecal administration of ZD 7288 increased pain thresholds in IBS-like rats in a dose-dependent manner. Visceral hypersensitivity includes allodynia and hyperalgesia. Allodynia indicates that an originally non-noxious stimulation induces pain, and hyperalgesia indicates that an originally noxious stimulation induces a supernormal reaction[21,30,31]. Neonatal CRD in the present study may result in allodynia and hyperalgesia, which is consistent with previous studies[20]. ZD 7288, an HCN channel blocker, attenuated visceral pain at 20-40 (non-noxious stimulation) and 60-80 mmHg CRD (noxious stimulation). Therefore, ZD 7288 attenuated allodynia and hyperalgesia in rats with chronic visceral pain. Our results suggest that ZD 7288, an HCN channel blocker, is a useful drug for the treatment of chronic visceral pain in the future.
Neonatal CRD produced allodynia and hyperalgesia in the present study, which supports the hypothesis that early life stress may trigger visceral hyperalgesia and colonic dysfunction[32]. Our AWR scores were ca. 1.8, 3.1, 3.8, and 3.9 at 20-80 mmHg CRD in IBS-like rats, which is partially different from the results obtained by Li et al[33], who reported AWR scores of ca. 0.9, 2.5, 3.1 and 3.5 in visceral hypersensitivity rats. These differences may be attributed to the distinct models. Li et al[33] used neonatal maternal deprivation to produce visceral hypersensitivity, and our study used neonatal CRD to induce IBS-like symptoms. These results demonstrate that neonatal CRD may induce allodynia and hyperalgesia, but neonatal maternal deprivation may preferentially induce hyperalgesia. Zhou et al[4] proposed that a 9-d heterotypic intermittent stress generates visceral hypersensitivity. The results of these three studies suggest that stimulus intensity is more important than stimulus type for the induction of visceral hypersensitivity.
EMG and AWR scores are two different methods to assess visceral hypersensitivity in rats[4,20,33]. AWR scores are semi-measurement data, and it is sometimes difficult for an observer to distinguish the difference between scores of 3 and 4. EMG recording results are pure measurement data that are not influenced by objective factors. EMG recording is a relatively sensitive method to evaluate visceral pain. However, other signals easily interfere with EMG recordings during the experimental process. Most AWR scores in our study were consistent with the EMG findings. Therefore, the combined application of EMG and AWR scores improves the credibility of results.
In conclusion, intrathecal administration of ZD 7288, an HCN channel blocker, attenuated visceral hypersensitivity in IBS-like rats without motor disorders. Therefore, ZD 7288 might be a potential treatment for IBS.
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder that is characterized by chronic visceral pain, bloating and altered bowel habit. Several treatments, such as anti-spasm medications, antidepressants, and probiotics, are efficacious for IBS, but patients and clinicians question their efficacy due to the recurrence of abdominal pain. Hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channels are called pacemakers of pain. ZD 7288, an HCN channel blocker, may reduce neuropathic pain and provide significant analgesic effects. However, the role of ZD 7288 in chronic visceral pain remains unknown.
The present study used ZD 7288 to treat visceral hypersensitivity in rats with IBS-like symptoms induced using neonatal colorectal distention. Treatment with 50-100 nmol/L ZD 7288 attenuated chronic visceral pain and increased pain thresholds in IBS-like rats.
This is the first study to demonstrate that ZD 7288 treatment produces an analgesic effect on visceral hyperalgesia in rats with IBS-like symptoms induced using neonatal colorectal distention.
This study showed that ZD 7288 administration produced an analgesic effect on visceral hyperalgesia in IBS-like rats and provide scientific evidence for ZD 7288 as a novel treatment for visceral pain in IBS.
IBS is a common gastrointestinal disorder that is characterized by chronic visceral pain, bloating and altered bowel habit. The HCN channel is a hyperpolarization-activated cyclic nucleotide-gated channel. The HCN current is a crucial determinant of the firing frequency of action potentials. ZD 7288 is an HCN channel blocker.
This study describes an analgesic effect of ZD 7288 in IBS-like rats and elucidates that ion channels may be the primary determinants of neuronal excitability. The HCN channel current creates a more positive resting membrane potential and up-regulates neuronal excitability. The presented results are important for clinicians and the fundamental scientific community.
| 1. | Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2013;14:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Camilleri M. Review article: new receptor targets for medical therapy in irritable bowel syndrome. Aliment Pharmacol Ther. 2010;31:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 3. | Zhou EH, Liu HR, Wu HG, Shi Y, Wang XM, Tan LY, Yao LQ, Zhong YS, Jiang Y, Zhang LL. Suspended moxibustion relieves chronic visceral hyperalgesia via serotonin pathway in the colon. Neurosci Lett. 2009;451:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Zhou YY, Wanner NJ, Xiao Y, Shi XZ, Jiang XH, Gu JG, Xu GY. Electroacupuncture alleviates stress-induced visceral hypersensitivity through an opioid system in rats. World J Gastroenterol. 2012;18:7201-7211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Lin C, Al-Chaer ED. Differential effects of glutamate receptor antagonists on dorsal horn neurons responding to colorectal distension in a neonatal colon irritation rat model. World J Gastroenterol. 2005;11:6495-6502. [PubMed] |
| 6. | Takasu K, Ono H, Tanabe M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain. Pain. 2010;151:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain. 2007;131:243-257. [PubMed] |
| 8. | Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455-472. [PubMed] |
| 10. | Jiang YQ, Sun Q, Tu HY, Wan Y. Characteristics of HCN channels and their participation in neuropathic pain. Neurochem Res. 2008;33:1979-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Emery EC, Young GT, McNaughton PA. HCN2 ion channels: an emerging role as the pacemakers of pain. Trends Pharmacol Sci. 2012;33:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Wickenden AD, Maher MP, Chaplan SR. HCN pacemaker channels and pain: a drug discovery perspective. Curr Pharm Des. 2009;15:2149-2168. [PubMed] |
| 13. | Harris NC, Constanti A. Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J Neurophysiol. 1995;74:2366-2378. [PubMed] |
| 14. | Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169-1178. [PubMed] |
| 15. | Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y. Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain. 2008;137:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Wan Y. Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain. Sheng Li Xue Bao. 2008;60:579-580. [PubMed] |
| 17. | Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol. 2009;31-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 18. | Lewis AS, Chetkovich DM. HCN channels in behavior and neurological disease: too hyper or not active enough? Mol Cell Neurosci. 2011;46:357-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73-82. [PubMed] |
| 20. | Luo XQ, Cai QY, Chen Y, Guo LX, Chen AQ, Wu ZQ, Lin C. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord contributes to chronic visceral pain in rats. Brain Res. 2014;1542:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [PubMed] |
| 22. | Morteau O, Hachet T, Caussette M, Bueno L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci. 1994;39:1239-1248. [PubMed] |
| 23. | Wesselmann U, Baranowski AP, Börjesson M, Curran NC, Czakanski PP, Giamberardino MA, Ness TJ, Robbins MT, Traub RJ. Emerging therapies and novel approaches to visceral pain. Drug Discov Today Ther Strateg. 2009;6:89-95. [PubMed] |
| 24. | Du L, Wang SJ, Cui J, He WJ, Ruan HZ. The role of HCN channels within the periaqueductal gray in neuropathic pain. Brain Res. 2013;1500:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Cui KM, Li WM, Gao X, Chung K, Chung JM, Wu GC. Electro-acupuncture relieves chronic visceral hyperalgesia in rats. Neurosci Lett. 2005;376:20-23. [PubMed] |
| 26. | Lembo AJ, Conboy L, Kelley JM, Schnyer RS, McManus CA, Quilty MT, Kerr CE, Drossman D, Jacobson EE, Davis RB. A treatment trial of acupuncture in IBS patients. Am J Gastroenterol. 2009;104:1489-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Traub RJ, Zhai Q, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression in the spinal cord and the visceromotor reflex. Neuroscience. 2002;113:205-211. [PubMed] |
| 28. | Soleimannejad E, Naghdi N, Semnanian S, Fathollahi Y, Kazemnejad A. Antinociceptive effect of intra-hippocampal CA1 and dentate gyrus injection of MK801 and AP5 in the formalin test in adult male rats. Eur J Pharmacol. 2007;562:39-46. [PubMed] |
| 29. | Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS, Xing GG. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009;215:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Zhuo M. A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells. 2007;23:259-271. [PubMed] |
| 31. | Li XY, Ko HG, Chen T, Collingridge GL, Kaang BK, Zhuo M. Erasing injury-related cortical synaptic potentiation as a new treatment for chronic pain. J Mol Med (Berl). 2011;89:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJ. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2007;292:G849-G856. [PubMed] |
| 33. | Li L, Xie R, Hu S, Wang Y, Yu T, Xiao Y, Jiang X, Gu J, Hu CY, Xu GY. Upregulation of cystathionine beta-synthetase expression by nuclear factor-kappa B activation contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Mol Pain. 2012;8:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
P- Reviewers: Hauser G, Yang CH S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Zhang DN