INTRODUCTION
Gastric cancer an inflammation-associated cancer etiologically related to infection with the human gastric bacterial pathogen, Helicobacter pylori (H. pylori)[1]; gastric cancer is also the second leading cause of cancer-related deaths worldwide. H. pylori infection is typically acquired in childhood and can then be life-long. The infection is associated with infiltration of the gastric mucosa with both acute and chronic inflammatory cells. This inflammatory process results in progressive damage to the gastric mucosa and to transformation of the normal acid secreting mucosa into metaplastic epithelia consisting of combinations of pyloric (spasmolytic polypeptide-expressing) and intestinal metaplasia and ultimately to gastric cancer. Chronic atrophic gastritis is thus the soil from which gastric cancer arises.
Ultimately worldwide eradiation of H. pylori, the fundamental cause of gastric cancer, will prevent the entire process and gastric cancer will become a rare disease. Until then, we must deal with the innumerable people now living with active H. pylori infection who will develop gastric cancer. Treatment choices for gastric cancer depend on tumor type and stage. Currently, the only hope for cure rests on removal of the malignant tissue either endoscopically or by surgical resection. For advanced disease, treatment is largely palliative and consists of a combination of surgery, chemotherapy, and radiation. Overall, the results of current therapy for advanced disease are poor with low 5 years survivals. Immunotherapy provides another dimension to the prevention and management of gastric cancer and offers hope of breaking through current constraints.
HUMAN IMMUNE SYSTEM AGAINST TUMORS
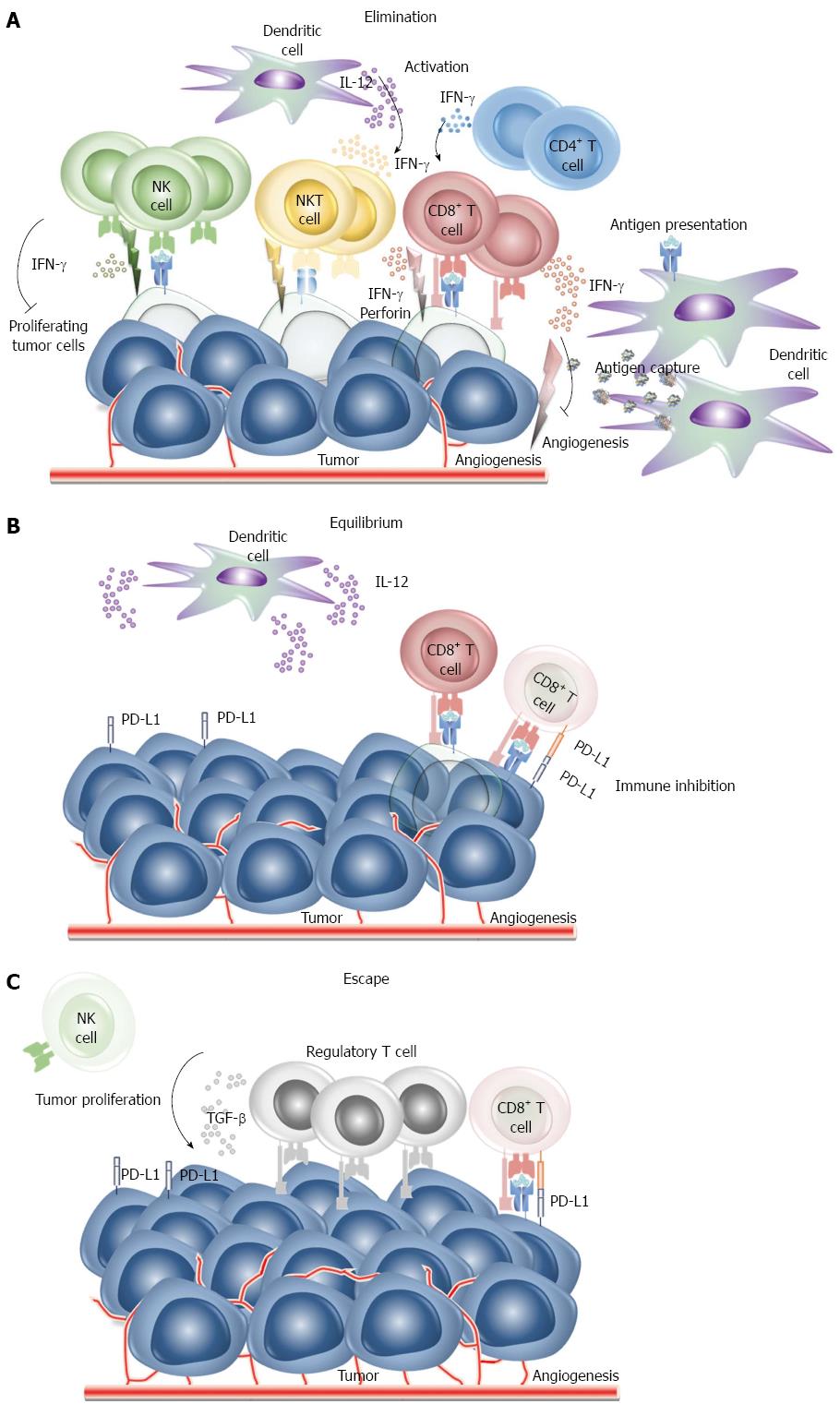
The immune system is designed to discriminate “self” from “non-self” such that when something is recognizes as non-self, the immune system attempts to eliminate it. The immune system can be thought of as patrolling the body to recognize and destroy pathogens as well as nascent transformed cells. Cancers are caused by the progressive growth and spread of the progeny of single transformed cell. It is likely that tumor cells appear daily in healthy individuals but in the vast majority of instances they are removed by the immune system and do not develop into clinical malignancies. This ability of the immune system to detect tumor cells as non-self and destroy them is called “immunosurveillance”[2]. It is currently thought that immunosurveillance primarily functions by immunoediting. “Cancer immunoediting” has been described as both the host protective and as promoting the ability of the tumor to resist the immune response. Immunoediting goes through three main phases: elimination, equilibrium and escape. Tumors are recognized by innate and adaptive immune cells which recognize the local tissue damaged caused when the growing tumors begins to remodel the stromal. Innate and adaptive immune cell, natural killer (NK) cells, NK T cells, CD8+ T cells, CD4+ T cells, secret interferon (IFN)-γ which inhibits angiogenesis and proliferation of tumor cells. Macrophages and dendritic cells are also recruited and secret cytokines to activate immune cells to phagocytize and remove dead tumor cells. If successful progression to clinical cancer is prevented (Figure 1A). Tumor cells killed in the process are digested by dendritic cells for presentation to T cells. If some tumor cells survive the elimination phase, immunoediting enters the equilibrium phase during which the residual tumor cells remain in equilibrium under pressure from the immune system. This phase it typically the longest of the three phases of cancer immunoediting. CD8+ T cells and dendritic cells which secret IFN-γ and interleukin (IL)-12, respectively maintain the tumor cells in a state of functional dormancy. During this time, because the tumor cells are highly heterogenetic and genetically instable, they may change their characteristics/populations in response to immune system editing and escape suppression (Figure 1B). In an immunosuppressed state within the tumor microenvironment allowing the tumor cells to escape from the immune system and begin to grow. The proliferation of immune cells is also reduced and tumor-specific effector cells experience apoptosis such that regulatory T cells (Tregs) associated immunosuppression occurs (Figure 1C). Cancer immunotherapy is designed to prevent the immunoediting process by enhancing the ability of the immune system to destroy the tumor.
Figure 1 Cancer immunoediting phases.
A: Phase 1: Elimination. Tumor cells are recognized by innate and adaptive immune cell and are destroyed before they can become a clinical malignancy. Modified after Dunn GP[69]; B: Phase 2: Equilibrium. If tumor cells are not destroyed in the elimination phase, the tumor may enter an equilibrium phase. Genetic change and/or resistant to immune detection occur in the equilibrium phrase and tumor cells are maintained chronically. Modified after Dunn GP[69]; C: Phase 3: Escape. The tumor microenvironment allows that tumor cells to grow and change to become poorly immunogenic. The tumor microenvironment becomes immunosuppressive. Modified after Dunn GP[69]. NK: Natural killer; PD: Programmed death; IFN: Interferon; IL: Interleukin.
The ultimate goal of immunotherapy is to achieve cancer cures by inducing an effective immune response against the tumor cells. Immune therapy of cancer is based on using the normal immune system to eliminate or control a malignancy. The presence of a tumor means the tumor cells are either not recognized as non-self or possess mechanisms to evade or overcome immunosurveillance. Research in cancer immunology is currently focused how overcome these blocks and to train the immune system to identify and target cancers for elimination. Further advances are predicated on better understanding of how to overcome the ability of tumor cells to evade being eliminated.
Theoretically, if specific antigens can be identified in the precursor lesions leading to cancer, the immune responses could also be utilized eliminate them which would prevent tumors from ever developing. Gastric cancer is potentially an ideal target for such preventive immunotherapy as it has a long latent period and clearly recognizable premalignant lesions which if successfully targeted could prevent progression to frank cancer.
WHAT IS IMMUNOTHERAPY?
Traditionally, immunotherapy considered to have begun in 1798 when Edward Jenner showed that inoculation with cowpox could prevent smallpox in humans. Since that time, a variety of different immunotherapies have been utilized to control diseases. Initially the focus was on vaccination and serum therapies. The history of cancer immunotherapy began in 1891 when William B Coley injected streptococcal organisms into a patient with inoperable cancer[3] resulting in shrinkage of the malignant tumor. That experiment suggested that it might also be possible to utilize natural defense mechanisms to rid the body of a malignancy. Success with tumor immunotherapy has been slow as the immune system is exquisitely regulated with multiple checkpoints and feedbacks to prevent damage to the host. Further success requires a detailed understanding of the immune system which is only now beginning to be achieved.
Current immunotherapies are often based on use of monoclonal antibodies, cytotoxic immunocytes, or gene transferred vaccines. Monoclonal antibodies can also be used as alternatives to the traditional approach of administering small molecules (i.e., drugs) to inhibit factors critical for tumor growth and survival. The human monoclonal antibody SC-1 was isolated from a patent with ring cell carcinoma and SC-1 antibody inhibited tumor cell growth by inducing apoptosis of tumor cells[4]. Solid tumors require growth of blood vessels to survive and grow. Vascular endothelial growth factor (VEGF) plays an important role in this process by stimulating new blood vessel formation (i.e., angiogenesis) and anti-VEGF antibody has been used as immunotherapy to bind the growth factor thus inhibit angiogenesis. While, the search for targets that when inhibited by monoclonal antibodies reduce tumor growth is a major effort in cancer research, this review focuses on cellular immunotherapy. Monoclonal antibodies may still play a role because antibodies directed against tumor-specific antigens can be used to target the cellular immune system to destroy tumors. For example, the receptor human epidermal growth factor receptor 2 (HER-2/neu) is often overexpressed in breast cancer. Administration of anti-HER-2/neu monoclonal antibodies results in the antibodies binding to tumor cells which is followed by the attraction and activation of effector cells, such as NK cells and monocytes (via their Fc receptors) and ultimately, lysis of the tumor cells.
CELLULAR IMMUNOTHERAPY
The cellular immune response and can employ the innate (e.g., NK cells, macrophages, and eosinophils) or adaptive (CD8+ and CD4+ cells) immune response, or both. The response is mounted when specialized cytotoxic cells are induced to recognize and directly attack tumor cells based on expression of antigens on the tumor cell surface called tumor rejection antigens. Tumor rejection antigens are peptides of tumor cell proteins that are recognized by the immune system when presented to T cells by major histocompatibility complex (MHC) molecules. These peptides then become the targets of a tumor-specific T cells response. The actual strategy requires both choice of the target peptide and the immunocytes for therapy (i.e., success requires both identifying a target and a strategy to attack that target). Because tumors primarily consist of self the possibility remains that any attempt at immunotherapy against a tumor would also attack normal non-tumor tissues. Clinically successful immunotherapy must therefore be able to tread the delicate line between attacking the tumor while doing minimal damage to normal tissues.
STRATEGIES AND CURRENT APPROACHES FOR IMMUNOTHERAPY IN GASTRIC CANCER
Current cellular immune strategies rely on the use of immunocytes designed to either activate tumor specific cytotoxic T cells to lyse tumor cells, or to specifically bind to target molecules or proteins expressed on the malignant tumor cells. A number of tumor rejection antigens have been identified. Experimental vaccination strategies have included use of whole protein and peptide vaccines and are based on identification of peptides recognized by cytotoxic T lymphocytes and helper T lymphocytes. Tumor rejection antigens melanoma-associated antigen 3 (MAGE-3) and HER-2/neu are examples of antigens selectively expressed in human tumors including gastric cancer which can be recognized by cytotoxic T cells.
ADOPTIVE CELL THERAPY
The transfusion of tumor-specific T cells into a cancer patient is called “adoptive cell therapy”. A number of different cell types can be used such as killer cells, lymphokine-activated killer cells[5], tumor infiltration lymphocytes (TILs)[6], anti-CD3 monoclonal antibody-induced killer cells[7], and cytokine induced killer cells[8] (Figure 2). The first trial of adoptive cell therapy in humans utilized lymphokine-activated killer cells. In that study patients with metastatic melanoma were treated with the combination of lymphokine-activated killer cells plus IL-2[9]. IL-2 was used to ensure the survival and sustained activation of the infused lymphokine-activated killer cells. IL-2 is a cytokine produced by human T lymphocytes that is necessary for the growth, proliferation, and differentiation of T cells to become effector T cells and was approved for the treatment of metastatic melanoma in 1998[10]. This approach has resulted in marked tumor regression in up to or approximately 30% of the patients with renal-cell, melanoma, colorectal, non-Hodgkin’s lymphoma, and lung cancer showing proof of principle[11].
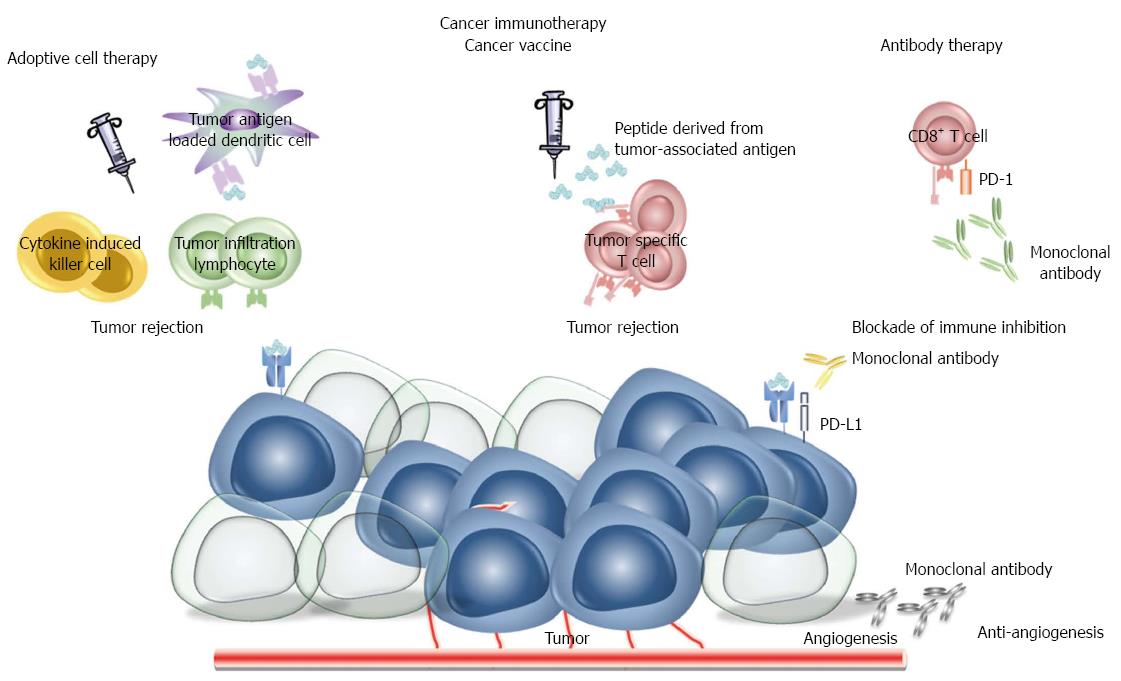
Figure 2 Cancer immunotherapy.
Immunological approaches to cancer therapy are based on use of cytotoxic immunocytes (cytokine induced killer cells, tumor infiltration cells, tumor antigen loaded dendritic cells), cancer vaccines, monoclonal antibodies. Therapeutic vaccines enhance pre-existing immunity and lead to a more robust antitumor immune response whereas monoclonal antibodies are used to inhibit critical molecules for tumor growth and survival. PD: Programmed death.
TILs are lymphocytes isolated from the patient’s tumor. TILs have been used for immunotherapy of gastric cancer[12]. TILs are potentially especially useful because they already recognize some tumor-specific antigens in that tumor. Adoptive immunotherapy with TILs has provided promising results in preclinical studies in sarcoma and colonic adenocarcinoma[13]. A clinical study of adoptive immunotherapy with tumor-associated lymphocytes in combination with chemotherapy in gastric cancer resulted in a longer 50% survival with the combination of adoptive immunotherapy and chemotherapy than with chemotherapy alone[14].
Cytokine induced killer cells are rapidly proliferating lymphocytes with strong anti-tumor activity. These cells are generated by the in vitro expansion of peripheral blood lymphocytes using the combination of anti-CD3 antibodies and IL-2. The antigen receptor molecules on T cells are non-covalently associated on the cell surface with the CD3 molecular complex and perturbation of the complex with anti-CD3 monoclonal antibodies induces T cell activation[15]. Clinical studies have confirmed a survival benefit in gastric cancer patients treated with chemotherapy combined with cytokine induced killer cells compared to chemotherapy alone[16,17].
CANCER VACCINES
Cancer vaccines are designed to activate and expand tumor-specific T cells as effector T cells. Therapeutic vaccines can enhance pre-existing immunity, induce novel immunity, or lead to a more robust anti-tumor immune response (Figure 2). In order to induce tumor-specific T cells, peptides derived from tumor-associated antigens must be presented to T cells by professional antigen-presenting cells, such as dendritic cells, which are the most powerful and efficient antigen-presenting cells able to activate naïve and memory T cells[18]. Immature dendritic cells with high phagocytic capacity are localized to sites where tumor cells grow. They take up antigens which are digested into small oligopeptides which are then loaded onto the MHC class I molecule for presentation to CD8+ cytotoxic T cells or to MHC class II molecules for presentation to CD4+ helper T cells. The process can also be done in vitro. For this, monocytes are obtained by apheresis and are induced to form immature dendritic cells with cytokines (GM-CSF, IL-4). The immature dendritic cells are then cultured in vitro with tumor lysates or peptides derived from tumor-associated antigens and the cytokine tumor necrosis factor (TNF)-α, IL-1 or IFN-γ. The mature dendritic cells that develop are then injected to patients by the intradermal or intravenous routes where they present antigens to T cells to induce a robust anti-humor immune response.
Tumor-associated antigens are defined as antigens expressed on tumor cells that can elicit an immune response in the host. Thousands of potential tumor associated antigens have been identified and many studies have confirmed that cytotoxic T cells activated by immunogenic peptides derived from tumor-associated antigens presented on the surface of tumor cells with MHC-I are capable of lysing tumor cells[19-22]. Both protein and peptide targets have been used to attempt to stimulate a specific immune response in gastric cancer. Those experiments have been based on peptides derived from the tumor associated antigen HER2/neu-derived peptide[19] and MAGE[23-27] which are restricted to MHC class I have been shown to induce cytotoxic T cells against tumors. Gastric cancers typically overexpress HER-2/neu and vaccination using dendritic cells pulsed with HER-2/neu peptide has resulted in tumor regression. MAGE-3 peptide/chitosan-deoxycholic acid vaccine-loaded nanoparticles have also been used to simulate an antitumor immune response and successfully produced regression of tumor growth in a mouse model of gastric cancer[28].
Peptides derived from human vascular endothelial growth factor (VEGF) receptor 1 and vascular endothelial growth factor receptor 2 combined with chemotherapy (S-1 plus cisplatin) have been shown to induce a VEGF-specific cytotoxic lymphocyte response in patients with advanced gastric cancer resulting in a partial response in 55% of patients as well as prolonged overall survival[29] suggesting that cancer vaccines combined with standard chemotherapy may be a promising strategy for the treatment of advanced cancer.
RNA-BASED VACCINES
Dendritic cells incubated with mRNA are capable of presenting the encoded antigen[30] making mRNA-based gene transfer vaccine an attractive possibility for immunotherapy[31,32] (Figure 2). Dendritic cells transfected with mRNA coding for a tumor-associated antigen or whole tumor RNA have been able to induce potent antigen- and tumor-specific T cell responses. The generation of immune responses with naked but stabilized mRNA has also been accomplished in mouse models[33-37] and clinical trials have been encouraging in melanoma[38,39] and renal cell carcinoma[40].
There are a number of potential advantages of RNA-based vaccines. For example, naturally transient and cytosolic active mRNA molecules are considered to be a possibly safer pharmaceutical because of expression is transient and the absence of genomic integration. The mRNA application also allows targeting multiple tumor-associated antigens simultaneously[39]. RNA vaccination does not cause severe side effects such as the generation of autoimmune disease or anti-DNA antibodies and finally, unlike peptide-based vaccinations it is not MHC-restricted.
HOW CANCERS EVADE THE IMMUNE RESPONSE
Immune escape and immunosuppressive tumor network
The key cells of the immune system for tumor surveillance are T cells and NK cells. However, despite the theoretical advantages of immunotherapy, current approaches often do not stimulate immunity efficiently and the tumors continue to grow despite the presence of an immune response[41-43]. Multiple mechanisms have been identified allowing tumors to escape rejection by the immune system[44-46]. Theoretically, downregulation or loss of HLA class I antigen in cancers would be an important evasion mechanism and has been reported. However, expression of HLA class I antigens has not been shown to correlate with any important clinical or pathologic parameters of gastric cancers[47]. Other mechanisms are downregulation of antigen expression on tumor cells and production of immunosuppressive cytokines [transforming growth factor (TGF)-β1, IL-10, IL-6, VEGF, prostaglandin] by the tumor.
There is considerable current interest in Tregs and MSCsas major components of the immune suppressive tumor microenvironment. Tregs cells inhibit cytotoxic lymphocytes and/or helper T activity as well as NK cells. Tregs are characterized by the CD4+CD25+FOXP3+ phenotype and normally play an indispensable role in maintaining immunological tolerance to self-antigens and in suppressing excessive immune responses that would be deleterious to the host. Regulatory immune cells, mostly Tregs, have been identified as the major regulatory component of the adaptive immune response and are also involved in H. pylori-related inflammation and bacterial persistence[48]. For example, H. pylori-induced gastritis is regulated by Tregs. Tregs play an important role in the equilibrium between H. pylori and immune system and a better understanding of the role of these cells in immunosuppression in the tumor environment should lead to approaches to blunt or eliminate Treg-associated immunosuppression.
Recently, the role of mesenchymal- or bone marrow-derived stem cells (BM-MSCs) for the malignant transformation has been studied[49,50] and are known to migrate to tumor issues[51]. BM-MSCs into a chronic H. pylori-infected mouse model showed the generation of an immunosuppressive environment. The local and systemic immunosuppression mediated by BM-MSCs likely contributed to an environment that is compatible with the development of H. pylori-induced gastric cancer[52]. It has been demonstrated that this cell population can serve as a “seeding point” for gastric carcinogenesis in animal models but the relevance with respect to human disease still remains unclear[50]. Development of immunotherapies targeted to Tregs and BM-MSCs is an attractive new strategy to activate antitumor immunity in patients with cancer.
Eliminating both tumor and lymphocyte-mediated immune suppressive mechanisms without damaging normal cells also holds promise. Specifically, the blockade of secreted immunosuppressive molecules, (e.g., TGF-β1, IL-10 or prostaglandins) may be required in addition to eliminating Tregs.
Immune checkpoint
Immune checkpoints are inhibitory pathways hardwired into the immune system that are crucial for maintaining self-tolerance and modulating the duration and amplitude of physiological immune responses in order to minimize collateral tissue damage. Thus, immune checkpoints play critical roles for physiological homeostasis especially in protection of tissues from damage when the immune system responds to infections. These checkpoints may also allow immune escape in cancer.
Checkpoint pathways are regulated by ligand/receptor interactions. For example, programmed death-1 receptor (PD-1) and CTL-associated antigen 4 (CTLA-4) are inhibitory molecules whose presence on lymphocytes signifies a blunted immune response. PD-1 negatively regulates T cell responses and downregulation and eventually apoptosis is initiated following binding of a PD-1 ligand with PD-1. PD-1 ligands, PD-L1 or PD-L2, are frequently expressed on tumor cells and can thus thwart the immune response. One approach to overcome this inhibition of the immune response has been to target immune checkpoints with blocking monoclonal antibodies (mAb) (Figure 2). For example, PD-1 mAb binds to the PD-1 receptors on T cells and inhibits their binding to the ligands on tumor cells thus preventing the tumors from down regulating the cytotoxic lymphocyte response. This approach has been successful clinically. For example, anti-CTLA-4 mAb is the basis for immunotherapy producing a survival benefit in advanced melanoma[53,54]. Phase I clinical trials of anti-PD-L1 mAb are under investigation for gastric cancer. Other mechanisms focus on the T-cell immunoglobulin domain and mucin domain 3 for promoting inflammation or to restrain a T helper 1 cell response. Inducible T-cell co-stimulator is a CD28-superfamily co-stimulatory molecule expressed on activated T cells and considered important for Th2 cell, B and T lymphocyte attenuator whose activation inhibits the function of tumor-specific cytotoxic T cells[55]. These co-stimulatory and co-inhibitory receptors modulate the function of both antigen-presenting cells and T cells. The available immunostimulatory monoclonal antibodies have not proved sufficient suggesting that there must be other target molecules that are extracellularly accessible and are candidates for manipulation with monoclonal antibodies in cancer therapy. We expect more will be discovered and developed in the future.
FUTURE PROSPECTS FOR IMMUNOTHERAPY
Biomarkers are needed to predict good candidates for immunotherapy
The use of immunotherapy would be enhanced if one could identify biomarkers predictive of response and thus allow matching of treatments with suitable patients. HER2 has proven to be an excellent biomarker in breast cancer. Trastuzumab (herceptin), a humanized anti-HER2 receptor monoclonal antibody, has been shown to improve the outcome in patients with HER2-positive metastatic breast cancer[56]. The combination of pertuzumab (HER2-targeted humanized monoclonal antibody) plus trastuzumab plus docetaxel has been compared with placebo plus trastuzumab plus docetaxel as first-line treatment for HER2-positive metastatic breast cancer and significantly prolonged progression-free survival[57]. HER2 is also expressed in gastric cancer suggesting that this approach may show therapeutic efficacy. There is significant current interest in identifying predictive biomarkers in cancer in general and in gastric cancer in particular.
Recently, DNA microarray technology have been developed and extensively used to search for new biomarkers for individualized therapies[58-62]. Gene expression profiles in tumor tissues, TILs and peripheral blood have been reported to clearly reflect clinical outcomes and/or responses to treatments in cancer patients. Furthermore, expression array data of peripheral blood and TILs have also shown an association with survival and immune response[60,62]. Gene expression profiling is developing into a mainstream tool for the assessment of immune system and monitoring immune responses to drugs or therapies.
Engineered cellular immunotherapy
Rapid advances in understanding of the details of the molecular events and regulatory pathways involved in effective use of cytotoxic cells as anti-tumor therapy have prompted work on developing customized or engineered cells. The ideal regimen would be one that targeted antigens that are specific to a particular type of malignancy and then engineer cells programmed to evade the tumors repertory of anti-immune defenses and to respond to those antigens. Challenges include (1) identification of one or preferably several antigens specific to the tumor; (2) programming the appropriate cytotoxic cells to respond to only those antigens; (3) ensuring that the cytotoxic cells were capable of avoiding the tumor’s defenses; and (4) engineering signals to initiate the process as well as signals to end the attack if or when this becomes desirable.
Genetic tools have been developed to engineer T-cell specificity and enhance T cell function. Chimeric antigen receptors are receiving increasing attention and becoming a promising new therapeutic method. Chimeric antigen receptors lead to enhanced proliferation, cytotoxicity, and persistence in vivo. Apheresed T cells from a patient are stimulated with CD3 antibody and IL-2. Activated cells are then transduced with the chimeric antigen receptors using a retro- or lentiviral platform. Because the chimeric antigen receptor is integrated into the T-cell genome, all daughter cells that are generated during this expansion also express the chimeric antigen receptor. Chimeric antigen receptor-transduced T cells are then infused into patients[63]. Early clinical studies have revealed a very encouraging therapeutic efficacy of chimeric antigen receptor-mediated immunotherapy in a variety of cancers including lymphoma, chronic lymphocytic leukemia, melanoma, and neuroblastoma[64]. Despite the promising results obtained from clinical trials with infusion of chimeric antigen receptor-modified T cells, some severe adverse events have been reported[65-67]. Recent reports have highlighted key issues and future directions to avoid these adverse events[68]. Selection of candidate target antigens is essential for improved efficacy and safety of the chimeric antigen receptor-based therapy.
Although one can also theoretically strip the effector cells of all checkpoints, it is important that such cells have as much tumor specific ability as possible to prevent the cells from becoming indiscriminate killers. The fundamental problem is to unleash an attack capable of elimination of the target but one that is restrained and does not do irreversible harm to the host. Ideally, one would like to be able to control all of the elements of the process from choosing the most tumor specific antigens and then be able to engineer the cells to respond only to those antigens and at the same time be able to control when and where the attach is focused. Thus, the cells might be engineered with a safety switch that can be switched to on and initiate the process and then switched off if the cells move out of the area or the process is completed. The “off” switch might consist of implementation of a suicide program that completely eliminated the cells.
Identification of one or preferably several antigens specific to the tumor
Because the primary tumor or metastasis may be difficult to sample, the challenges to identification of the appropriate antigens include obtaining access to the tumor or tumor cells to identify critical antigens specific that tumor (e.g., cancer testis antigens) or identification of antigens that are expressed on the majority of similar tumors (e.g., universal antigens). Advances in obtaining circulating tumor cells and analyzing them would help not only monitoring efficacy of therapies, but also useful to identify appropriate, effective and specific tumor antigen or molecules for metastasis. It also expected to provide selection of appropriate therapy for individual patients by characterization of circulation tumor cells.
CONCLUSION
The practice and theory of cancer immunotherapy has seen major advancements during the past 20 years. However, many hurdles to remain to be overcome before cancer immunotherapy becomes the first line and most reliable and effect cancer treatment. In view of the complexity and diversity of tumors and immune cell repertoires, it would be of critical importance to identify new target molecules or develop new ways to utilize already know targets, and expand knowledge of the effectiveness of combinations of immunotherapies with conventional therapies. In addition, it essential to identify reliable biomarkers to identify candidates that would most benefit from immunotherapy, and/or early diagnosis to prevent cancer progression as a vital part of that therapy.
P- Reviewers: Aoyagi K, Baba H, Vieth M S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN