Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18296
Revised: June 28, 2014
Accepted: July 16, 2014
Published online: December 28, 2014
Processing time: 246 Days and 23.2 Hours
AIM: To study the cancer stem cell population in esophageal cancer cell lines KYSE-150 and TE-1 and identify whether the resulting stem-like spheroid cells display cancer stem cells and radiation resistance characteristics.
METHODS: A serum-free medium (SFM) suspension was used to culture esophageal cancer stem cell lines and enrich the esophageal stem-like spheres. A reverse transcription polymerase chain reaction assay was used to detect stem cell gene expression in the spheroid cells. Radiosensitivity of stem-like spheres and parental cells were evaluated by clonogenic assays. Furthermore, different cells after different doses of irradiation were tested to evaluate the change in sphere formation, cell cycle and CD44+CD271+ expression of tumor stem-like spheroid cells using flow cytometry before and after irradiation.
RESULTS: The cells were observed to generate an increased number of spheres in SFM with increasing cell passage. Radiation increased the rate of generation of stem-like spheres in both types of cells. The average survival fraction (SF2) of the cultured KYSE-150 compared with TE-1 stem-like spheres after 2 Gy of radiation was 0.81 ± 0.03 vs 0.87 ± 0.01 (P < 0.05), while the average SF2 of KYSE-150 compared with TE-1 parental cells was 0.69 ± 0.04 vs 0.80 ± 0.03, P < 0.05. In the esophageal parental cells, irradiation dose-dependently induced G2 arrest. Stem-like esophageal spheres were resistant to irradiation-induced G2 arrest without significant changes in the percentage population of irradiated stem-like cells. Under irradiation at 0, 4, and 8 Gy, the CD44+CD271+ cell percentage for KYSE150 parental cells was 1.08% ± 0.03% vs 1.29% ± 0.07% vs 1.11% ± 0.09%, respectively; the CD44+CD271+ cell percentage for TE1 parental cells was 1.16% ± 0.11% vs 0.97% ± 0.08% vs 1.45% ± 0.35%, respectively. The differences were not statistically significant. Under irradiation at 0, 4, and 8 Gy, the CD44+CD271+ cell percentage for KYSE-150 stem-like spheres was 35.83% ± 1.23% vs 44.9% ± 1.67% vs 57.77% ± 1.88%, respectively; the CD44+CD271+ cell percentage for TE1 stem-like spheres was 16.07% ± 0.91% vs 22.67% ± 1.12%, 16.07% ± 0.91% vs 33.27% ± 1.07%, respectively. The 4 and 8 Gy irradiated KYSE-150 and TE-1 stem-like spheres were compared with the 0 Gy irradiated group, and the differences were statistically significant (P < 0.05).
CONCLUSION: The KYSE-150 and TE-1 stem-like spheres are more radioresistant than their parental cells which may suggest that cancer stem cells are related to radioresistance.
Core tip: Radiotherapy is one of the major treatments for esophageal squamous cell cancer. However, resistance to radiotherapy is considered to be one of the main reasons for poor prognosis and high mortality rates in patients with esophageal cancers. In recent years, cancer stem cells have received significant attention in cancer research. Cancer stem cells are found tolerant to both radiotherapy and chemotherapy. In this study, we report the isolation of cancer stem cells from human esophageal cancer cell lines KYSE150 and TE1. The biological characteristics of cancer stem cells and their resistance to radiation are discussed.
- Citation: Wang JL, Yu JP, Sun ZQ, Sun SP. Radiobiological characteristics of cancer stem cells from esophageal cancer cell lines. World J Gastroenterol 2014; 20(48): 18296-18305
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18296.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18296
Esophageal cancer (EC) is one of the six most common malignant tumors worldwide[1]. There is increasing evidence to show that a cell subset in the tumor tissue may play an important role in tumorigenesis, neoplasia and metastasis. This subset of cells is highly metastatic and resistant to chemo-radiotherapy and these cells are known as cancer stem cells (CSCs)[2-6]. In addition, studies have demonstrated that CSCs are tolerant to both radiotherapy and chemotherapy. Although researchers have isolated CSCs from various tumor tissues and cell lines[7-11], isolation, enrichment and culture of CSCs from esophageal cancer are still a challenge which needs to be addressed in order to study the molecular mechanisms involved in esophageal cancer.
With the rapid advancements in radiobiology and accumulation of clinical data, it is now believed that an accelerated repopulation of surviving CSCs in conventional fraction radiotherapy is the main reason for the failure of conventional fraction radiotherapy. Since the accelerated repopulation of cancer cells may primarily occur 3-5 wk after conventional fraction radiotherapy, the use of hyper-fractionated accelerated radiation therapy with an increased frequency, abbreviated treatment course, but similar total radiation dose as the conventional fraction radiotherapy, may prevent the accelerated repopulation of cancer cells[12-14]. Zhang et al[15] reported that a group of radiation-resistant cells displayed similar characteristics to CSCs after continuous segmentation irradiation performed for esophageal cancer. These cells demonstrated higher telomerase activity, enrichment of side- population cells and higher expression of β-catenin, OCT3/4 and β-integrin. Cancer cells can be killed by radiation, whereas CSCs are resistant to radiation. DNA damage repair in CSCs is one of the main reasons leading to failure of radiotherapy[16,17]. Repair of DNA damage is achieved by activating a series of effector molecules in the cell cycle checkpoint, such as effect enzyme Chk2 and Chkl[18,19]. Cancer stem cells develop into new neoplasms through proliferation and differentiation resulting in tumor recurrence. McCord et al[20] demonstrated that CD133+ deficient malignant glioma stem cells do not respond to DNA damage and are sensitive to radiotherapy, supporting the important role of DNA damage checkpoint response in radioresistance.
In this study, we attempted to isolate a cancer stem cell-enriched mass from human esophageal cancer cell lines, KYSE150 and TE1, and thereby provide a satisfactory cell model for in vitro studies related to specific gene expression and abnormal signal transduction pathways involved in esophageal CSCs. Furthermore, we demonstrated that the cell mass isolated from esophageal cancer cell lines possesses cancer stem cell characteristics such as high invasion and self-renewal. We also tried to use CD271+ CD44+ as a cancer stem cell surface marker. The isolated cell mass was also resistant to radiation. Thus, we demonstrated that the esophageal cancer stem cell mass is responsible for the development of resistance to radiation.
Human esophageal cancer cell lines KYSE150 and TE1 were purchased from Shanghai Biological Cell Bank (Shanghai, China). Cells were cultured in 1640 medium (Gibco, United States) containing 10% fetal calf serum supplemented with 100 U/mL penicillin and 100 mg/L streptomycin and incubated at 37 °C, 5% CO2 and 100% humidity. Cells were passaged every 2 d. Cells in the logarithmic phase were chosen for the experiments. Serum-free medium (SFM) consisted of RPMI-1640 (1:1), B27 (1:50) (Invitrogen, United States), epidermal growth factor (20 ng/mL) (PeproTech, United States), basic fibroblast growth factor (20 ng/mL) (PeproTech, United States), insulin 5 μg/mL, transferrin 10 μg/mL and 0.5% bovine serum albumin. The cells were resuspended in SFM and plated in low adhesion 6-well culture plates (Corning, United States) at 1 × 105 cells/well. The cells were then incubated at 37 °C. Fresh SFM (1 mL) was added every other day to replenish the old medium.
Logarithmic phase KYSE150 and TE1 cells were collected. Monolayer adherent cultured KYSE150 and TE1 cells were digested using 0.25% pancreatin containing 0.02% EDTA. The cells were plated in low adhesion 6-well culture plates at 1000 cells/well. Cell spheres were collected 3-4 d after routine culture and then mechanically dispersed to form a single cell suspension. Next, the cells were resuspended in the SFM mentioned above and passaged at a proportion of 1:1. Three generations of cell spheres were counted continuously. Cell spheres formed 10 d after culture in SFM were placed in SFM again and were differentiated. Morphological changes were observed under an inverted microscope.
The two types of cells and cell spheres were respectively made into two single cell suspensions. The cell suspensions were placed in 96-well plates at a density of 1 × 104 cells/well in a volume of 200 μL/well. Medium only was added to the control group. The cells were incubated at 37 °C and 5% CO2. The assay was performed on pore plates at 24 h intervals for 6 d. The mean values of each assay were recorded. The assay was performed as follows: 20 μL (5 mg/mL) MTT (Sigma, United States) was added to 180 μL basal culture medium and incubated at 37 °C for 4 h. Thereafter, the medium was removed and 150 μL DMSO was added to each well and shaken for 10 min. Optical density (A, 490 nm) was measured using a microplate reader. The cell growth curve was plotted with A on the Y axis and time on the X axis.
The bottom of the Transwell chamber (Millipore) was coated with Matrigel (BD, United States) dissolved in SFM with RMPI 1640 (1:4). After allowing the gel to set, the remaining supernatant was aspirated. KYSE-150 and TE-1 parent cells and cell spheres from the logarithmic phase were dispersed into a single cell suspension and counted. Cells 1 × 104 and serum free 1640 medium were added to the inside of the Transwell invasion chamber, while 1640 medium was supplemented with 10% fetal calf serum and added to the outer chamber. Each group of cells was taken out of the chamber after 12 h of routine culture. Cells on top of the microporous membrane were wiped off and stained with crystal violet, followed by rinsing with PBS. Cells on the lower side of the membrane were observed under microscope. In each slice, 5 fields showing cells in relatively high concentration were randomly selected and the invasive cell population was counted to obtain a mean value. Each group of experiments was repeated 3 times.
KYSE150 and TE1 parent cells and cell spheres in logarithmic growth phase, passaged for at least three generations were collected. Cells were digested and centrifuged to obtain a cell precipitate. Total RNA was isolated using Trizol. Reverse transcription was performed according to the manufacturer’s instructions in the reverse transcription polymerase chain reaction (RT-PCR) kit (Thermo, United States). The reaction conditions used were as follows: Incubation at 60 °C for 60 min, and then 25 °C for 5 min. Reverse transcription and amplification were conducted using the RT-PCR kit and the pre-composed primer (Table 1). PCR cycle parameters were as follows: initial denaturation, 94 °C for 5 min; 94 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, cycled 30 times followed by a final incubation for 7 min at 72 °C. The PCR products were stored at 4 °C. Ten microliters PCR product and 2 μL 6 × loading buffer were added to each well for electrolysis on a 1% sepharose gel. The gel was stained with ethidium bromide for DNA visualization. Semi-quantitative analysis of the bands was performed using Bandscan 5.0 gel image analysis software. All primers were synthesized by Shanghai Sheng Gong Co. (Shanghai, China).
| Gene product | Forward (F) and reverse (R) primers (5'→3') | Size (bp) |
| BMI-1 | F: GAGCTAAATCCCCACCTGATGTG | 631 |
| R: 5'-GTGGACCATTCCTTCTCCAGG | ||
| SOX-2 | F: 5'-CCAGGAGAACCCCAAGATGCACA | 533 |
| R: 5'-GCTGCGAGTAGGACATGCTGTAGG | ||
| GAPDH | F: 5'-GGAAGGTGAAGGTCGGAGT | 134 |
| R: 5'-TGGGTGGAATCATATTGGAA |
The ability of KYSE150 and TE1 cells to form spheres after exposure to radiation was determined. Twenty-four hours after exposure to a radiation dose of 2, 4, 6, 8 and 10 Gy, KYSE150 and TE1 cells were digested, centrifuged and resuspended in SFM. The cells were then seeded in low adhesion 6-well plates at 10000 cells/well. After 10 d, cell spheres formed from the cancer cells were counted. Cancer cells with no exposure to radiation served as controls. Methods of irradiation: a Primus-H medical linear accelerator (Siemens, Germany) was employed for radiation, with a 6 MV X-ray, 30 cm × 30 cm beam size, 1 Gy/min dosage rate with the radiation source at a distance of 100 cm from the cell growth surface. The X-ray irradiation device was provided by the Second People’s Hospital of Changzhou, China (Primus-H medical linear accelerator produced by Siemens).
Adherent culture cells and cell spheres were digested and evenly inoculated into 6-well plates at different densities (5 × 102, 1 × 103, 2 × 103, 5 × 103, 1 × 104 and 2 × 104 cells) and subjected to different irradiation doses (0, 2, 4, 6, 8 and 10 Gy). Three samples were used in each group and each irradiation dose. The cells were then cultured for 14 d after irradiation. Next, the cells were fixed in methanol and stained with crystal violet. Cell colonies with more than 50 cells were counted under microscopy. Planting efficiency in different experimental conditions was calculated. Planting efficiency, PE (%) = colony number/inoculated cells × 100% and survival fraction, SF = colony number in one irradiation dose group/(inoculated cells in the group ×PE in the non-irradiation group). A cell survival curve was fitted using a multi-target single-hit model S = 1 - (1 - e-D/Do)N on Graphpad Prism 5 Demo software. The radiobiological parameters, D0, Dq and SF2, were assayed and calculated. Independent experiments were repeated 3 times.
Adherent culture cells and esophageal cancer cell spheres cultivated for more than 3 generations in a logarithmic growth phase were disrupted into single cell suspensions and the cell density was adjusted to l × l06/mL. Cells were collected and digested 24 h after exposure to radiation at doses of 0, 2, 4 and 6 Gy. Then 70% precooled ethanol was added to the cells which were kept overnight at -20 °C. The cells were stained for 1 h in 10 μg/mL propidium iodide and 5 μg/mL RNaseA at 4 °C in the dark. Cell cycle changes were detected using flow cytometry.
Suspended cultured esophageal cancer cell spheres passaged after 3 generations and cultured adherent cancer cells were collected. The cells were digested or mechanically dispersed into single cell suspensions 24 h after irradiation at 0, 4 and 8 Gy. Cell density was adjusted to 1 × l06/100 μL and monoclonal antibodies, rat against human, marked by CD44-FITC (Abcam, United States) and CD271-PEcy7 (BD, United States) were added. No antibodies were added to the control group. The cells were then incubated for 30 min at 4 °C in the dark, rinsed twice in PBS followed by detection using flow cytometry. All experiments were carried out in triplicate within 1 h of staining.
The results were analyzed by IBM SPSS 20.0 software. Data are expressed as mean ± SD. T tests were used for comparison of two groups and one-way analysis of variance was used for multiple data comparison. The Q test was used for pairwise comparisons. P < 0.05 was considered statistically significant.
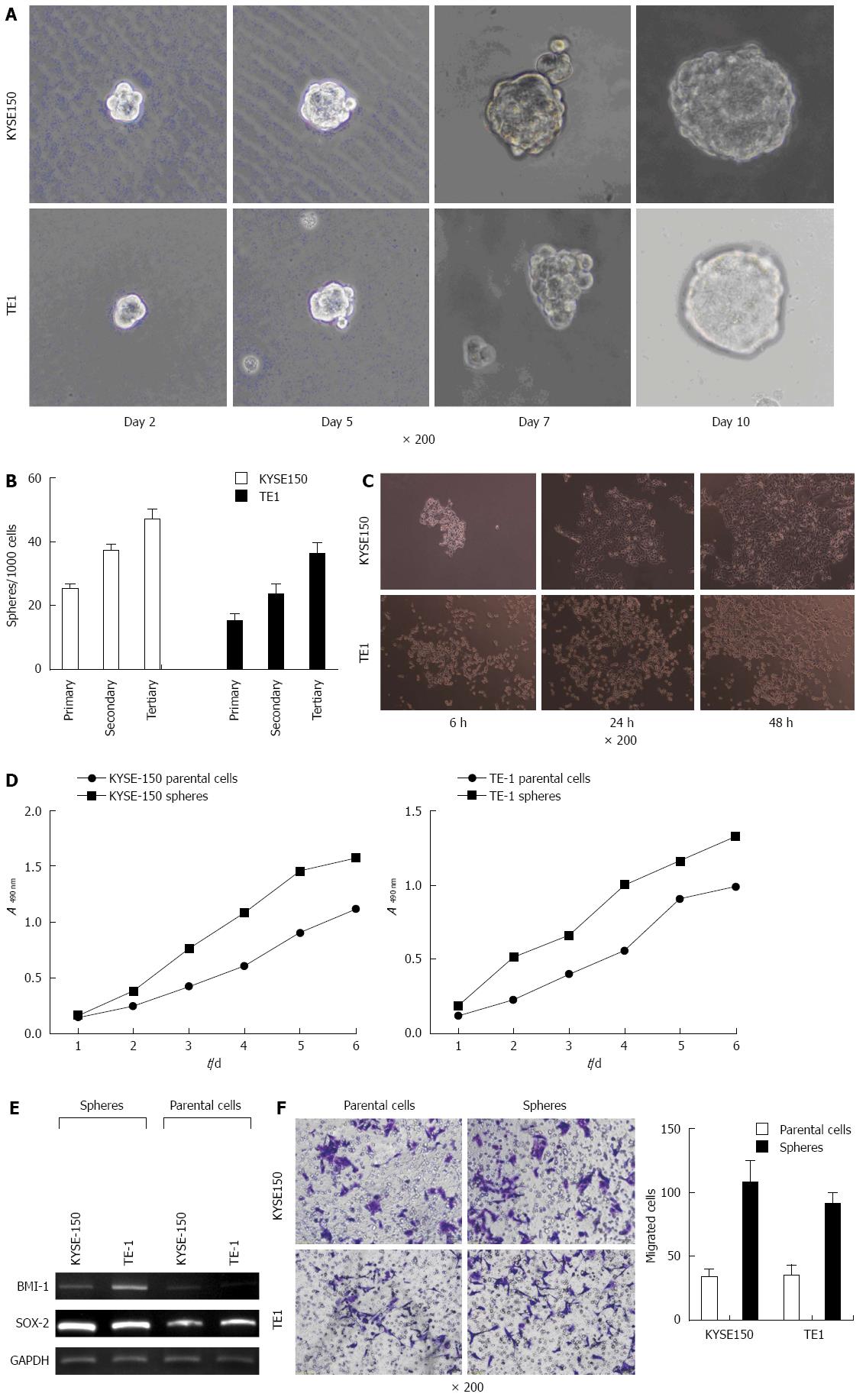
Observation under an inverted phase contrast microscope indicated cell clusters of various sizes suspended in SFM. The edge of the cell spheres displayed high refractivity. Cell spheres gradually increased in size and attained a more regular spherical shape after prolonged culture (Figure 1A). Each group of cell spheres was passaged for 3 generations. The cell sphere formation rate increased with passage number (P < 0.05, Figure 1B). Microscopic observation indicated that most cell spheres adhered after 6 h, with irregular forms (Figure 1C), and the number of adherent cells increased markedly after 24 h. Migrated cells were radially distributed and after 48 h, most cell spheres disappeared. Cells grew adherent to the wall, with cellular morphology similar to those in normal serum culture.
The MTT colorimetric method was used to measure the optical densities of cell spheres and adherent cells after different time periods. The results indicated that the multiplication capacity of KYSE150 and TE1 cell spheres was significantly higher than that of normal adherent cells (Figure 1D). Objective bands with uniform brightness were seen at 134 bp in all the experimental groups. For GAPDH, objective bands with non-uniform brightness were seen at 631 bp and at 533 bp in groups BMI-1 and SOX-2 (Figure 1E). The expression of stem cell genes, BMI-1 and SOX-2, in KYSE150 and TE1 cell spheres was much higher than the expression level in parent cells. Transwell migration experiments demonstrated a much higher invasion capacity of KYSE150 and TE1 cell spheres when compared with the parent cells (P < 0.05, Figure 1F).
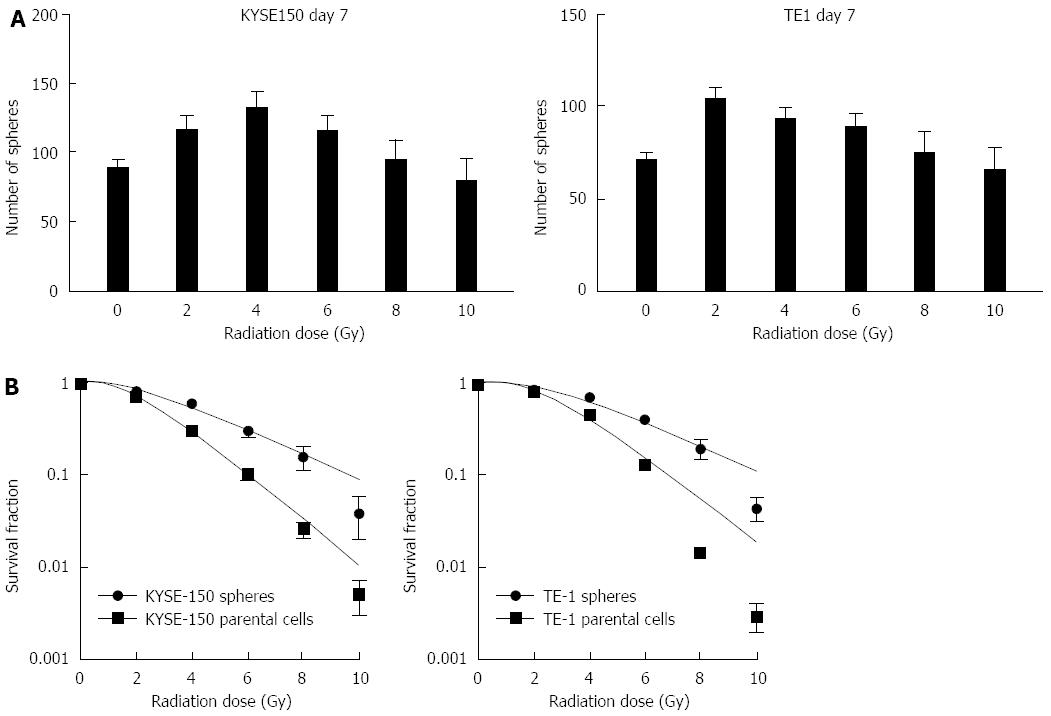
The formation rate of cell spheres increased after irradiation at different doses. The number of cell spheres obtained after irradiation of KYSE150 cells (with irradiation doses of 2, 4 and 6 Gy) was more than the non-irradiated cells. The number of TE1 cell spheres irradiated at 2 and 4 Gy was more than the non-irradiated controls (P < 0.05, Figure 2). These findings suggest that irradiation increased the rate of cell sphere formation in KYSE150 and TE1 cell lines in vitro. Following irradiation with five different irradiation doses, cell spheres and parent cells were cultured for 14 d. KYSE150 stem-like spheres and parental cells SF2Gy were 0.81 ± 0.03 vs 0.69 ± 0.04, TE1 stem-like spheres and parental cells SF2Gy were 0.87 ± 0.01 vs 0.80 ± 0.03, and the differences were statistically significant (P < 0.05). KYSE150 stem-like spheres and parental cells D0 were 3.09 ± 0.14 vs 1.98 ± 0.2, TE1 stem-like spheres and parental cells D0 were 3.03 ± 0.12 vs 1.84 ± 0.10, and the differences were statistically significant (P < 0.05). KYSE150 stem-like spheres and parental cells Dq were 2.52 ± 0.26 and 1.89 ± 0.17, TE1 stem-like spheres and parental cells Dq were 3.11 ± 0.15 and 2.54 ± 0.17, respectively (P < 0.05).
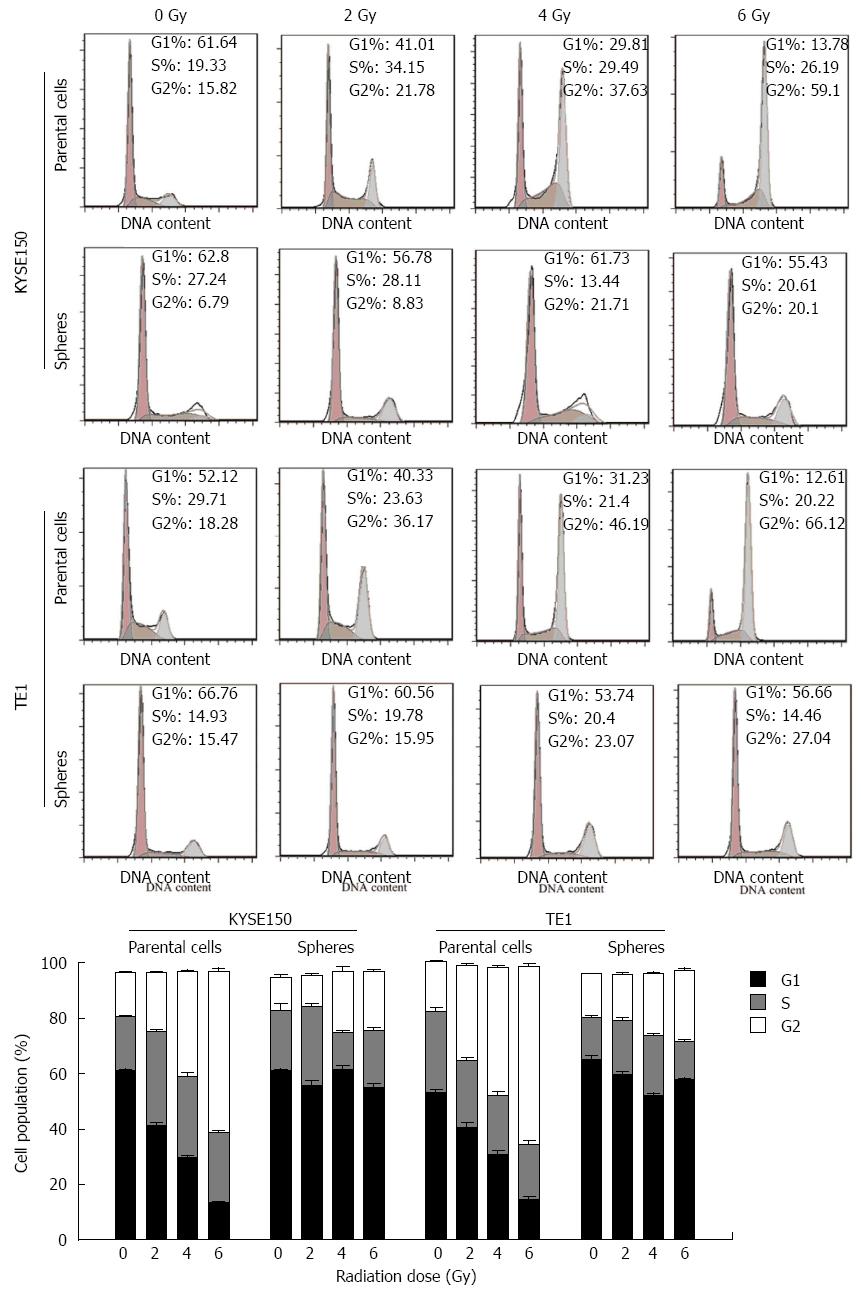
Irradiation retarded the G2 phase of parent cells in KYSE150 and TE1 cell lines. A higher dose of irradiation had a more obvious effect on inhibition of the cell cycle. Cell spheres in KYSE150 and TE1 cell lines displayed no G2 phase retardation (Figure 3). Under the same irradiation dose, the anti-proliferative effects of radiation on CSCs were significantly weaker than those on esophageal cancer parent cells (P < 0.05).
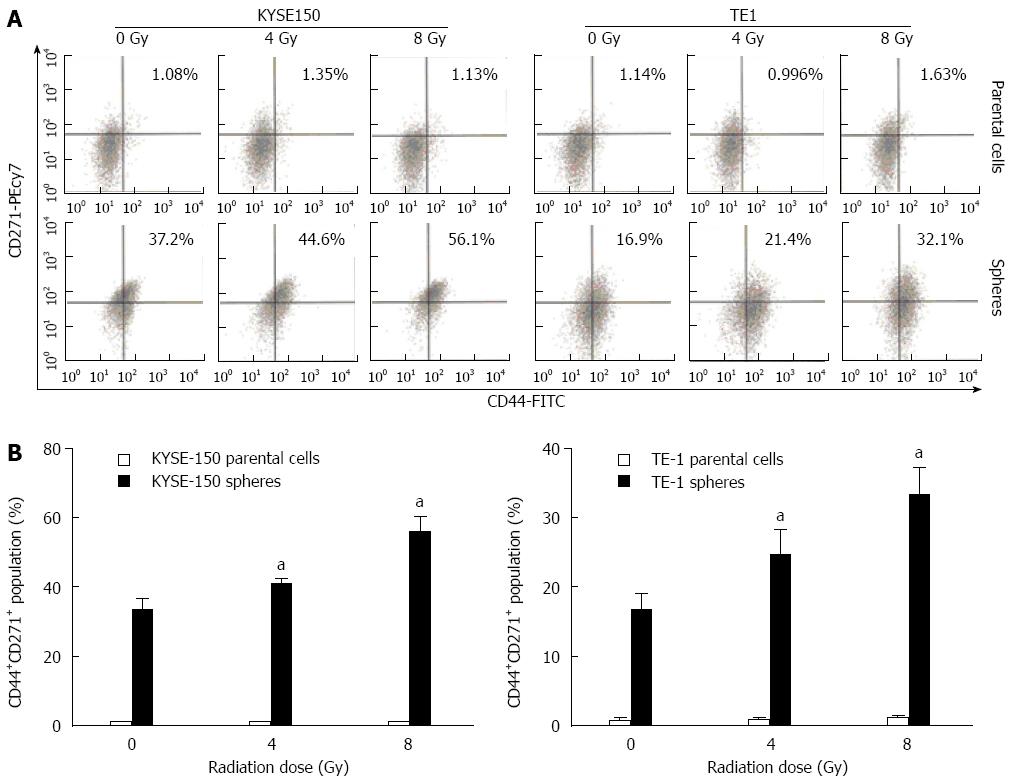
The expression of CD44+, CD271+ and CD44+CD271+ in KYSE150 and TE1 parent cells were markedly lower than those in the cell spheres (Figure 4). Under different irradiation doses, the proportion of CD44+CD271+cells in KYSE150 and TE1 parent cells exposed to 0, 4 and 8 Gy irradiation were not different among the groups. However, the proportion of CD44+CD271+ cells in KYSE150 and TE1 cell spheres was significantly different among all groups (P < 0.05, Figure 4A and C).
Cancer stem cells are thought to be the root cause of tumorigenesis, invasion, metastasis and resistance to routine chemo-radiotherapy[21-23]. As cancer cells are believed to originate from CSCs, these cells are also known as tumor initiating cells[24,25]. Many studies have proved that CSCs present in solid tumors lead to resistance to radiotherapy or chemotherapy, which accounts for the failure of contemporary treatments[26-28]. Thus, it is imperative to study the influence of radiotherapy on cancer cells as well as the effects on the proliferation and cell cycle of CSCs in order to elucidate the mechanisms responsible for resistance to radiation. This could also provide insights regarding new targets for further studies on esophageal cancer radioresistance.
There are several features associated with CSCs[29-31]: (1) self-renewal ability: CSCs can develop daughter cells identical to the last generation, thus maintaining continuous tumor growth; (2) differentiation potential: CSCs can develop into diverse differentiated cells to form new tumors in vivo; (3) high tumorigenicity: Even the injection of a few CSCs into experimental animals can result in a tumor, whereas higher numbers of cancer cells need to be injected in order to form tumors; and (4) drug resistance: Most cancer stem cell membranes express ATP-binding cassette membrane transport proteins. These proteins can transport and excrete substances including metabolites, drugs, toxic substances, endogenous lipids and polypeptides, thus making CSCs resistant to many anti-cancer drugs.
The effects of radiotherapy are mainly achieved by damage to the cell DNA, which affects the proliferation state of cells and changes the cell cycle, resulting in cell death or apoptosis. CSCs are usually in G0/G1 phase, a relatively stationary cell cycle phase[32]. CSCs express high levels of adenosine triphosphate binding proteins and have a high DNA repair capacity. Therefore, it is plausible that the mechanism of radiation resistance in CSCs is associated with the repair of damaged DNA and the influence of transduction pathways such as cell cycle control, cell apoptosis and cell proliferation. Bao et al[19] reported an association between CD133+ cells and cell survival after exposure to radiation. They demonstrated that CD133+ glioblastoma multiforme cells, isolated after radiation therapy of glioblastoma and heterotransplanted to in vitro cell cultures and in vivo cancer cells demonstrated a higher cancer cell survival rate after radiotherapy.
At present, the sorting methods for CSCs primarily include magnetic activated cell sorting, flow cytometry and the SFM. The device and magnetic beads for magnetic activated cell sorting are expensive, while sorting by flow cytometry has high requirements regarding the device and technique. Thus, the application of both methods is limited. Cancer stem cells can be cultured using the suspension culture method in SFM to form stem cell spheres in order to isolate the cancer stem cell mass. This method has been used to isolate, culture and identify CSCs in various squamous-cell carcinomas[33-36]. By the SFM culture method, we successfully obtained cell spheres from two esophageal cancer cell lines, which were significantly different from homologous cells obtained from SSM culture. The expression of genes characteristic of stem cells was much higher than that of cell spheres, suggesting an enrichment of the cancer stem cell mass in cell spheres from those in parent cells; MTT and Transwell chamber experiments demonstrated that cell spheres isolated by suspension culture were highly proliferative and invasive.
Exposure to a specific dose of irradiation increased the rate of sphere formation in both cell lines, indicating that irradiation in vitro markedly enhanced the sphere formation ability of KYSE150 and TE1 cell lines. We also found that under conditions of irradiation, esophageal cancer parent cells were retarded in G2 phase more obviously when compared to the cell spheres in the cancer stem cell mass. Furthermore, esophageal cancer stem cell spheres were resistant to G2 phase retardation and displayed no obvious changes in cell cycle distribution. At present, there are no precise markers on the surface of esophageal CSCs. However, some studies have indicated that esophageal squamous carcinoma CD44[37] and CD271 (P75NTR/NGFR)[38,39] positive cells display some features of CSCs such as tumorigenesis, differentiation, drug resistance and self-renewal. Therefore, we chose these as reference markers to reflect cancer stem cell characteristics in esophageal squamous carcinoma cells. We found that irradiation (4 and 8 Gy) significantly increased CD44+CD271+cell mass in cell spheres. Furthermore, in esophageal cell spheres, CD44+CD271+cell number was higher in comparison with the parent cells.
In conclusion, the serum-free suspension culture discussed in this study is a low cost, affordable and feasible method to isolate CSCs. Through SFM suspension culture, cell spheres rich in esophageal CSCs can be isolated and display a high proliferative capacity. These cells can also be passaged and amplified stably in vitro. Therefore, this culture method can be temporarily used as a tool for esophageal cancer stem cell studies. The mechanism of CSCs radioresistance may be associated with the repair of damaged DNA indicating the involvement of signal transduction pathways such as cell cycle regulation and proliferation. In this study, we found that esophageal cancer cell spheres with stem cell characteristics were more resistant to radiation than their respective parent cells. At present, studies on the radioresistance of esophageal CSCs are still in their infancy and there are still many open questions that need to be answered regarding the characteristics of esophageal CSCs.
Cancer persistence and recurrence are the major factors affecting the efficacy of radiation therapy in esophageal cancer. The reason for this is the radiation resistance of esophageal cancer. In recent years, cancer stem cells have become a hot topic in the field of cancer research. To date, there are no research reports on the role of cancer stem cells (CSCs) in esophageal cancer radiation resistance.
CSCs have been demonstrated to be a target in radiation therapy, however, few reports on esophagus CSCs as a radiation-resistance agent are available, and their influence on radiation remains undetermined.
In this study, the authors found more CSCs in esophageal cancer (EC) spheres than in the EC parental cell lines. The stem-like spheres were more radioresistant than their parental cells, which may suggest that CSCs are related to radioresistance.
CSCs status during radiotherapy is of great significance in predicting prognosis.
CSCs are cancer cells that possess characteristics associated with normal stem cells. CSCs are therefore tumorigenic (tumor-forming), in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types.
This study focuses on sphere formation and radio-sensitivity of two esophageal cancer cell lines, as well as their influence on self-renewal of sphere-type cultures, cell cycle and cell surface markers.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 870] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 3. | Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 575] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 4. | Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1518] [Cited by in RCA: 1793] [Article Influence: 128.1] [Reference Citation Analysis (0)] |
| 5. | Fujita T. Subpopulations of circulating cancer stem cell-like cells. Ann Surg. 2014;259:e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Zhang CC. Novel signaling axis in CML-initiating cells. Blood. 2014;123:2443-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Sonderegger S, Pollheimer J, Knöfler M. Wnt signalling in implantation, decidualisation and placental differentiation--review. Placenta. 2010;31:839-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Stoica G, Lungu G, Martini-Stoica H, Waghela S, Levine J, Smith R. Identification of cancer stem cells in dog glioblastoma. Vet Pathol. 2009;46:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [PubMed] |
| 10. | O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [PubMed] |
| 11. | El-Khattouti A, Selimovic D, Haïkel Y, Megahed M, Gomez CR, Hassan M. Identification and analysis of CD133(+) melanoma stem-like cells conferring resistance to taxol: An insight into the mechanisms of their resistance and response. Cancer Lett. 2014;343:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Meijneke TR, Petit SF, Wentzler D, Hoogeman M, Nuyttens JJ. Reirradiation and stereotactic radiotherapy for tumors in the lung: dose summation and toxicity. Radiother Oncol. 2013;107:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Moore KL, Brame RS, Low DA, Mutic S. Experience-based quality control of clinical intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Gomez DR, Hunt MA, Jackson A, O’Meara WP, Bukanova EN, Zelefsky MJ, Yamada Y, Rosenzweig KE. Low rate of thoracic toxicity in palliative paraspinal single-fraction stereotactic body radiation therapy. Radiother Oncol. 2009;93:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Zhang X, Komaki R, Wang L, Fang B, Chang JY. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clin Cancer Res. 2008;14:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Debeb BG, Xu W, Woodward WA. Radiation resistance of breast cancer stem cells: understanding the clinical framework. J Mammary Gland Biol Neoplasia. 2009;14:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 644] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 18. | Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2102] [Cited by in RCA: 1961] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 19. | Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-760. [PubMed] |
| 20. | McCord AM, Jamal M, Williams ES, Camphausen K, Tofilon PJ. CD133+ glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin Cancer Res. 2009;15:5145-5153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Filipovic A, Stebbing J, Giamas G. Cancer stem cells--therapeutic targeting or therapy? Lancet Oncol. 2013;14:579-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Bütof R, Dubrovska A, Baumann M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother Oncol. 2013;108:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Peitzsch C, Kurth I, Kunz-Schughart L, Baumann M, Dubrovska A. Discovery of the cancer stem cell related determinants of radioresistance. Radiother Oncol. 2013;108:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1011] [Cited by in RCA: 1176] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 25. | Potter NE, Greaves M. Cancer: Persistence of leukaemic ancestors. Nature. 2014;506:300-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, Wang Z, Yu J, Shi Jz, Wang C, Fu Wh, Chen Zw, Yang J. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett. 2012;322:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, Sung PS, Song MJ, Lee BC, Hwang D. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | López J, Poitevin A, Mendoza-Martínez V, Pérez-Plasencia C, García-Carrancá A. Cancer-initiating cells derived from established cervical cell lines exhibit stem-cell markers and increased radioresistance. BMC Cancer. 2012;12:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [PubMed] |
| 30. | Zhou J, Wang CY, Liu T, Wu B, Zhou F, Xiong JX, Wu HS, Tao J, Zhao G, Yang M. Persistence of side population cells with high drug efflux capacity in pancreatic cancer. World J Gastroenterol. 2008;14:925-930. [PubMed] |
| 31. | She JJ, Zhang PG, Wang X, Che XM, Wang ZM. Side population cells isolated from KATO III human gastric cancer cell line have cancer stem cell-like characteristics. World J Gastroenterol. 2012;18:4610-4617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936-4941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 33. | Lim YC, Oh SY, Cha YY, Kim SH, Jin X, Kim H. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011;47:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One. 2011;6:e16466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 35. | Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085-4095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 36. | Zhang L, Jiao M, Li L, Wu D, Wu K, Li X, Zhu G, Dang Q, Wang X, Hsieh JT. Tumorspheres derived from prostate cancer cells possess chemoresistant and cancer stem cell properties. J Cancer Res Clin Oncol. 2012;138:675-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Zhao JS, Li WJ, Ge D, Zhang PJ, Li JJ, Lu CL, Ji XD, Guan DX, Gao H, Xu LY. Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44. PLoS One. 2011;6:e21419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Okumura T, Shimada Y, Imamura M, Yasumoto S. Neurotrophin receptor p75(NTR) characterizes human esophageal keratinocyte stem cells in vitro. Oncogene. 2003;22:4017-4026. [PubMed] |
| 39. | Huang SD, Yuan Y, Liu XH, Gong DJ, Bai CG, Wang F, Luo JH, Xu ZY. Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer. 2009;9:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
P- Reviewer: Freter R, Greenberger JS S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Wang CH