Published online Feb 21, 2014. doi: 10.3748/wjg.v20.i7.1852
Revised: November 20, 2013
Accepted: December 12, 2013
Published online: February 21, 2014
Processing time: 218 Days and 19.5 Hours
AIM: To determine whether the application of post-operative intravenous (IV)-iron for acute isovolemic anemia after gastrectomy for cancer may be effective.
METHODS: Among 2078 gastric cancer patients who underwent surgery between February 2007 and August 2009 at the National Cancer Center Korea, 368 patients developed post-operative anemia [hemoglobin-(Hb)-level < 9 g/dL] within the first postoperative week. Patients requiring transfusions were excluded. IV-iron was administered to 63 patients (iron group). Sixty patients were observed without treatment (observation group). The clinical outcomes of the groups were compared concerning clinicopathologic data, morbidity, and changes in Hb levels using Fisher’s exact test, Student’s t-test and the Z-test.
RESULTS: The initial Hb level was higher in the iron group than in the observation group (7.3 ± 1.0 g/dL vs 8.4 ± 0.5 g/dL, P < 0.001). The slope of the changes in the Hb level was significantly higher in the iron group than in the observation group (0.648 ± 0.054 vs 0.349 ± 0.038, P < 0.001). The Hb level 1 and 3 mo post-operatively increased from 10.7 ± 1.3 to 11.9 ± 1.3 g/dL in the iron group (P = 0.033) and from 10.1 ± 1.0 to 10.8 ± 1.4 g/dL in the observation group (P < 0.001). The postoperative hospital stay was significantly longer in the iron group than in the observation group (10.5 ± 6.8 d vs 7.6 ± 5.5 d, P = 0.011). There were no significant differences in the major and surgical complications between the groups (6.3% vs 13.3%, P = 0.192; 9.5% vs 3.3%, P = 0.164).
CONCLUSION: IV-iron supplementation may be an effective treatment for post-operative isovolemic post-gastrectomy anemia and may be a better alternative than observation.
Core tip: Acute isovolemic anemia frequently occurs after major surgery. Intravenous iron supplementation was more effective in elevating the hemoglobin level than observation, and the complications were comparable to observation in 123 acute post-gastrectomy anemia patients.
- Citation: Yoon HM, Kim YW, Nam BH, Reim D, Eom BW, Park JY, Ryu KW. Intravenous iron supplementation may be superior to observation in acute isovolemic anemia after gastrectomy for cancer. World J Gastroenterol 2014; 20(7): 1852-1857
- URL: https://www.wjgnet.com/1007-9327/full/v20/i7/1852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i7.1852
Perioperative anemia occurs in 25%-75% of cancer patients, and the prevalence of anemia in the immediate post-operative period after major surgery is reported to be as high as 90%[1]. Because acute blood loss normally leads to intraoperative hypovolemia, volume expanders (crystalloids or colloids) are usually used to stabilize volume status. Transfusion is often deferred until the amount of bleeding is considered excessive according to transfusion guidelines[2,3]. Under such conditions, acute isovolemic anemia develops. Postoperative acute isovolemic anemia can affect the recovery and quality of life of patients by subtly slowing reaction time, deteriorating memory, increasing the heart rate and decreasing oxygen levels[4,5].
Acute isovolemic anemia can frequently occur after gastrectomy for gastric cancer due to the invasiveness of the procedure. Oncologic gastrectomy with a D2 lymphadenectomy is considered the standard treatment for gastric cancer, and there is thus a high probability of blood loss with subsequent acute isovolemic anemia. Some surgeons balance acute isovolemic anemia with a red blood cell (RBC) transfusion. The postoperative transfusion rates for patients with colorectal and gastric cancers have been reported to be 10%-38% and 21%, respectively[6,7]. Other surgeons prefer observation for postoperative anemia because spontaneous correction occurs within several months if oral intake is adequate. Moreover, RBC transfusion can result in potential complications and is associated with poor prognosis[8-11]. Indeed, many patients do not recover from postoperative anemia.
Refractory iron deficiency anemia thus remains a clinical problem particularly in the postoperative setting. In recent years, intravenous (IV)-iron and red cell substitutes, such as erythropoietin stimulants and artificial oxygen carriers, have been developed and used under clinical investigation[12]. An IV-iron sucrose infusion is considered safe and effective in patients on dialysis[13] and before orthopedic surgery[14]. However, treatment with IV-iron for acute isovolemic anemia after oncologic gastrectomy has not been investigated.
The purpose of this retrospective analysis was to determine whether the postoperative use of IV-iron for acute severe isovolemic post-gastrectomy anemia in patients not requiring urgent transfusion may be effective.
This retrospective case-control study was approved by the Institutional Review Board of the National Cancer Center Korea (NCC NCS-10-388). The study protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Between February 2007 and August 2009, 2.078 patients underwent surgery for gastric cancer at the National Cancer Center in Korea. Three hundred sixty-eight patients (17.7%) exhibited hemoglobin (Hb) levels < 9.0 g/dL during the postoperative period and were diagnosed with anemia. We excluded 245 patients from this analysis with documented substitutions of a RBC-unit pre-, intra- or post-operatively; preoperative iron treatments; unstable vital signs (hypotension or tachycardia); dyspnea; heart disease (angina and myocardial infarction); more than one treatment modality for postoperative anemia; and any other surgical procedure during the follow-up period. Thus, 123 patients who were either treated with an IV-infusion of iron (iron group) or who underwent clinical observation without treatment (observation group) were enrolled in this analysis.
The target Hb level was considered 12.0 g/dL. Sixty-three patients were treated with IV-iron sucrose, and 60 patients were observed without treatment. The iron group received 10 mL of Fe3+ (200 mg) mixed with 100 mL of normal saline every other day and had a mean IV infusion of 13.7 ± 8.3 ampules of iron sucrose.
All postoperative adverse events requiring treatment or hospitalization were considered relevant complications. Surgical complications included ileus, anastomotic site leakage, anastomotic site stenosis, fluid collection and abscess formation. Non-surgical complications included pleural effusion, voiding difficulty, brain infarction and stress-induced cardiomyopathy. Postoperative clinically unapparent events not requiring hospitalization or treatment such as minor pleural effusion, atelectasis or abdominal fluid collections without signs of infection were omitted from the analysis as relevant complications.
Possible adverse events related to IV-iron administration, including nausea/vomiting, headache, anaphylactic reaction, dyspnea, abdominal pain and allergic reactions, such as urticaria and fever, were retrospectively reviewed.
The following parameters were recorded and analyzed using a Pearson χ2 test or Fisher’s exact test: patient age and gender, clinicopathologic data and morbidity. Student’s t test was used to analyze the Hb levels before treatment and throughout the hospital stay after treatment. The Z test was performed to determine whether a significant difference existed between the groups with respect to the slopes of the changes in the Hb level during follow-up. A P value < 0.05 was considered statistically significant. The slopes of the two groups were estimated using a linear regression test.
The mean patient ages were 63.2 ± 12.3 and 64.4 ± 10.2 years in the iron and observation groups, respectively (P = 0.560). The gender ratio was not significantly different between the groups (P = 0.331). There were no statistically significant differences between the groups with respect to the type of surgical procedure and combined resection (P = 0.184 and P = 0.610, respectively). The cancer stage was more advanced in the iron group than in the observation group (P = 0.036). The number of patients undergoing adjuvant chemotherapy in the iron group was significantly higher than that in the observation group (P = 0.002). Demographic data are presented in Table 1.
| Variables | Iron group (n = 63) | Observation group (n = 60) | P value |
| Age (yr), mean ± SD | 63.2 ± 12.3 | 64.4 ± 10.2 | 0.5601 |
| Gender (male/female) | 37:26 | 30:30 | 0.3312 |
| Operation type | 0.8782 | ||
| Subtotal gastrectomy | 38 | 36 | |
| Total gastrectomy | 25 | 24 | |
| Stage of cancer (AJCC 6th) | 0.0362 | ||
| I and II | 28 (44.4) | 38 (63.3) | |
| III and IV | 35 (55.6) | 22 (36.7) | |
| Combined resection | 0.6102 | ||
| None | 48 (76.2) | 48 (75) | |
| Done | 15 (23.8) | 12 (25) | |
| Adjuvant chemotherapy | 0.0022 | ||
| No | 39 (61.9) | 52 (86.7) | |
| Yes | 24 (38.1) | 8 (13.3) | |
The Hb and hematocrit level before treatment was lower in the iron group than in the observation group (7.3 ± 1.0 g/dL vs 8.4 ± 0.5 g/dL, P < 0.001; 23.8 ± 3.3 g/dL vs 26.4 ± 1.9 g/dL, P < 0.001). The mean corpuscular volume in the iron group before treatment did not differ from that of the observation group (P = 0.13), and the mean corpuscular hemoglobin concentration in the iron group was lower than that in the observation group (P < 0.001). Hyperchromic anemia was ruled out for all patients in the present analysis (Table 2).
| Iron group | Observation group | P value1 | |
| (n = 63) | (n = 60) | ||
| Hematologic laboratory data | |||
| Hemoglobin (g/dL) , mean ± SD | 7.3 ± 1.0 | 8.4 ± 0.5 | < 0.001 |
| Hematocrit, mean ± SD | 23.8 ± 3.3 | 26.4 ± 1.9 | < 0.001 |
| Mean MCV (fL), mean ± SD | 85.1 ± 8.9 | 87.4 ± 7.8 | 0.130 |
| Mean MCHC (g/dL), mean ± SD | 31.0 ± 1.3 | 31.9 ± 1.4 | < 0.001 |
| Morbidity | |||
| Surgical | 4 (6.3) | 8 (13.3) | 0.192 |
| Non-surgical | 8 (12.7) | 3 (5.0) | 0.135 |
| Major | 6 (9.5) | 2 (3.3) | 0.164 |
| Minor | 16 (25.4) | 10 (16.7) | 0.236 |
| Adverse events | 0 (0.0) | NA | |
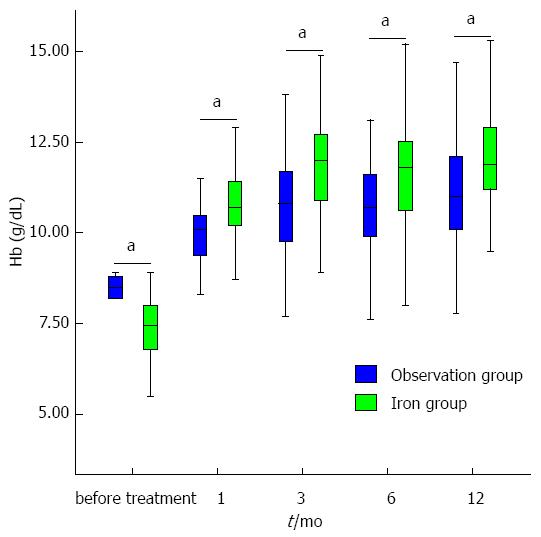
The Hb level was higher in the iron group one month post-operatively than in the observation group (10.7 ± 1.3 and 10.1 ± 1.0 g/dL, respectively, P = 0.033). Three months post-operatively, the Hb level was higher in the iron group than in the observation group (11.9 ± 1.3 and 10.8 ± 1.4 g/dL, respectively, P < 0.001). Six months post-operatively, the Hb level was again higher in the iron group than in the observation group (11.5 ± 1.3 g/dL vs 10.7 ± 1.3 g/dL, P = 0.003). At 12 mo post-operatively, the Hb level was higher in the iron group than in the observation group (12.0 ± 1.4 and 11.1 ± 1.5 g/dL, respectively, P = 0.003) (Figure 1).
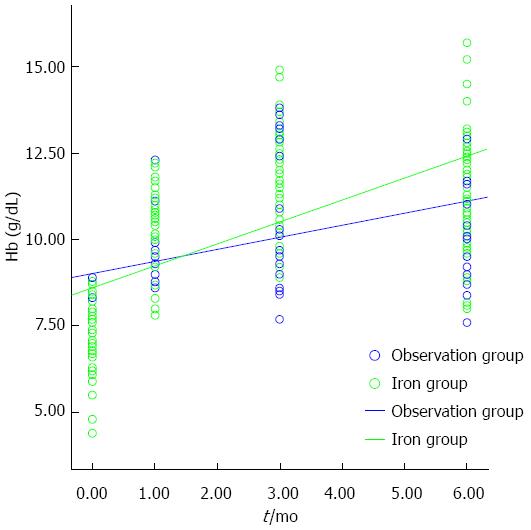
The slopes (β± SE) for the changes in the Hb levels in the iron and observation groups were 0.628 ± 0.054 and 0.349 ± 0.038 according to a linear regression test (P < 0.001 and P < 0.001, respectively). The slope in the iron group was significantly steeper than that in the observation group, as determined by Z-test (Z-score 2.777, P = 0.006), indicating that the Hb level in the iron group increased more rapidLy than in the observation group. No patient suffered from chronic anemia (Hb level < 9 g/dL one year after treatment) in the iron group compared with six patients (10.5%) in the observation group (P = 0.012) (Figure 2).
The postoperative hospital stay was significantly longer in the iron group than in the observation group (10.5 ± 6.8 and 7.6 ± 5.5 d, respectively, P = 0.011).
Table 2 demonstrates no significant differences between the two groups with respect to surgical complications (6.3% in the iron group and 13.3% in the observation group; P = 0.192) and non-surgical complications (12.7% in the iron group and 5.0% in the observation group; P = 0.135). There were no significant differences between the groups with respect to major and minor complications (P = 0.164 and P = 0.236, respectively). There were no 30-d mortalities in either of the groups. There were no patients with adverse events related to IV-iron.
Recently, several clinical studies on IV-iron treatment in surgery have been reported[14-16]. Theusinger et al[14] reported on the beneficial effects of preoperative IV-iron administration in orthopedic patients, and Van Wyck et al[15] reported the advantages of IV-iron for postpartum anemia. In contrast, a consensus statement by Beris et al[16] reported only moderate- to low-quality evidence for IV-iron application in surgical patients until further data from prospective randomized controlled trials become available. However, there are few studies on acute post-operative anemia in patients with gastric cancer. This retrospective analysis was designed to evaluate the efficacy of IV-iron for the treatment of post-operative anemia in patients with gastric cancer. The current analysis demonstrated that the Hb level in the iron group increased more rapidly than in the observation group and that there were no significant differences between the groups with respect to complications or any adverse events related to iron application. The results of the present analysis are consistent with those of earlier studies that reported that IV-iron sucrose treatment for patients with dialysis-associated anemia, pre-arthroplasty and pre-hysterectomy resulted in increased Hb levels[13-15].
When intra-operative bleeding occurs, anesthesiologists do not use packed RBCs as long as the patient’s vital signs are stable and the amount of bleeding is not considered excessive. As a consequence, postoperative acute isovolemic anemia has been largely neglected by surgeons. We found that one, three and six months postoperatively, the Hb level in the observation group increased spontaneously. However, the Hb level in the observation group was lower than that in the iron group at every timepoint and increased more slowly than did the levels in the iron group. These findings suggest that the active treatment of acute isovolemic anemia by the administration of IV-iron might improve postoperative recovery because impaired cognitive function and circulatory homeostasis might be restored earlier[4,5].
The postoperative Hb levels in the iron group and the observation group differed significantly before any treatment, which might imply a selection bias. Further, there were more advanced cancers in the iron group. It is possible that advanced gastric cancer influences iron metabolism and that preoperative Hb levels might thus have been lower. It is also conceivable that due to the larger number of advanced cancers, surgical invasiveness and thus blood loss was higher in the iron group. Another finding was that the number of patients undergoing adjuvant chemotherapy was significantly higher in the iron group. Interestingly, though chemotherapy may influence erythropoiesis, the Hb levels in the iron group recovered more quickly than they did in the observation group, which might suggest the beneficial effect of IV-iron administration.
The present analysis also reveals that IV-iron use was commonly effective irrespective of the preoperative iron deficiency. Although some studies for different surgical procedures reported that iron supplementation after surgery is unnecessary for recovery if the iron level before surgery is adequate, those prior studies were not focused on the specific clinical setting of acute isovolemic anemia[17,18]. The pre- and immediate postoperative iron level should be checked to determine whether IV-iron supplementation for acute isovolemic anemia is equally effective for preoperative iron deficiency and normal iron storage in a future prospective study.
Although the Hb level after transfusion therapy might increase more rapidly than the level following iron treatment, transfusion alone does not replenish the iron stores, which could eventually be depleted for several months[19]. Iron supplementation is necessary to restore the Hb level effectively. Furthermore, for post-gastrectomy patients, the diet is severely restricted during the early postoperative months, and iron absorption is reduced. Furthermore, the clinical observation of acute post-operative isovolemic anemia without iron supplementation may be tolerable for some patients to return to a normal Hb level but may result in chronic anemia in other patients. The present analysis revealed that 10.5% of the patients in the observation group developed chronic anemia (Hb < 9 g/dL) by 12 mo post-operatively. On the contrary, no patient in the iron group developed chronic anemia.
Many physicians may be reluctant to use iron dextran as a transfusion alternative because iron dextran is known to cause life-threatening anaphylactic reactions in up to 0.6% of treated patients because of the high molecular weight[20]. Furthermore, IV-iron is believed to produce oxidative stress, inflammation, endothelial dysfunction, and renal injuries[21]. However, a recent study reported that the rates of adverse drug effects, life-threatening events, and allergic reactions for iron sucrose are extremely rare: 19.8 per million, 0.6 per million and 2.0 per million, respectively[22]. In the current analysis with iron sucrose, there were no adverse events, such as severe anaphylactic reactions or mortality. The rate of adverse events of low molecular weight IV-iron was recently reported to be lower than that of high molecular weight IV-iron[23].
The Hb levels became steady three months postoperatively irrespective of iron supplementation. As Liedman et al[24] demonstrated, energy intake decreases in the early postoperative period after gastrectomy due to the difficulty of food intake and remained constant or increased only after the first three months after surgery, a dietary factor that could possibly explain these findings. Most importantly the Hb level in the iron group was higher than in the observation group at 3, 6, 9 and 12 mo postoperatively and may support the idea that iron supplementation in the early postoperative period might be helpful in maintaining normal Hb levels.
The current retrospective analysis has several limitations. The data were retrospectively collected from a prospectively documented database. Furthermore, the clinical outcomes of earlier Hb level recovery were not evaluated, and the quality of life of the patients was not measured and compared. It is thus debatable whether earlier Hb recovery actually translates into measurable patient benefits. Further, no data were collected concerning the patients’ ferritin, iron, and TIBC levels. Finally, IV-iron was used based on clinical experience. The authors are well aware of the limitations of this retrospective analysis, but the main intention was to demonstrate that IV-iron administration for acute isovolemic anemia may be more beneficial than clinical observation alone. Therefore, our institution is initiating a randomized controlled trial to clarify these uncertainties.
In conclusion, the current analysis demonstrates that IV-iron supplementation might be an effective treatment for postoperative isovolemic post-gastrectomy anemia and appears to be a better alternative than clinical observation. An additional prospective trial is needed to determine the proper indications of IV-iron in postoperative isovolemic anemia for gastric cancer patients.
Perioperative anemia occurs in 25%-75% of cancer patients, and the prevalence of anemia in the immediate postoperative period after major surgery is reported to be as high as 90%. Because acute blood loss normally leads to intraoperative hypovolemia, volume expanders (crystalloids or colloids) are usually used to stabilize volume status. Transfusion is often deferred until the amount of bleeding is considered excessive according to transfusion guidelines.
The current analysis demonstrates that intravenous (IV)-iron supplementation might be an effective treatment for postoperative isovolemic post-gastrectomy anemia and appears to be a better alternative than clinical observation. An additional prospective trial is needed to determine the proper indications of IV-iron in postoperative isovolemic anemia for gastric cancer patients.
Acute isovolemic anemia frequently occurs after major surgery. IV-iron supplementation was more effective in elevating the hemoglobin level than observation, and the complications were comparable to observation in 123 acute post-gastrectomy anemia patients.
This retrospective analysis was conducted to determine whether the postoperative use of IV-iron for acute severe isovolemic post-gastrectomy anemia in patients not requiring urgent transfusion may be effective. This study enrolled 63 patients with IV-iron sucrose treatment and 60 patients without treatment. Then the authors observed the Hb levels for a period of time. As a consequence, Hb-level in the iron group increased more rapidly than in the observation group. In conclusion, IV-iron supplementation might be an effective treatment for postoperative isovolemic post-gastrectomy anemia and appears to be a better alternative than clinical observation.
| 1. | Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:58S-69S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Murphy MF, Wallington TB, Kelsey P, Boulton F, Bruce M, Cohen H, Duguid J, Knowles SM, Poole G, Williamson LM. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 242] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | American Association of Blood Banks. Circular of information for the use of human blood and blood components. Available from: http://www.aabb.org/Documents/About_Blood/Circulars_of_Information/coi0702.pdf Accessed January 2013. |
| 4. | Weiskopf RB, Kramer JH, Viele M, Neumann M, Feiner JR, Watson JJ, Hopf HW, Toy P. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92:1646-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Weiskopf RB, Feiner J, Hopf HW, Viele MK, Watson JJ, Kramer JH, Ho R, Toy P. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology. 2002;96:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:1468-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Adachi Y, Mimori K, Mori M, Maehara Y, Sugimachi K. Morbidity after D2 and D3 gastrectomy for node-positive gastric carcinoma. J Am Coll Surg. 1997;184:240-244. [PubMed] |
| 8. | Weber RS, Jabbour N, Martin RC. Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol. 2008;15:34-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med. 2007;131:708-718. [PubMed] |
| 10. | Wu HS, Little AG. Perioperative blood transfusions and cancer recurrence. J Clin Oncol. 1988;6:1348-1354. [PubMed] |
| 11. | Hyung WJ, Noh SH, Shin DW, Huh J, Huh BJ, Choi SH, Min JS. Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol. 2002;9:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Goodnough LT, Shander A, Brecher ME. Transfusion medicine: looking to the future. Lancet. 2003;361:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Charytan C, Levin N, Al-Saloum M, Hafeez T, Gagnon S, Van Wyck DB. Efficacy and safety of iron sucrose for iron deficiency in patients with dialysis-associated anemia: North American clinical trial. Am J Kidney Dis. 2001;37:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Theusinger OM, Leyvraz PF, Schanz U, Seifert B, Spahn DR. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology. 2007;107:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Van Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: a randomized controlled trial. Obstet Gynecol. 2007;110:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Beris P, Muñoz M, García-Erce JA, Thomas D, Maniatis A, Van der Linden P. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Karkouti K, McCluskey SA, Ghannam M, Salpeter MJ, Quirt I, Yau TM. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Can J Anaesth. 2006;53:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | van Iperen CE, Kraaijenhagen RJ, Biesma DH, Beguin Y, Marx JJ, van de Wiel A. Iron metabolism and erythropoiesis after surgery. Br J Surg. 1998;85:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1002] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 20. | Hamstra RD, Block MH, Schocket AL. Intravenous iron dextran in clinical medicine. JAMA. 1980;243:1726-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Bishu K, Agarwal R. Acute injury with intravenous iron and concerns regarding long-term safety. Clin J Am Soc Nephrol. 2006;1 Suppl 1:S19-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 24. | Liedman B, Andersson H, Berglund B, Bosaeus I, Hugosson I, Olbe L, Lundell L. Food intake after gastrectomy for gastric carcinoma: the role of a gastric reservoir. Br J Surg. 1996;83:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
P- Reviewers: Aoyagi K, Baba H, Symeonidis NG, Wang SK S- Editor: Gou SX L- Editor: A E- Editor: Ma S