Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18189
Revised: July 3, 2014
Accepted: September 5, 2014
Published online: December 28, 2014
Processing time: 245 Days and 6.8 Hours
AIM: To investigate the role of caspase-12 and its downstream targets in carbon tetrachloride (CCl4)-induced hepatocyte apoptosis.
METHODS: The role of caspase-12 was determined by using caspase-12 knock-out (-/-) mice. CCl4 (300 μL/kg body weight) or vehicle (corn oil) was administered to caspase-12+/+ or caspase-12-/- mice as a single intraperitoneal injection. The animals were sacrificed 24 h after the CCl4 treatment. Blood was collected to evaluate liver function by the measurement of the activity of alanine aminotransferase. Liver samples were used for the measurements of reactive oxygen species using plasma malondialdehyde as biomarker, hepatocyte apoptosis was evaluated via terminal transferase-mediated dUTP nick-end labeling and controlled by morphologic study, and cytochrome C release and caspase activations were measured by Western blotting.
RESULTS: Administration of a low dose of CCl4 resulted in hepatocyte apoptosis and acute liver injury in wild-type mice. CCl4 also induced the generation of reactive oxygen species and induction of endoplasmic reticulum stress in the liver followed by activations of caspase-12, -9 and -3 as well as release of small amounts of cytochrome C. However, in the CCl4-treated caspase-12-/- mice, activation of caspase-9 and -3 were significantly attenuated (P < 0.05); no effect was seen in cytochrome C release. CCl4-induced apoptosis and liver damage was markedly reduced in caspase-12-/- mice compared to caspase-12+/+ mice (P < 0.05). The active form of caspase-8 was not detected in either caspase-12+/+ or caspase-12-/- mice. There was no significant different in the formation of reactive oxygen species in the livers of caspase-12+/+ and caspase-12-/- mice treated with CCl4.
CONCLUSION: Caspase-12 plays a pivotal role in CCl4-induced hepatic apoptosis through the activation of the downstream effector caspase-3 directly and/or indirectly via capase-9 activation.
Core tip: Sublethal doses of carbon tetrachloride (CCl4) induced significant hepatocyte apoptosis and acute liver injury in the wild-type mice. CCl4 also induced the generation of reactive oxygen species in the liver followed by activation of caspase-12, -9 and -3. Loss of caspase-12 in knock-out mice attenuated CCl4-induced activation of caspase-9 and -3, significantly reduced apoptosis, and preserved liver function. Caspase-8 was not detected, indicating that it does not play a significant role in this model of hepatocyte apoptosis. The data indicate that caspase-12 plays a pivotal role in CCl4-induced hepatocyte apoptosis through the downstream activation of the effector caspase-3 directly and/or indirectly via caspase-9 activation.
- Citation: Liu H, Wang Z, Nowicki MJ. Caspase-12 mediates carbon tetrachloride-induced hepatocyte apoptosis in mice. World J Gastroenterol 2014; 20(48): 18189-18198
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18189
Toxic liver damage may result in acute liver failure, hepatic fibrosis and carcinogenesis[1]. Hepatotoxicity remains a major reason for drug withdrawal from pharmaceutical development and clinical use[1]. Hepatocyte apoptosis is an important contributing factor to acute liver injury in a variety of liver diseases including toxic effects of drugs, alcohol, viral infection, non-alcoholic steatosis and cholestasis[2-4]. From the studies over last decade, hepatocyte apoptosis appears to be the first cellular response to toxic damage and is thought to be the main mode of cell death in liver diseases[4,5].
Apoptosis is executed through the activation of caspase cascades, via the apoptotic pathways. The pathways start with activation of an initiator caspase by different stimuli, caspase-8 in membrane-mediated, caspase-9 in mitochondrial-mediated, and caspase-12 in endoplasmic reticulum (ER) stress-mediated pathways[6-8]. The active initiator caspases then activate effector caspase-3, -6 or -7 which cleave key substrates required for normal cellular functions leading to apoptosis[6,7,9-12].
Carbon tetrachloride (CCl4)-induced hepatic injury has been widely used to study the mechanisms of hepatotoxic injury and repair. Treatment with a sublethal dose of CCl4 results in massive apoptotic damage in the liver[13,14]. Previous studies have shown the activation of caspase-3 and -9 in the liver of CCl4-treated mice or rats[15,16]. However, the role of caspase-12 and its down-stream targets in CCl4-induced hepatocyte apoptosis have not been defined.
Procaspase-12 is predominantly located on the cytoplasmic side of the ER and expressed at high levels in muscle, liver and kidney[8], and is activated by ER stress[17,18]. The initial event in the liver of CCl4-treated animals is generation of reactive oxygen species (ROS) within the ER resulting from the interaction of CCl4 and cytochrome P450 (CYP)[19,20]. ER is highly sensitive to environmental insults such as oxidative stress which lead to ER stress[21,22]. Nakagawa et al[8] have demonstrated that ER-stress inducer tunicamycin-induced apoptosis in embryonic fibroblasts and renal tubular epithelial cells was significantly attenuated in caspase-12 knockout (-/-) mice. In the same study, treatment of thymocytes isolated from wild-type and caspase-12-/- mice with anti-Fas antibody (activating the membrane-dependent pathway) or dexamethasone (activating the mitochondria-dependent pathway through cytochrome C release) developed similar amounts of apoptosis[8]. The authors suggest that caspase-12 is involved in ER stress-induced apoptosis independent of membrane-mediated and mitochondrial pathways. In a cisplatin model of renal tubular apoptosis, we demonstrated that activation of caspase-12 prior to the activations of caspase-3 and -9 and transfection of anti-caspase-12 antibody into renal tubular epithelial cells prevented the activation of procaspase-12 and significantly attenuated cisplatin-induced renal tubular apoptosis[23]. The direct role of caspase-12 in hepatocyte apoptosis was not explored previously. Hence, the current study was to examine if caspase-12 plays essential role and its downstream targets in CCl4-induced hepatocyte apoptosis using caspase12-/- mice.
Caspase-12-/- mice were purchased from the Mutant Mouse Regional Resource Center (Chapel Hill, NC, United States), which were developed on a C57BL/6J background as described[8]. The litter resuscitated from cryo-archive was genotyped, and the breeding was carried out by monogamous mating. A pair of male and female homozygous caspase-12-/- mice was kept in the same cage for mating. Pups were weaned at an age of 3 wk and separated according to gender. Animals were maintained under 12 h light/dark cycles with unlimited access to food and water. Male mice at 8 wk of age, weighing 25-30 g, were used for the experiments. All experimental procedures were conducted in accordance with our institutional guidelines and approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center (Permit Number: 1107B).
Our previous study indicated that a large dose of CCl4 [1000 μL/kg body weight (BW), equivalent to 1.59 g/kg BW; ip] induced severe liver damage and massive necrosis. Therefore, a pilot study was performed to determine the appropriate dose and time points for significant induction of hepatocyte apoptosis in littermate capsase-12+/+ mice. CCl4 (Sigma-Aldrich, St. Louis, MO, United States) was administered as a single ip dose of different volumes (100, 300 and 500 μL/kg BW, equivalent to 159, 477 and 795 μg/kg BW; dissolved in corn oil) or vehicle (corn oil). The animals were sacrificed at 6, 12, 24 and 48 h after CCl4 injection. Blood and liver samples were collected for the measurements of liver injury and apoptosis.
Alanine aminotransferase (ALT) activity was measured on a Roche-cobas c501 analyzer (Roche Diagnostics, Basel, Switzerland) using Roche-cobas ALTL reagents. The assay is linear from 5-700 U/L and exhibits a slope of 1.003 (r = 0.996).
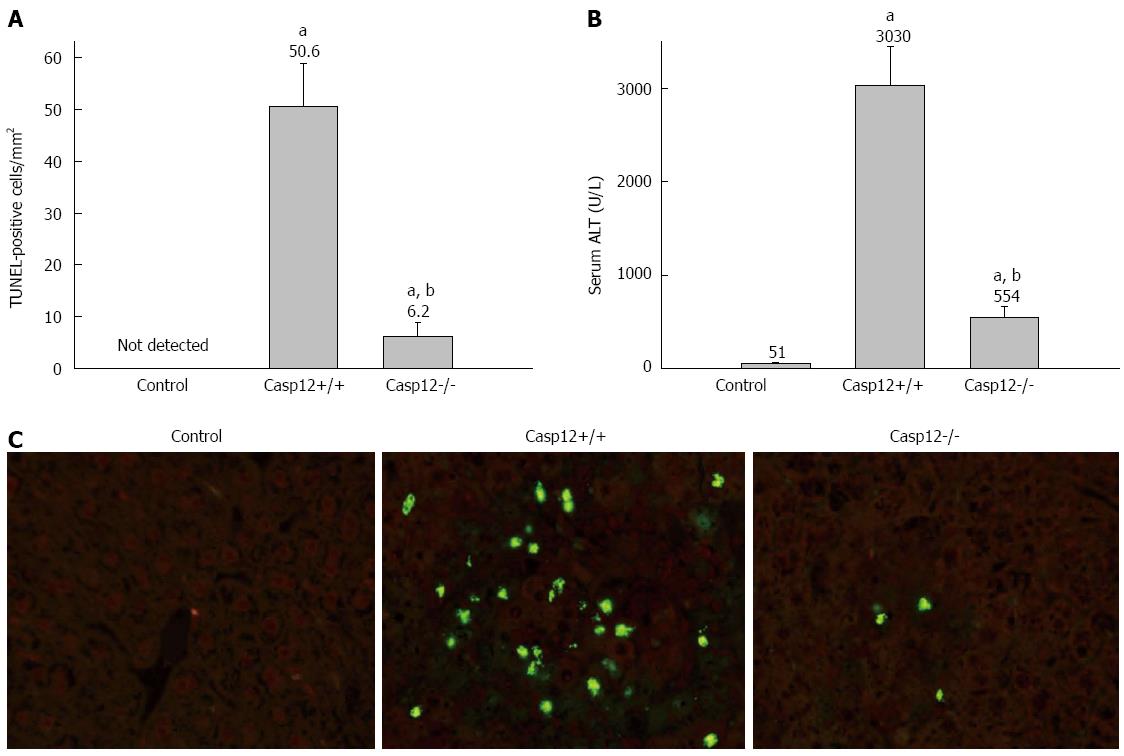
Detection of apoptotic cells by terminal transferase-mediated dUTP nick-end labeling (TUNEL) is extensively used for the quantification of apoptotic cells in tissue or cell culture. However, there have been concerns about its sensitivity and specificity. In the current study, apoptotic cells were quantified by a modified TUNEL protocol, and were controlled by morphologic study. TUNEL stain on mouse liver sections was performed by use of ApoAlert DNA Fragmentation Assay Kit (Clontech of Takara Bio Inc., Shiga, Japan) following the manufacturer’s protocol and with modifications as described by Tamura et al[24] and Labat-Moleur et al[25]. Apoptosis was quantified as TUNEL-positive cells/mm2. In morphologic examination, the following findings were considered to represent apoptosis: condensation of chromatin and cytoplasm, margination of nuclei, cell blebbing, and apoptotic bodies[26].
Malondialdehyde (MDA), an intermediate of lipid peroxidation, was determined as a measure of lipid peroxidation following the protocol described by Wenger et al[27]. Briefly, liver tissue was rapidly frozen at the time of harvest and stored in liquid nitrogen. The tissue was homogenized in an ice-cold buffer (20 mmol/L PBS pH 7.4, 140 mmol/L NaCl, 0.01% butylated hydroxyanisole, 0.25 mol/L DMSO), and then centrifuged at 900 ×g for 10 min; 20 μL of liver homogenate was incubated at 90 °C for 60 min in 0.8 mL reaction mixture of 1.35 mol/L acetic acid, 0.15 mol/L sodium dodecyl sulphate, 20 mol/L 1-thiobarbituric acid (pH 3.5). After cooling the reaction tubes in ice water, 0.2 mL distilled water and 1 mL n-butanol/pyridine mixture (15:1) were added. This mixture was mixed by vortexing and then centrifuged. The organic layer was taken for fluorometric measurements of the thiobarbituric acid reactive substances fluorescence (excitation, 515 nm; emission, 553 nm), the concentration of which was calculated using 1.1.3.3-tetraethoxypropane dissolved in acetic acid as a standard.
Cell fractions were prepared as described previously[8]. Liver tissue was homogenized in an extraction buffer and cell fractions were obtained by differential centrifugations.
Western blot was performed by the chemiluminescence method as described elsewhere[28]. The antibodies used were as follows: rabbit anti-caspase-12 (AB3612, Lot 22101182; Chemicon of EMD Millipore, Billerica, MA, United States) and mouse anti-actin (MAB1501; Chemicon); rabbit anti-caspase-3 (sc-7148), caspase-8 (sc-7890), caspase-9 (sc-8355), and rabbit anti-cytochrome C (sc-7159) from Santa Cruz Biotechnology (Dallas, TX, United States); anti-glucose-regulated protein 78 (GRP78) (NBP1-06274; Novus Biologicals, Littleton, CO, United States); anti-CCAAT/-enhancer-binding protein homologous protein (CHOP) (#2895; Cell Signaling Technology, Danvers, MA); anti-serine/threonine-protein kinase/endoribonuclease (IRE-1) (ab37073; Abcam, Cambridge, United Kingdom).
Genomic DNA was isolated from a small section of mouse tail by using a GentraPure gene Mouse Tail Kit (Qiagen, Venlo, Limburg, Netherlands). PCR was performed by a GoTaq Flexi DNA Polymerase kit (Promega Corp., Madison, WI, United States) and using three primers: GS (E), GCCAGGAGGACACATGAAAGAGATC; GS (E, T), AGCTGTTCCTGGGAAT TGGCAATG; Neo, GGGTGGGATTAGATAAATGCCTGCTCT. For routine genotyping, a PCR protocol employing a mixture of the three primers was used to detect wild allele (246 bp) and mutant allele (414 bp). PCR products were visualized on a 2% agarose gel with ethidium bromide. Caspase-12 expression in mice was further confirmed by RT-PCR and Western blot.
RNA was isolated from the liver tissue using TRIzol reagent (Gibco of Thermo Fisher Scientfic, Waltham, MA, United States). Reverse transcription of 0.5 μg of total RNA and PCR were carried out by a one-step RT-PCR protocol using AccessQuick RT-PCR system (Promega Corp.). Caspase-12 primers are: 5’-GAAGGAATCTGTGGGGTGAA (F), 5’-AGGCCTGCATGATGAGAATC (R) (product size about 133 bp). GAPDH primers include: 5’-AAGATGGTGAAGGTCGGTGT (F), 5’-TTGATGGCAACAATGTCCACT (R) (product size about 98 bp). The PCR products were visualized on a 2% agarose gel using ethidium bromide and ultraviolet transillumination.
Values are expressed as the mean ± standard error. Statistical analysis was performed using an unpaired t test (for two groups only) and analysis of variance (for more than two groups). Statistical significance was considered at P < 0.05. Calculations were made with SigmaStat 3.5 (Systat Software Inc., San Jose, CA, United States).
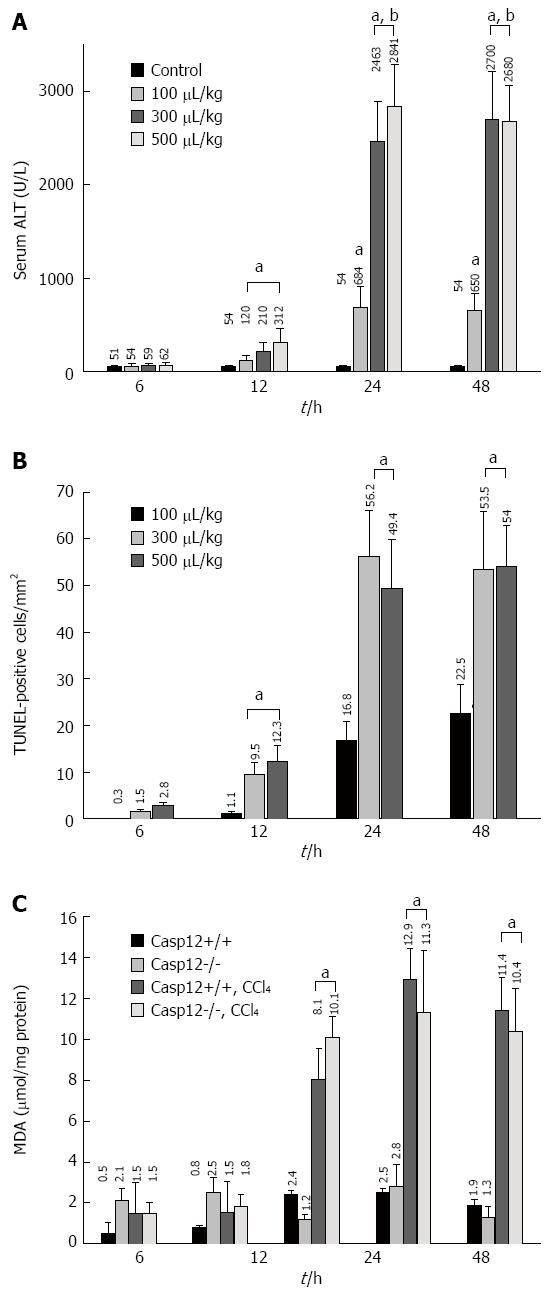
We conducted a pilot study to determine the appropriate dose and time points for significant induction of apoptosis in the liver of wild-type mice. CCl4-induced hepatic injury as measured by ALT was noted starting at 12 h and this damage became more severe at 24 and 48 h (Figure 1A). The increases of ALT in 300 and 500 μL/kg BW treated groups were similar, and they were significantly higher than that of 100 μL/kg group (P < 0.05). TUNEL staining demonstrated that there were virtually no positive apoptotic cells in the liver of control mice. Very few apoptotic cells (1-5 per mm2) were observed in the liver section of the mice 6 h after the CCl4 injection. A significant amount of apoptotic cells occurred after 12 h and increased to about 50 TUNEL-positive cells per mm2 at 24 and 48 h following the CCl4 treatment (P < 0.05) (Figure 1B). The TUNEL stain result was controlled by morphologic examination, which showed that about 85% TUNEL-positive cells exhibited the morphologic features of apoptotic cells. It was determined that CCl4 administered as a single dose of 300 μL/kg (equivalent to 477 μg/kg BW) ip induced hepatocyte apoptosis at about 50 TUNEL-positive cells/mm2 at 24 h after the injection, and this was the dose used for the subsequent animal experiments.
MDA was determined as a measure of lipid peroxidation. Lipid peroxidation started to increase 6 h after CCl4 treatment in caspase-12+/+ and -12-/- mice. It was significantly increased at 12 h, continued to rise at 24 h, and maintained until 48 h following the admission of CCl4 (P < 0.05) (Figure 1C). There was no significant difference in MDA generation between caspase-12+/+ and 12-/- mice.
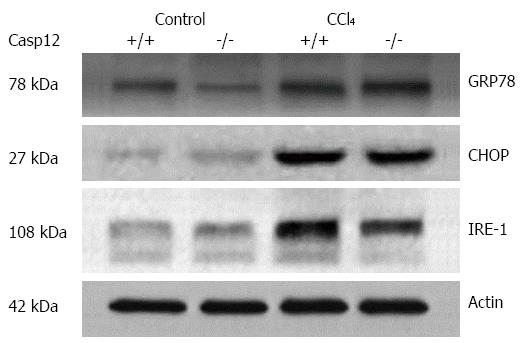
To examine the ER stress response induced by CCl4, we analyzed several key ER stress markers in CCl4-treated mice by Western blot. As expected, GRP78, CHOP and IRE-1 were upregulated in the liver tissue from both caspase-12+/+ and -12-/- mice treated with CCl4 (Figure 2). Caspase-12 gene knockout has no effect on the inductions of these proteins suggesting that activation of caspase-12 is a downstream event of ER stress.
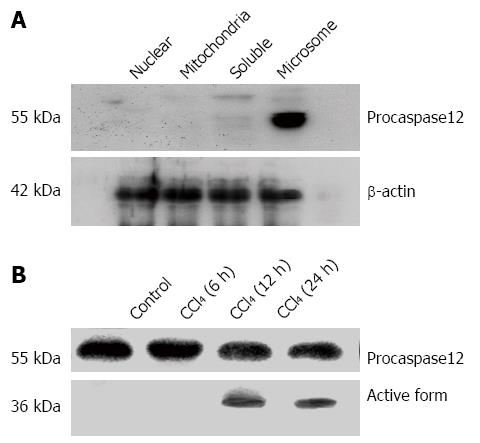
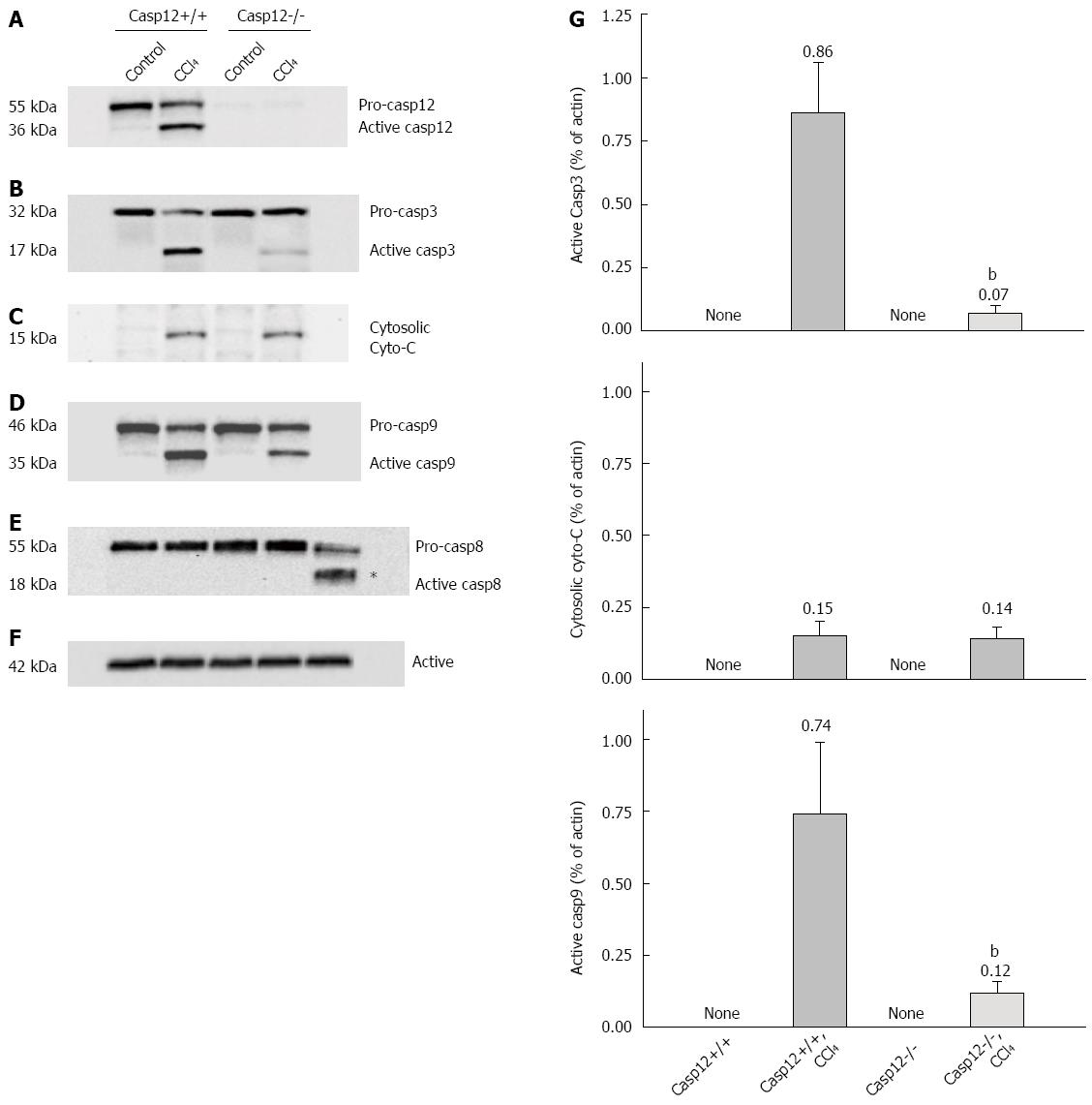
Procaspase-12 was identified and localized in microsomal fraction of wild-type mouse liver homogenate, but not in the nuclear, mitochondria or soluble cell factions (Figure 3A). As shown in Figure 3B, procaspase-12 (55 kDa) was cleaved to the active form (36 kDa). CCl4 activated caspase-12 in the liver of caspase-12+/+ mice 12 and 24 h after the treatment (Figure 3B).
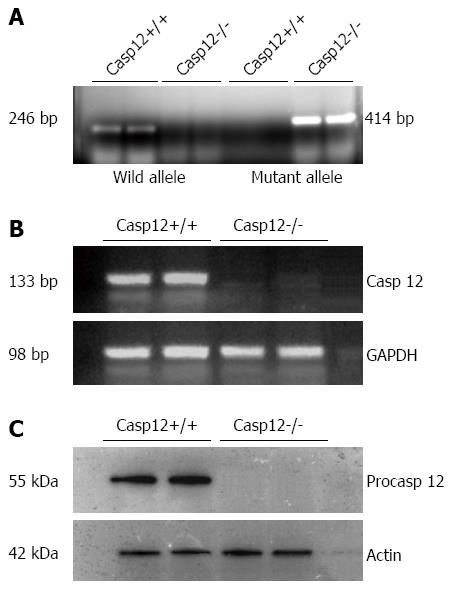
Initially, we confirmed the caspase-12-/- mice by genotyping, RT-PCR and Western blot using the tissue from a small section of the tail. Genotyping results indicated the absence of the wild-type allele and presence of the mutant allele in the caspase-12-/- mice (Figure 4A). The absence of caspase-12 in the knockout mice was confirmed by RT-PCR for mRNA expression (Figure 4B), and by Western blot for its protein expression (Figure 4C).
To examine the role of caspase-12 in CCl4-induced liver apoptosis, caspase-12+/+ or caspase-12-/- mice were injected with single dose of CCl4 (300 μL/kg BW, ip), and the animals were sacrificed at 24 h following the injection. CCl4 induced marked hepatic apoptosis in caspase-12+/+ mice, and this was significantly attenuated in the caspase-12-/- mice (P < 0.05) (Figure 5A, C). CCl4 injection resulted in marked liver damage in caspase-12+/+ mice as measured by serum ALT, and this injury was substantially reduced in caspase-12-/- mice (Figure 5B).
Western blot analysis was performed to examine activation of key caspases and cytochrome C release in response to the CCl4 treatment. As shown in Figure 6, caspase-12 was activated (presence of cleaved form of caspase-12) in the CCl4-treated caspase-12+/+ mice (Figure 6A). CCl4 treatment also resulted in the activation of caspase-3 and-9 in the liver of caspase-12+/+ mice; activations of these caspases were significantly inhibited in caspase-12-/- mice (P < 0.05) (Figure 6B, D, G). The mitochondria-mediated apoptotic pathway involves release of mitochondrial protein cytochrome C, resulting in activation of caspase-9. In a Western blot analysis using soluble cell factions, CCl4 treatment induced a small amount of cytochrome C release from mitochondria, with no significant difference in the amount of cytochrome C released between caspase-12+/+ and caspase-12-/- mice (Figure 6C, G).
There was no cleaved form of caspase-8 either in caspase-12+/+ or caspase-12-/- mice following CCl4 treatment. To confirm this result, a positive control using liver tissue extract from a lipopolysaccharide/galactosamine-treated rat was included along with other samples. As shown in Figure 6E, active caspase-8 was generated following exposure to galactosamine/lipopolysaccharide, but not after administration of CCl4. The absence of active form of caspase-8 indicates the membrane-mediated pathway does not play a significant role in CCl4-induced hepatic apoptosis in the mice.
Apoptosis is the first cellular response to many toxic liver diseases[4]. CCl4 induced hepatic apoptosis as early as 6 h[14], far preceding the hepatic necrosis observed at 24 to 48 h following CCl4 treatment[19]. CCl4 is activated by CYP2E1, CYP2B1/CYP2B2 in the ER of hepatocytes to form the trichloromethyl radical and CCl3*. This radical can react with oxygen leading to the formation of the trichloromethylperoxy radical (CCl3OO*), a highly reactive species, which initiates a chain reaction of lipid peroxidation[29]. ROS are generated as early as 1 h after CCl4 administration and can serve as a cell-death signal to induce apoptosis[30]. ROS formation in the ER leads to perturbation of Ca2+ homeostasis, altered glycosylation and the accumulation of misfolded proteins causing ER stress[31]. Caspase-12 is localized to the cytosolic interface of ER, making it vulnerable to the ER stress and, when activated, leading to further activation of the caspase cascade[8]. In fact, our current study demonstrated that CCl4 treatment resulted in significant ROS generation and upregulation of ER stress response proteins GRP78, CHOP and IRE-1 in the liver tissue. Moreover, CCl4 induced a similar degree of ROS generation and ER stress in the liver of both caspase-12+/+ and -12-/- mice. These results suggest that caspase-12 activation is a downstream event following ER stress.
In the current study, we confirmed that procaspase-12 is localized to the microsomal fraction of the hepatocytes in the wild-type mice but not in the mitochondrial, nuclear, and soluble fractions by Western blot analysis. A sublethal dose of CCl4 induced ROS generation starting at 6 h followed by activation of caspase-12, hepatocyte apoptosis and liver injury at 12 h after CCl4 injection.
To explore the role of caspase-12 in CCl4-induced hepatocyte apoptosis, we conducted a study by using caspase-12-/- mice. A complete absence of caspase-12 in the knockout mice was confirmed by genotyping, Western blot and RT-PCR. CCl4-induced apoptosis in the liver of caspase-12-/- mice was significantly attenuated, and the liver damage (ALT level) was markedly reduced in the caspase-12-/- mice as compared with caspase-12+/+ mice. The nearly complete protection of caspase-12-/- mice against CCl4-induced hepatic apoptosis indicates that caspase-12 is a pivotal caspase in CCl4-induced apoptotic signaling.
Having demonstrated the role of caspase-12, we next examined its downstream targets in the apoptotic pathway. Caspase-9 was activated in the liver of wild-type mice treated with CCl4, and the activation of caspase-9 was significantly reduced in caspase-12-/- mice. This result implies that caspase-9 is a downstream target of the active caspase-12. It has been a common belief that cytochrome C-Apaf-1 complex, namely the apoptosome, is required for the activation of caspase-9 during apoptosis. However, an in vitro study showed that ER stress induced activations of caspase-12, -9 and -3 in cytochrome C free cytosols[12]. Apaf-1-/- fibroblasts are known to be resistant to apoptotic insults that initiate the mitochondria pathway; however, they are susceptible to apoptosis caused by ER stress inducers[18]. In a study using a cell-free system, addition of microsomes (isolated from ER-stress-induced cells) to an Apaf-1-/- cell extract lacking mitochondria or cytochrome C resulted in activation of caspase-9[18]. These results indicate caspase-12 can activate caspase-9 in a cytochrome C and Apaf-1 independent fashion. Morishima et al[12] have shown that recombinant caspase-12 specifically cleaves and activates procaspase-9 in cytosolic extracts of a murine myoblast cell line C2C12.
Agents that induce ER stress can also result in release of cytochrome C from mitochondria due to its membrane damage[32,33]. Indeed, in the present study, a small amount of cytochrome C release was observed in the liver of both wild-type and caspase-12-/- mice treated with CCl4. There was no difference in the amount of cytochrome C release between wild-type and caspase-12-/- mice. However, caspase-12-/- significantly reduced caspase-9 activation and provided marked protection against CCl4-induced apoptosis. It appears that this release of cytochrome C does not have a significant role in CCl4-induced apoptosis in the liver. As discussed earlier, cytochrome C is not required for caspase-9 activation under ER stress.
Caspase-3 is the key effector caspase that executes apoptosis through the cleavage of substrates required for normal cellular functions, such as cytoskeletal proteins, nuclear proteins and DNA-repairing enzymes[6]. It is well known that caspase-3 is activated by initiators such as caspase-8 and -9 through membrane- or mitochondrial-mediated pathways in response to different stimuli[6,9-12]. In the current study, caspase-8 was not activated in the CCl4-induced hepatocyte apoptosis. To confirm this result, a positive control using liver tissue extract from a lipopolysaccharide/galactosamine-treated rat was included in Western blot analyses along with other samples. The active caspase-8 was present in the liver of the animals exposed to lipopolysaccharide/galactosamine, but not in CCl4-treated mice. The absence of active form of caspase-8 indicates that the membrane-mediated pathway does not play a significant role in CCl4-induced hepatocyte apoptosis. The absence of caspase-8 activation in the apoptosis associated with ER-stress was also evidenced by our previous study[23] and by others[18] in different experimental animal models.
Previous studies demonstrated that activation of caspase-12 occurs prior to the activation of executioner caspase-3 in other animal models of apoptosis associated ER-stress[23,34]. Treatment of human neuroblastoma cells with ER-stress inducers resulted in the activation of caspase-3 without the activations of caspase-8 and -9, and cells with stable expression of caspase-12 were more vulnerable to ER stress, concomitant with increased activation of caspase-3[35]. Our current study shows that caspase-3 activation is significantly attenuated in caspase-12-/- mice accompanied by a marked reduction of hepatocyte apoptosis. These findings suggest that caspase-12 activates caspase-3 downstream.
Taken together, our data establish the essential role of caspase-12 in CCl4-induced hepatocyte apoptosis. We postulate that the ER-associated caspase-12 services as an initiator caspase that activates caspase-3 downstream directly and/or indirectly through activation of caspase-9, resulting in apoptosis. The primary effects of CCl4 in humans are on the liver, kidneys, and central nervous system. Human symptoms of acute (short-term) inhalation and oral exposures to CCl4 include headache, weakness, lethargy, nausea, and vomiting. Acute exposures to higher levels and chronic (long-term) inhalation or oral exposure to CCl4 produce liver and kidney damage in humans[36]. The United States Environmental Protection Agency has set a maximum allowable level of 0.005 mg/L for CCl4[36]. There are no known short-term or immediate illness symptoms reported in humans due to exposure at these levels[36]. However, exposure to CCl4 at the low level for a longer time could result in pathologic changes in the liver of humans. More clinical and animal studies are needed to investigate the long-term consequences of minimal exposure.
We thank Dr. Steven A. Bigler (Pathologist, University of Mississippi Medical Center) for his help on the quantification of apoptotic cells in liver tissue, and Dr. Patrick Kyle for the measurement of ALT. We also thank Lan Chen for her help with editing the manuscript.
Carbon tetrachloride (CCl4) is a toxic agent that causes damages to liver, kidneys, and the central nervous system in humans. Many studies have investigated CCl4-induced acute liver failure and hepatic necrosis at lethal doses. Currently, the use of CCl4 has been limited in many countries because of its toxicity. The United States Environmental Protection Agency has set a maximum allowable level of 0.005 mg/L for CCl4. There are no known short-term or immediate illness symptoms reported in humans due to exposure at this level. However, animal studies have shown that CCl4 induces hepatic apoptosis at sublethal doses. The mechanism involving CCl4-induced hepatocyte apoptosis is not well understood.
Apoptosis is executed through the activation of caspase cascades via apoptotic pathways. The pathways start with activation of an initiator caspase by different stimuli, caspase-8 in membrane-mediated, caspase-9 in mitochondrial-mediated, and caspase-12 in endoplasmic reticulum (ER) stress-mediated pathways. The active initiator caspases then activate effector caspases-3, -6 or -7, which cleave key substrates required for normal cellular functions leading to apoptosis. The initial event in the liver of CCl4-treated animals is generation of reactive oxygen species within the ER. The ER is highly sensitive to environmental insults such as oxidative stress, which lead to ER stress. Procaspase-12 is predominantly located on the cytoplasmic side of the ER, and it is activated by ER stress. The direct role of caspase-12 and its downstream targets in CCl4-induced hepatocyte apoptosis have not been examined previously.
This is believed to be the first study to investigate the direct role of caspase-12 and its downstream targets in CCl4-induced hepatocyte apoptosis using caspase-12 knockout animals. The authors have demonstrated that caspase-12 plays a pivotal role in CCl4-induced hepatocyte apoptosis through the downstream activation of the effector caspase-3 directly and/or indirectly.
Understanding the mechanism of CCl4-induced hepatocyte apoptosis is important for the development of potential therapeutic strategies, such as the use of antioxidant, chemical ER chaperones and/or caspase-12 inhibitor for preventing/lessening CCl4-induced liver apoptosis and injury at the minimal exposure.
The ER is a major intracellular site for calcium storage and the production of secretory proteins through protein synthesis, folding and modification. Disturbance in any of the functions will disrupt proper protein folding and increase the load of unfolded or misfolded protein; the condition is called ER stress. The unfolded protein response (UPR) is a cellular response to ER stress. UPR has three aims: initially to restore normal cellular function by decreasing protein translation, degrading misfolded proteins, and activating the signaling pathways that lead to increasing the production of molecular chaperones involved in protein folding. If these objectives are not achieved or the disruption is prolonged, the UPR aims towards apoptosis.
The manuscript provides novel information. It is well written. The design, materials and methods are satisfactory. The results support the conclusions drawn.
P- Reviewer: Breitkopf-Heinlein K, Jeschke MG S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 879] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 2. | Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 4. | Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Bilodeau M. Liver cell death: update on apoptosis. Can J Gastroenterol. 2003;17:501-506. [PubMed] |
| 6. | Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 2010] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 7. | Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549-11556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1507] [Cited by in RCA: 1517] [Article Influence: 56.2] [Reference Citation Analysis (11)] |
| 8. | Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2547] [Cited by in RCA: 2617] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 9. | Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5101] [Article Influence: 182.2] [Reference Citation Analysis (12)] |
| 10. | Fraser A, Evan G. A license to kill. Cell. 1996;85:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 473] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Chinnaiyan AM, Dixit VM. The cell-death machine. Curr Biol. 1996;6:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 265] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287-34294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 707] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 13. | Zahedi K, Barone SL, Xu J, Steinbergs N, Schuster R, Lentsch AB, Amlal H, Wang J, Casero RA, Soleimani M. Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G546-G560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Schattenberg JM, Nagel M, Kim YO, Kohl T, Wörns MA, Zimmermann T, Schad A, Longerich T, Schuppan D, He YW. Increased hepatic fibrosis and JNK2-dependent liver injury in mice exhibiting hepatocyte-specific deletion of cFLIP. Am J Physiol Gastrointest Liver Physiol. 2012;303:G498-G506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Tien YC, Liao JC, Chiu CS, Huang TH, Huang CY, Chang WT, Peng WH. Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int J Mol Sci. 2011;12:4053-4067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 18. | Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836-21842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Zimmerman HJ. Hepatotoxicity: The adverse effects of drugs and other chemicals on the liver. New York: Appleton-Century-Crofts 1978; 349-369. |
| 20. | Recknagel RO, Glende EA, Hruszwcyz AM. Free Radicals in Biology: Chemical mechanisms in carbon tetrachloride toxicity. New York: Academic Press 1977; 97-132. [DOI] [Full Text] |
| 21. | Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102-S109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 456] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 22. | Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 1936] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 23. | Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol. 2005;16:1985-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Tamura T, Said S, Lu W, Neufeld D. Specificity of TUNEL method depends on duration of fixation. Biotech Histochem. 2000;75:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Brambilla E, Negoescu A. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J Histochem Cytochem. 1998;46:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 259] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994;71:219-225. [PubMed] |
| 27. | Wenger FA, Kilian M, Mautsch I, Jacobi CA, Steiert A, Peter FJ, Guski H, Schimke I, Müller JM. Influence of octreotide on liver metastasis and hepatic lipid peroxidation in BOP-induced pancreatic cancer in Syrian hamsters. Pancreas. 2001;23:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Bradd SJ, Dunn MJ. Analysis of membrane proteins by western blotting and enhanced chemiluminescence. Methods Mol Biol. 1993;19:211-218. [PubMed] |
| 29. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1171] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 30. | Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal. 2002;4:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Paschen W, Frandsen A. Endoplasmic reticulum dysfunction--a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem. 2001;79:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Häcki J, Egger L, Monney L, Conus S, Rossé T, Fellay I, Borner C. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene. 2000;19:2286-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 33. | Ito Y, Pandey P, Mishra N, Kumar S, Narula N, Kharbanda S, Saxena S, Kufe D. Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol. 2001;21:6233-6242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004;357:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 208] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Carbon tetrachloride (Update). Atlanta, GA: Public Health Service, U.S. Department of Health and Human Services 1994; . |