Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.2062
Revised: November 1, 2013
Accepted: November 18, 2013
Published online: February 28, 2014
Processing time: 170 Days and 20.7 Hours
AIM: To investigate the effect of T helper (Th) 17/T regulatory (Treg) cells on hepatic fibrosis in mice and its possible mechanism.
METHODS: Hepatic fibrosis was induced by intraperitoneal injection of carbon tetrachloride. Hepatic pathological changes were observed by hematoxylin and eosin staining; the protein levels of interleukin (IL)-6, transforming growth factor (TGF)-β and α-smooth muscle actin (SMA) in liver tissue were determined by Western blotting; and the frequency of Th17 and Treg cells in the liver was estimated by flow cytometry. In addition, hepatic stellate cells were isolated from healthy mouse liver and co-cultured with Th17 or Treg cells. Immunofluorescence staining and Western blotting were performed to determine the change in HSC activation.
RESULTS: In the model group, there were different degrees of fibroplasia, degeneration and necrosis. The protein levels of IL-6, TGF-β and α-SMA in liver tissue were significantly higher than those in the control group at 12 wk (P < 0.05). Compared with the control group, the frequency of Th17 cells in the model group was increased but the frequency of Treg cells decreased gradually. Furthermore, at 4, 8 and 12 wk, there were significant differences in the number of Th17 cells (0.52% ± 0.16%, 1.46% ± 0.24%, and 2.60% ± 0.41%, respectively, P < 0.05) and Treg cells (2.99% ± 0.40%, 2.16% ± 0.50%, and 1.49% ± 0.34%, respectively, P < 0.05). In vitro, Th17 cells promoted, whereas Treg cells inhibited the expression of α-SMA, both in a dose-dependent manner, compared with the control group.
CONCLUSION: Th17/Treg imbalance exists in mice with liver fibrosis, which potentially promotes liver fibrosis via HSC activation.
Core tip: It has been reported that T helper (Th) 17/T regulatory (Treg) cell imbalance is closely related to many autoimmune diseases. The role of Th17/Treg imbalance in liver fibrosis has seldom been reported. Our study focused on the change in Th17/Treg balance in a liver fibrosis model in mice, and explored the possible mechanism through which the development of fibrosis is regulated. The frequency of Th17 cells increased, while the frequency of Treg cells decreased in liver fibrosis. These changes promote the occurrence of liver fibrosis via hepatic stellate cell activation.
- Citation: Sun XF, Gu L, Deng WS, Xu Q. Impaired balance of T helper 17/T regulatory cells in carbon tetrachloride-induced liver fibrosis in mice. World J Gastroenterol 2014; 20(8): 2062-2070
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/2062.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.2062
Liver fibrosis is a chronic progressive disease that is characterized by the formation and accumulation of extracellular matrix that lead to the remodeling of the hepatic architecture. It is the final common pathway in a variety of chronic liver diseases that can be reversed at an early stage, but when it is irreversible, the patients with liver fibrosis are at increased risk of developing cirrhosis. However, the pathogenesis of fibrosis is not entirely clear at present.
Helper CD4+ T cells can orchestrate host immune responses through the release of distinct cytokine profiles. Recent studies have described two additional subsets-interleukin (IL)-17-producing CD4+ T helper (Th) 17 cells and T regulatory (Treg) cells[1]. Th17 cells expressing retinoic-acid-related orphan receptor (ROR)-γt play critical roles in the development of autoimmunity and allergic reactions by producing IL-17[2-4], while Treg cells expressing the forkhead/winged helix transcription factor P3 (FoxP3) have an anti-inflammatory role and maintain tolerance to self-components[5] by contact-dependent suppression or releasing anti-inflammatory cytokines [IL-10 and transforming growth factor (TGF)-β][6,7]. Recently, many studies have found that imbalance of Th17/Treg cells is closely related to a variety of autoimmune diseases[8-11]. However, the role of Th17/Treg imbalance in liver fibrosis has seldom been reported.
The objectives of this study were to evaluate whether Th17/Treg balance is disrupted in mice with liver fibrosis, and to explore the potential mechanism through which Th17/Treg imbalance promotes the development of liver fibrosis. We used carbon tetrachloride (CCl4) to induce liver fibrosis in a mouse model, and mice were sacrificed at 4, 8 and 12 wk. We first measured the protein levels of IL-6, TGF-β and α-smooth muscle actin (SMA) by Western blotting, and the frequency of Th17 and Treg cells in the liver was evaluated by flow cytometry. Finally, we investigated the effect of Th17 and Treg cells on the activation of hepatic stellate cells (HSCs) in vitro.
Male C57BL⁄6 mice (aged 6-8 wk, 18-21 g) were purchased from the Shanghai Slac Experimental Animal Centre (Shanghai, China). Mice were maintained under specific pathogen-free conditions with a 12-h light/dark cycle and unlimited supplies of food and water. The experimental procedures conformed to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals and were approved by the Research Ethics Committee of Renji Hospital [SYXY (hu) 2011-0121]. Sixty mice were randomly divided into a control group (n = 30) and model group (n = 30), and then the mice in each group were randomly divided into 4, 8 and 12-wk groups of 10 mice each.
Mice in the model group were injected intraperitoneally, twice a week, with 10 µL/g of 30% CCl4 (Shanghai Jiahe Biotechnology, Shanghai, China) dissolved in olive oil. Mice in the control group were given the same volume of olive oil for the indicated time intervals. Mice were sacrificed 72 h after the final CCl4 injection at 4, 8 and 12 wk, and liver tissues were collected. The liver tissues were divided into two parts. One part was kept for histological examination and Western blotting, and the other was used for the detection of Th17 and Treg cells.
The liver tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Slices 4-μm thick were prepared and stained with hematoxylin and eosin (HE) according to standard procedures. The degree of fibrosis was assessed based on Scheuer’s scoring system[12].
Total protein was extracted according to the manufacturer’s instructions (Pierce, United States) and the protein concentration was determined. Proteins were separated by 12% SDS-PAGE and then transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% non-fat milk for 2 h followed by incubation with primary antibody in Tris-buffered saline with Tween overnight at 4 °C (anti-IL-6 1:300 dilution; anti-TGF-β 1:300 dilution; and anti-α-SMA 1:500 dilution); all the antibodies were purchased from Abcam (Cambridge, United Kingdom). The membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (1:10000 dilution, LI-COR, Lincoln, NE, United States) The membrane was scanned by Odyssey machine and quantified using Image J version 1.4.3.67 software.
Single-cell suspensions were prepared from liver by dissecting the tissue into small pieces, grinding them, and then filtering them through stainless steel meshes. Lymphocytes were obtained through Percoll density gradient centrifugation (Beijing Dingguo Biotechnology, Beijing, China) from the cell suspensions. For Th17 cell detection, lymphocytes were stimulated for 5 h with 50 ng/mL phorbol myristate acetate (PMA), 1 µmol/L ionomycin (both from Sigma, St Louis, MO, United States) and 10 µg/mL brefeldin A (eBioscience, San Diego, CA, United States) in RPMI-1640 (Hyclone, Logan, UT, United States) supplemented with 10% fetal bovine serum (FBS; Hyclone). Upon harvest, cells were first stained with fluorescein isothiocyanate (FITC)-anti-CD4 at 4 °C for 20 min, then fixed in paraformaldehyde, permeabilized in Perm/Fix solution, and finally stained intracellularly with PE-anti-IL-17A. For Treg cell detection, lymphocytes were incubated with FITC-anti-CD4 and APC-anti-CD25 at room temperature for 20 min[13]. Cells stained with IgG isotype control were used as controls. All antibodies and fixation/permeabilization agents were purchased from Bioscience. Cells were analyzed by FACSCalibur flow cytometer. The percentage of positive cells was determined using CXP analysis software.
Mouse HSCs were isolated from the livers of C57BL⁄6 by in situ collagenase perfusion and differential centrifugation on Percoll density gradients (Beijing Dingguo Biotechnology) as described previously[14]. The fresh isolated HSCs were resuspended in RPMI-1640 (Hyclone) containing 10% FBS and penicillin/streptomycin, and then plated onto 24-well plates (plastic plates were used for self-activation of HSCs). The HSCs were cultured for 6 d and harvested for subsequent use. The purity of HSC cultures was > 92% as determined by the fluorescence of vitamin-A-containing lipid droplets[15-17]. All cells were cultured in a humidified incubator with 5% CO2 at 37 °C.
Th17 and Treg cells were isolated from the spleen of C57BL⁄6 mice using a cell isolation kit (Miltenyi Biotec, Bisley, Surrey, United Kingdom). For Th17 cell isolation, fresh spleen cells were stimulated with ionomycin (1 µg/mL) and PMA (10 ng/mL) for 3 h; labeled with Mouse IL-17 Catch Reagent, IL-17 Detection Antibody (Biotin) and Anti-Biotin-PE; and collected by magnetic separation. For Treg cell isolation, non-CD4+T cells were depleted after magnetic labeling of non-CD4+ cells and fluorescent labeling of CD25+ cells, finally the cells were labeled with Anti-PE MicroBeads, and CD4+CD25+ regulatory T cells were selected with MS Columns. Cells were cultured and expanded for 5 d using CD3/CD28 MACSiBead Particles and 2000 U/mL IL-2. RPMI-1640 medium supplemented with 10% FBS and penicillin/streptomycin were used for cultures. After culturing, cells were harvested and beads were removed.
Forty-eight-well plates (Corning, Corning, NY, United States) were used for cell co-cultures. First, the HSCs were plated at a density of 1 × 104 cells per well and cultured for 12 h. Th17 and Treg cells were added separately to the culture system. The cells were co-cultured for 3 d and expression of α-SMA was determined by Western blotting and immunofluorescene staining.
Immunofluorescence staining was performed as described previously[16,17]. The fixed cells were stained with rabbit anti-mouse α-SMA monoclonal antibody (1:100 dilution; Abcam) followed by CY3-conjugated goat anti-rabbit antibody (1:400 dilution) (Jackson ImmunoResearch, West Grove, PA, United States). DAPI was used for nuclear staining. Expression of α-SMA was observed under fluorescent microscopy and fluorescence intensity was determined by Image Pro Plus software.
Statistical analysis was performed using SPSS version 17.0 software. The results were presented as mean ± SD. One-way analysis of variance was applied, and P < 0.05 was considered statistically significant.
Hepatic fibrosis scores are shown in Table 1. In the model group, hepatic fibrosis score increased gradually after CCl4 injection; however, the scores in the control group were largely unchanged. These results indicated that olive oil was not harmful to the mouse liver.
| Time | Group | Number | Liver fibrosis score | |||
| - | + | ++ | +++ | |||
| 4 wk | Model | 9 | 2 | 7 | ||
| Control | 10 | 10 | ||||
| 8 wk | Model | 8 | 2 | 6 | ||
| Control | 10 | 10 | ||||
| 12 wk | Model | 6 | 1 | 5 | ||
| Control | 10 | 9 | 1 | |||
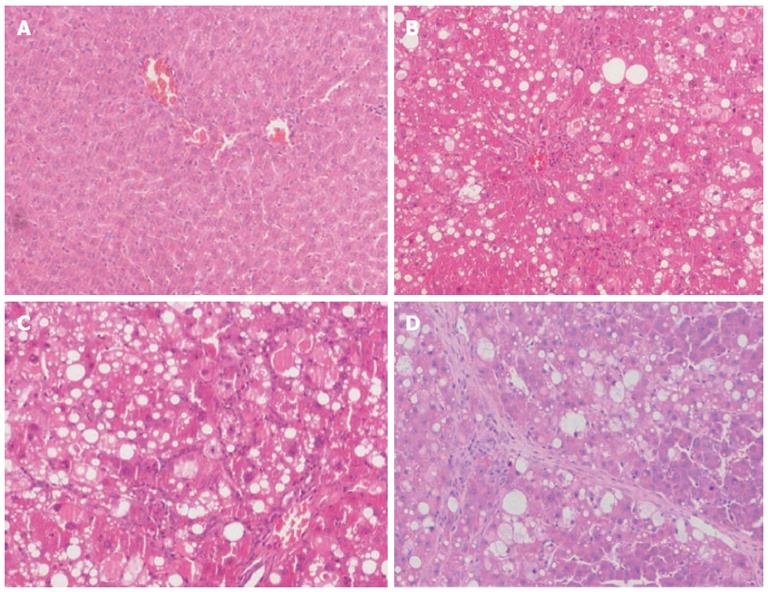
Mouse liver surface in the control group was smooth, the structure of the hepatic lobules and portal area was complete, and there was no cellular degeneration, inflammatory reaction and fiber cords as shown by HE staining (Figure 1A). After CCl4 administration, mice developed different degrees of hepatic inflammation, cellular degeneration, necrosis, and distorted hepatic architectural (Figure 1B-D). At the end of 12 wk, mouse liver showed diffuse hyperplasia of fibrous tissue, the arrangement of the hepatic cells was disordered, and there was a lot of degeneration and necrosis of liver cells, with deficiency of the central vein (Figure 1D). As the time of CCl4 injection was increased, the liver fibrosis model was gradually established.
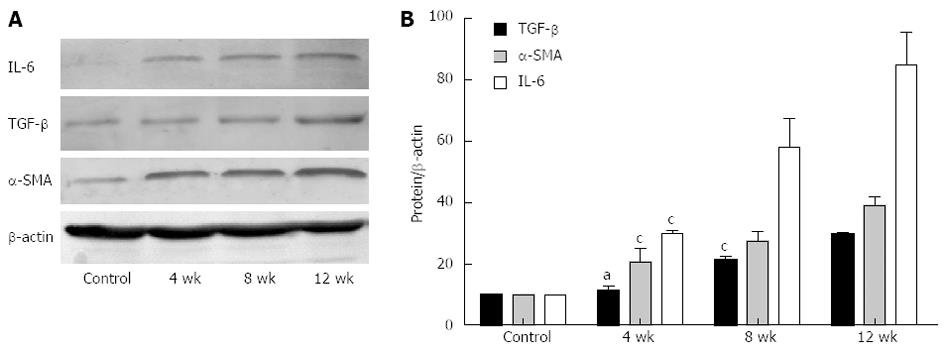
After CCl4 administration, the expression of IL-6, TGF-β and α-SMA was increased as compared with the controls (Figure 2A). Relative protein levels are shown in Figure 2B. Expression of TGF-β at 4 wk was higher than in the control group but the difference was not significant (P > 0.05). The level of TGF-β at 8 and 12 wk was significantly increased (P < 0.05). Compared with the controls, the expression of IL-6 and α-SMA in the model group was significantly increased (P < 0.05), and the differences at 4, 8 and 12 wk were significant (P < 0.05).
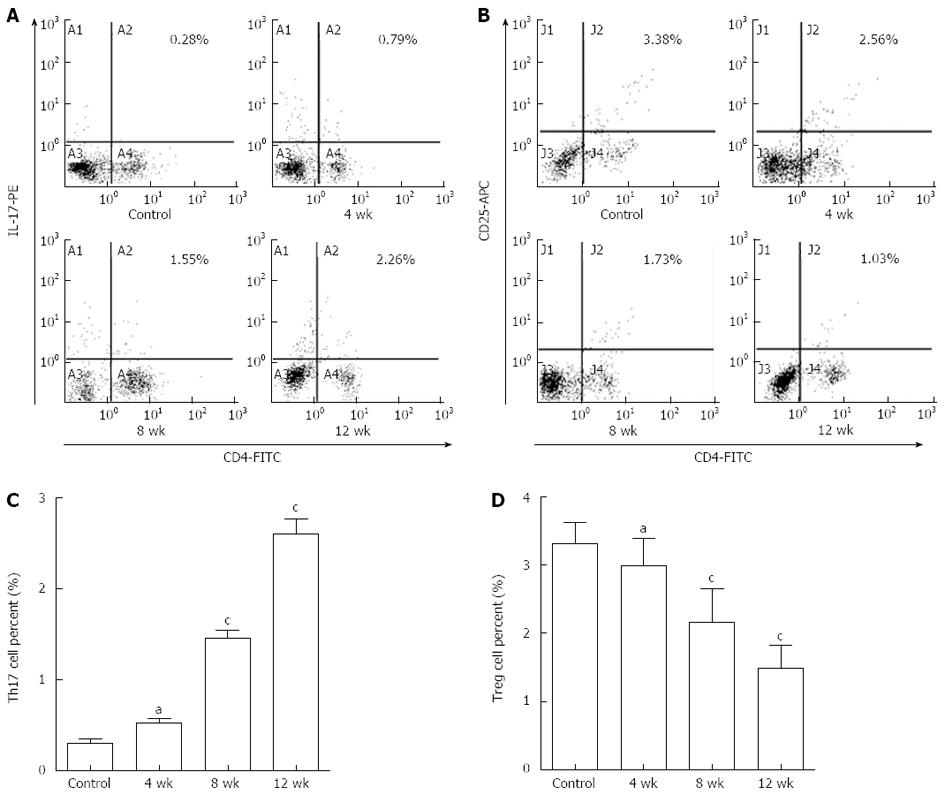
To investigate whether injection of CCl4 influenced the number of Th17 cells (CD4+IL-17+ T cells) and Treg cells (CD4+CD25+ Treg cells), the percentages of Th17 and Treg cells in mouse liver were measured by flow cytometry (Figure 3).
In the control group, the frequency of Th17 cells at 4, 8 and 12 wk was 0.30% ± 0.15%, 0.34% ± 0.16% and 0.26% ± 0.08%, respectively. The frequency of Treg cells was 3.31% ± 0.32%, 3.42% ± 0.27% and 3.25% ± 0.38%, respectively. The differences between the 4, 8 and 12-wk groups for the number of Th17 and Treg cells were not significant (P > 0.05). The results indicated that olive oil was not harmful to mouse liver. The data from the 4-wk group were used as controls for the model group.
In the model group (Figure 3A and C), the frequency of Th17 cells was increased gradually. The difference between the 4-wk group (0.52% ± 0.16%, n = 9) and the controls (0.30% ± 0.15%, n = 10) was not significant (P > 0.05). The frequency of Th17 cells was significantly increased in both the 8-wk (1.46% ± 0.24%, n = 8) and 12-wk (2.60% ± 0.41%, n = 6) groups compared with the controls (P < 0.01). Moreover, there was a significant difference between the 4-, 8- and 12-wk groups (P < 0.05).
As shown in Figure 3B and D, the frequency of Treg cells in the 4-wk group (2.99% ± 0.40%, n = 9) was not significantly decreased compared to the controls (3.31% ± 0.32%, n = 10) (P > 0.05). However, the frequency of Treg cells was significantly decreased in both the 8-wk (2.16% ± 0.50%, n = 8) and 12-wk (1.49% ± 0.34%, n = 6) groups (P < 0.01). Also, the difference between the 4-, 8- and 12-wk groups was significant (P < 0.05).
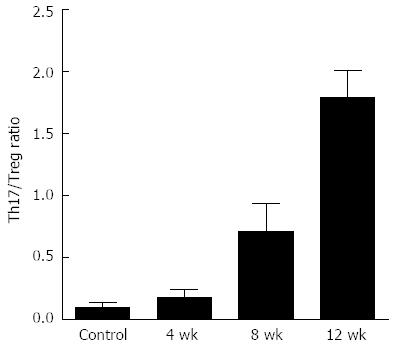
As shown in Figure 3, we detected the frequency of Th17 and Treg cells, and then we calculated the Th17/Treg cell ratios using the frequencies at different times. We found that the ratios in the model group were higher than in the controls (P < 0.05) (Figure 4) and the ratios increased gradually in the 4-, 8- and 12-wk groups. This indicated that the Th17/Treg cell balance in the liver was disrupted and more conducive to Th17 cell production and progression of fibrosis.
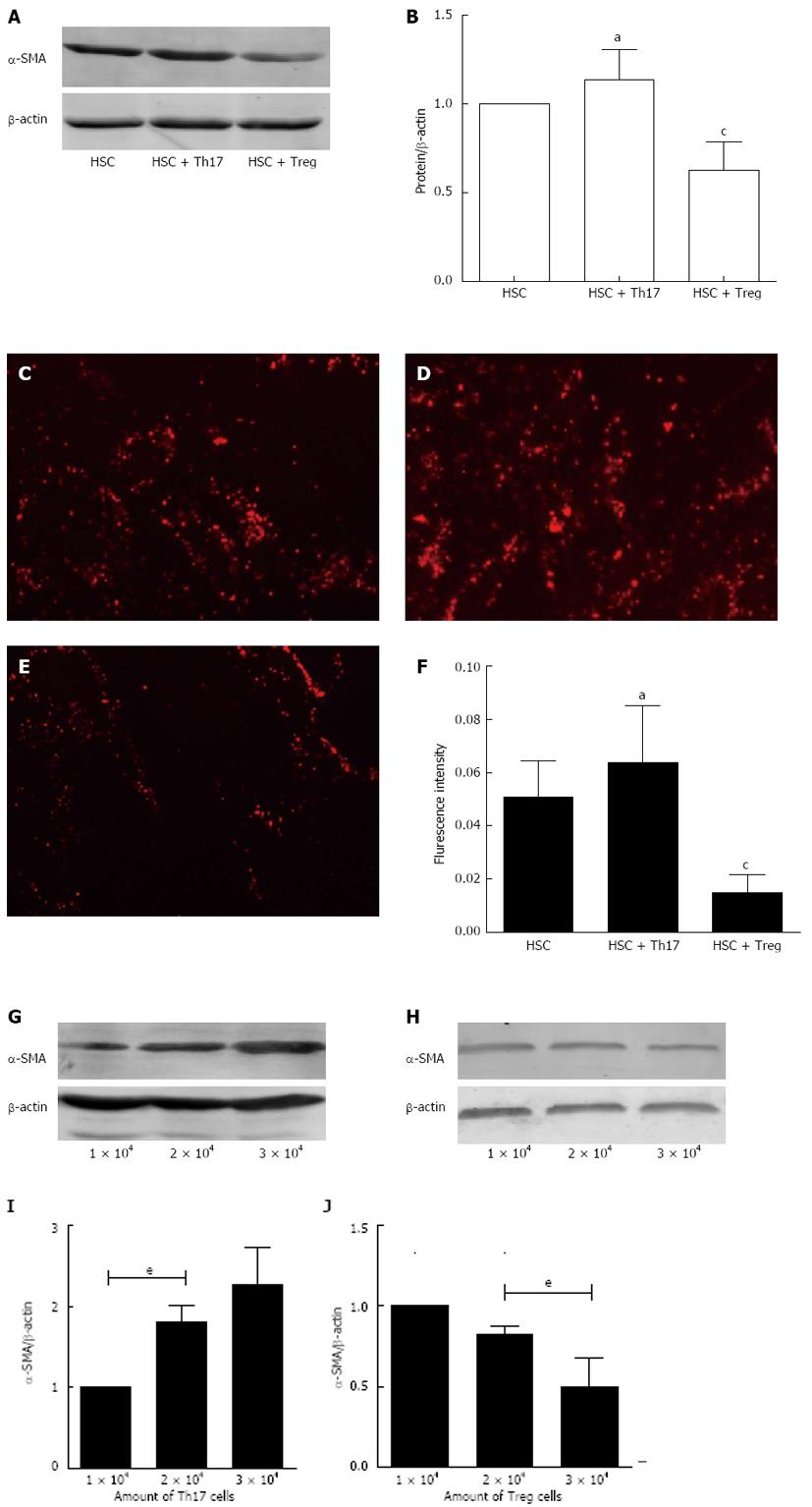
To study whether Th17/Treg cells could modulate the activation of HSCs, we isolated HSCs from the mouse liver, and treated then with Th17 cells (2 × 104/well) or Treg cells (2 × 104/well). Western blotting and immunofluorescence staining were performed to determine changes in the expression of α-SMA. As illustrated in Figure 5A and B, in comparison with the control group, α-SMA expression was induced following exposure to Th17 cells but the difference was not significant (P > 0.05). α-SMA expression was significantly reduced after exposure to Treg cells (P < 0.05). Figure 5C-E shows α-SMA expression after different treatments, and Figure 5F shows the fluorescence intensity of each group, which had the same trends as described above.
To detect further the influence of Th17 and Treg cells on HSCs, we cultured HSCs with various amounts of Th17 or Treg cells (1 × 104, 2 × 104 or 3 × 104/well). Results indicated that Th17 cells promoted, whereas Treg cells inhibited the expression of α-SMA in a dose-dependent manner.
Recent findings demonstrate that Th17 and Treg cells are T lymphocyte subgroups with unique immunoregulating functions and play opposing roles[18], and both of them participate in the regulation of a variety of diseases.
Wang et al[19] have found that the frequency of peripheral Th17 cells, as well as the level of IL-17 mRNA in PBMCs, was significantly increased in patients with acute-on-chronic hepatitis B liver failure compared with patients with chronic hepatitis B and healthy controls. Amoroso et al[20] suggested that the presence of Treg cells infiltrating the liver is associated with high levels of activated/effector T cells in the peripheral blood and lower activity of hepatitis. Therefore, Th17 and Treg cells are closely related with liver fibrosis. In addition, Th17 and Treg cells are both differentiated from naive CD4+ T cell precursors. TGF-β is a central cytokine to the differentiation of both cells and can induce the expression of FoxP3 and ROR-γt at the same time[21]. Low concentrations of TGF-β synergize with IL-6 to promote ROR-γt expression, thereby favoring Th17 cell differentiation. However, increased concentrations of TGF-β repress ROR-γt expression and favor the generation of Treg cells[22,23]. Considering all this evidence together, we hypothesize that an imbalance of Th17 cells and Treg cells may exist and play a role in regulating the immune response during liver fibrosis.
In our study, we determined the protein levels of IL-6 and TGF-β during liver fibrosis to understand their impact on Th17/Treg cell balance. Our data showed that after CCl4 administration, the expression of IL-6 and TGF-β was increased compared with the controls (Figure 2). In addition, the frequency of Th17 cells was increased while the frequency of Treg cells was decreased with the aggravation of liver fibrosis (Figure 3). To a large extent, the results of our study confirmed the above inference; that in the process of liver fibrosis, IL-6, TGF-β and other cytokines are produced in large quantities, and these changes are more conductive to the production of Th17 cells. Increased Th17 cells also induced the production of IL-6[24], so the Th17/Treg balance was disrupted (Figure 4), which led to immune disorder and promotion of liver fibrosis. Whether decreased Treg cells lead to the upregulation of TGF-β is controversial at present, and requires further study for confirmation.
As described previously, the transdifferentiation of HSCs into myofibroblasts is characterized by expression of α-SMA and is a critical event during liver fibrogenesis[25]. Our study also confirmed that the expression of α-SMA was increased gradually during the process of liver fibrosis (Figure 2), which indicates that the activation of HSCs in liver fibrosis is a continuous process. In addition, HSCs are not only the regulatory cells of liver inflammation but also the target cells of immunoregulation[26]. Li et al[27] have reported that CD4+CD25+ cells from chronic hepatitis B patients inhibit HSC activation in a dose-dependent manner, whereas recombinant IL-17 promotes the proliferation and activation of HSCs. Sun et al[28] have also found that IL-17 together with IL-17-activated monocytes promote the activation of HSCs in vitro.
To investigate whether Th17/Treg imbalance increased the activation of HSCs, we co-cultured HSCs with Th17 or Treg cells to explore their effects on HSCs. Our results indicated that Th17 cells promote HSC activation, while Treg cells have the opposite effect on HSC activation; both in a dose-dependent manner (Figure 5). In addition, we demonstrated that Th17 cells were increased in association with the reduction in Treg cells during liver fibrosis, and these changes were more conducive to HSC activation, which in turn, further promotes the development of fibrosis by secreting various inflammatory cytokines. Furthermore, fibrogenic factors (IL-6 and TGF-β) involved in the activation of HSCs induce the production of matrix proteins[29]. Therefore, a closed loop is formed between fibrogenic factors, Th17/Treg cells and HSCs, which influence each other and mediate the development of liver fibrosis jointly. However, the accurate mechanism by which Th17 and Treg cells play their pivotal roles in the activation of HSCs remains to be elucidated.
In summary, our data demonstrated that a Th17/Treg cell imbalance existed in mice with liver fibrosis. Therefore, the dynamic interaction between Th17 and Treg cells may be important in the development of liver fibrosis, suggesting a potential role for Th17/Treg imbalance in the pathogenesis of liver fibrosis.
Liver fibrosis is a chronic progressive disease that seriously affects human health, so it is important to prevent its occurrence. However, the pathogenesis of fibrosis is not entirely clear to date. T helper (Th) 17 and T regulatory (Treg) cells are T lymphocyte subgroups. Many studies have reported that Th17/Treg imbalance is closely related to many autoimmune diseases. However, its role in liver fibrosis has seldomly been reported.
The imbalance of Th17/Treg cells is closely related to a variety of autoimmune diseases. In the area of preventing liver fibrosis, the research hotpot is to explore how Th17/Treg balance changes and then prevent the occurrence of liver fibrosis by modulating the imbalance in Th17/Treg cells.
This is believed to be the first study to explore the effect of Th17/Treg cells in carbon tetrachloride (CCl4)-induced liver fibrosis in mice and its potential mechanisms. The results indicate that Th17/Treg imbalance exists and may play a potential role in the pathogenesis of liver fibrosis.
Correcting Th17/Treg imbalance may be a potential therapeutic approach in the management of liver fibrosis.
Th17 (IL-17-producing CD4+T helper cells) and Treg cells (regulatory T cells) are described as two additional subsets of T lymphocytes that can orchestrate host immune responses through releasing distinct cytokine profiles.
This was a good descriptive study in which the authors analyzed the effect of Th17/Treg cells in CCl4-induced liver fibrosis in mice. The results are interesting and suggest that Th17/Treg imbalance is a potential therapeutic target that could be used for preventing the occurrence of liver fibrosis.
| 1. | Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1425] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 2. | Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1119] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 4. | Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Lehtimäki S, Lahesmaa R. Regulatory T Cells Control Immune Responses through Their Non-Redundant Tissue Specific Features. Front Immunol. 2013;4:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603-2622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 242] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 4041] [Article Influence: 224.5] [Reference Citation Analysis (0)] |
| 8. | Lina C, Conghua W, Nan L, Ping Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:596-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Cai XY, Luo M, Lin XJ, Xu YL, Tang C, Ye JH, Li WN, He ZX, Yu N. [Expression and significance of Th17 and Treg cells in peripheral blood of patients with systemic lupus erythematosus]. Zhonghua Yi Xue Zazhi. 2012;92:460-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Szodoray P, Nakken B, Barath S, Csipo I, Nagy G, El-Hage F, Osnes LT, Szegedi G, Bodolay E. Altered Th17 cells and Th17/regulatory T-cell ratios indicate the subsequent conversion from undifferentiated connective tissue disease to definitive systemic autoimmune disorders. Hum Immunol. 2013;74:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Xing Q, Wang B, Su H, Cui J, Li J. Elevated Th17 cells are accompanied by FoxP3+ Treg cells decrease in patients with lupus nephritis. Rheumatol Int. 2012;32:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [PubMed] |
| 13. | Liu T, Chopra AK. An enteric pathogen Salmonella enterica serovar Typhimurium suppresses tumor growth by downregulating CD44high and CD4T regulatory (Treg) cell expression in mice: the critical role of lipopolysaccharide and Braun lipoprotein in modulating tumor growth. Cancer Gene Ther. 2010;17:97-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Copple BL, Bai S, Burgoon LD, Moon JO. Hypoxia-inducible factor-1α regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int. 2011;31:230-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Radaeva S, Wang L, Radaev S, Jeong WI, Park O, Gao B. Retinoic acid signaling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1. Am J Physiol Gastrointest Liver Physiol. 2007;293:G809-G816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Tabata C, Kubo H, Tabata R, Wada M, Sakuma K, Ichikawa M, Fujita S, Mio T, Mishima M. All-trans retinoic acid modulates radiation-induced proliferation of lung fibroblasts via IL-6/IL-6R system. Am J Physiol Lung Cell Mol Physiol. 2006;290:L597-L606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kono T, Kubo H, Shimazu C, Ueda Y, Takahashi M, Yanagi K, Fujita N, Tsuruo T, Wada H, Yamashita JK. Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arterioscler Thromb Vasc Biol. 2006;26:2070-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Zhao L, Qiu de K, Ma X. Th17 cells: the emerging reciprocal partner of regulatory T cells in the liver. J Dig Dis. 2010;11:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Wang LY, Meng QH, Zou ZQ, Fan YC, Han J, Qi ZX, Ge J, Xu AL, Wang SK, Wang K. Increased frequency of circulating Th17 cells in acute-on-chronic hepatitis B liver failure. Dig Dis Sci. 2012;57:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Amoroso A, D’Amico F, Consolo M, Skarmoutsou E, Neri S, Dianzani U, Spandidos DA, Mazzarino MC. Evaluation of circulating CD4+CD25+ and liver-infiltrating Foxp3+ cells in HCV-associated liver disease. Int J Mol Med. 2012;29:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Cao D, Börjesson O, Larsson P, Rudin A, Gunnarsson I, Klareskog L, Malmström V, Trollmo C. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol. 2006;63:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5550] [Article Influence: 277.5] [Reference Citation Analysis (0)] |
| 23. | Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol. 2007;82:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467-476. [PubMed] |
| 25. | Huang GC, Zhang JS, Tang QQ. Involvement of C/EBP-alpha gene in in vitro activation of rat hepatic stellate cells. Biochem Biophys Res Commun. 2004;324:1309-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Yi HS, Jeong WI. Interaction of hepatic stellate cells with diverse types of immune cells: foe or friend? J Gastroenterol Hepatol. 2013;28 Suppl 1:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Li J, Qiu SJ, She WM, Wang FP, Gao H, Li L, Tu CT, Wang JY, Shen XZ, Jiang W. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One. 2012;7:e39307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Chen MH, Chen JC, Tsai CC, Wang WC, Chang DC, Tu DG, Hsieh HY. The role of TGF-beta 1 and cytokines in the modulation of liver fibrosis by Sho-saiko-to in rat’s bile duct ligated model. J Ethnopharmacol. 2005;97:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
P- Reviewers: Muller MG, Ohkoshi S, Pan Q S- Editor: Cui XM L- Editor: A E- Editor: Zhang DN