Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.16935
Revised: April 24, 2014
Accepted: May 26, 2014
Published online: December 7, 2014
Processing time: 284 Days and 17.6 Hours
It is currently difficult for conventional treatments of acute pancreatitis (AP), which primarily consist of anti-inflammatory therapies, to prevent the progression of AP or to improve its outcome. This may be because the occurrence and progression of AP, which involves various inflammatory cells and cytokines, includes a series of complex immune events. Considering the complex immune system alterations during the course of AP, it is necessary to monitor the indicators related to immune cells and inflammatory mediators and to develop more individualized interventions for AP patients using immunomodulatory therapy. This review discusses the recent advances in immunomodulatory therapies. It has been suggested that overactive inflammatory responses should be inhibited and excessive immunosuppression should be avoided in the early stages of AP. The optimal duration of anti-inflammatory therapy may be shorter than previously expected (< 24 h), and appropriate immunostimulatory therapies should be administered during the period from the 3rd d to the 14th d in the course of AP. A combination therapy of anti-inflammatory and immune-stimulating drugs would hopefully constitute an alternative to anti-inflammatory drug monotherapy. Additionally, the detection of the genotypes of critical inflammatory mediators may be useful for screening populations of AP patients at high risk of severe infections to enable the administration of early interventions to improve their prognosis.
Core tip: In light of the complex immune system alterations that occur in acute pancreatitis (AP), it is necessary to develop more individualized interventions for AP patients by using immunomodulatory therapy instead of inflammatory drug monotherapy. We first suggest how we could monitor the immune status of these patients and identify optimal treatment methods. We also demonstrate for the first time that the detection of the genotypes of critical inflammatory mediators may be useful for screening populations of AP patients at high risk of severe infections.
- Citation: Li J, Yang WJ, Huang LM, Tang CW. Immunomodulatory therapies for acute pancreatitis. World J Gastroenterol 2014; 20(45): 16935-16947
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/16935.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.16935
Acute pancreatitis (AP) is a common acute abdominal disease. Despite advances in treatment, the mortality rate of severe acute pancreatitis (SAP) remains as high as 10%-30%, and multiple organ dysfunction is the main cause of death[1-3].
Currently, conventional treatments for AP include fluid infusion, inhibition of pancreatic secretion and organ support. However, fluid resuscitation cannot prevent pancreatic necrosis[4,5]. In addition, the inhibition of pancreatic secretions has always been considered one of the most important strategies for treating AP. Research has shown that cell apoptosis or necrosis occurs in AP patients, that the zymogen granules of acinar cells decrease in number and that pancreatic exocrine functions are inhibited[6]. Under such conditions, the inhibition of exocrine pancreatic function cannot prevent the progression of AP or improve its outcomes[6,7]. Because AP consists of chemical inflammation, the prophylactic use of antibiotics cannot lower the incidence of infection in a necrotic pancreas or the mortality of AP[8-10]. Although organ support and symptomatic treatment may help patients survive multiple organ failure[11,12], there is still a lack of effective treatment for AP.
Until now, conventional treatments for AP have mainly been anti-inflammatory therapies. However, their effects have not proven to be as satisfactory as expected[4]. In fact, numerous studies have shown that a variety of inflammatory cells and cytokines are involved in the occurrence and progression of AP, which comprises a series of complex immune events[13]. A thorough understanding of the AP immune response and its mechanism can aid in the development of better AP treatment strategies.
The onset of AP generally includes the following immunological stages: systemic inflammatory response syndrome (SIRS), compensatory anti-inflammatory response syndrome (CARS) and mixed anti-inflammatory response syndrome (MARS)[4,14-16]. In light of the complex immune system alterations that occur during the different phases of AP, a more individualized approach to AP, such as the use of immunomodulatory therapy, may be beneficial.
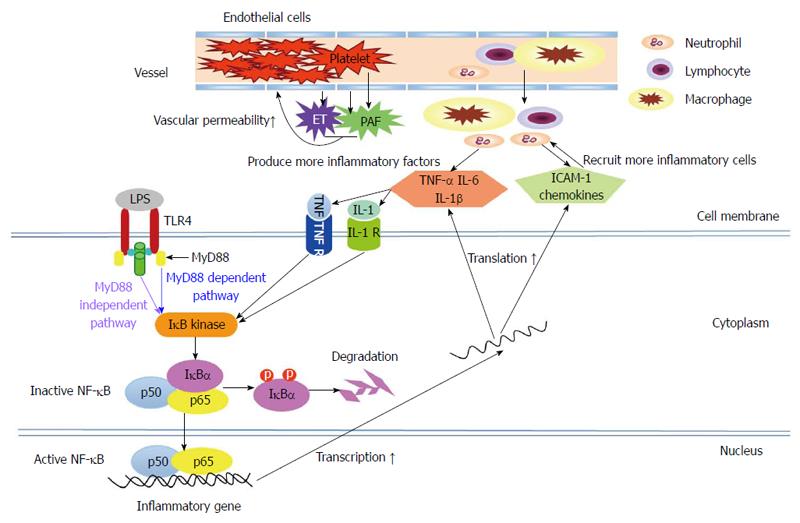
Following stimulation by various pathogenic factors, trypsin in the pancreatic acinar cells is prematurely activated due to neutrophil involvement[17]. This process is closely related to the following pathophysiological processes: high pancreatic duct pressure, the flow of Ca2+ into the pancreatic acinar cells and the activation of transcription factors, such as nuclear factor-κB (NF-κB)[6,12,18]. NF-κB is a core molecule of the innate immune response. The amplification of inflammatory signals generated by NF-κB is able to produce a large number of inflammatory factors, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and monocyte chemotactic protein (MCP)-1[19]. In the presence of these cytokines and chemokines, more neutrophils and lymphocytes gather in the pancreas and intestinal tracts, thus amplifying the inflammatory response[20]. When these pro-inflammatory cytokines are released into the blood, the inflammation is no longer confined to the pancreas, and, consequently, SIRS develops. The activated neutrophils and monocytes are then able to release proteases and oxygen free radicals. These inflammatory mediators can then cause damage to the vascular endothelial cells, increases in vascular endothelial permeability and the accumulation of a large amount of fluid in the tissue. As a result of these processes and other microvascular dysfunctions, tissue hypoxia and significant organ dysfunction develop[16].
In addition, intestinal ischemia-reperfusion during AP can significantly upregulate the expression of pattern recognition receptors, such as toll-like receptors (TLRs), in the intestinal mucosa, potentially leading to an overactive intestinal mucosal immune response and the rapid progression of AP[21]. Animal experiments have shown that TLR4-deficient mice rarely develop SAP[22]. Furthermore, TLR4 gene polymorphisms have been found to be associated with susceptibility to severe infections in AP patients[23].
With the release of pro-inflammatory cytokines, anti-inflammatory cytokines are produced in the body. The outcome of this disease depends on the balance between the inflammatory and anti-inflammatory responses. When the anti-inflammatory response is strong enough, patients may recover. On the contrary, when the anti-inflammatory response is not sufficiently strong, an excessive inflammatory response can lead to early organ dysfunction and SAP. During periods of overactive compensatory anti-inflammatory responses, the following changes may occur in AP: anergy in lymphocytes, thymus and spleen atrophy and a decrease in the number of peripheral lymphocytes (mainly the T lymphocytes), which could be associated with a depletion of lymphocytes or with lymphocyte apoptosis[13,24-27]. The abovementioned changes during immunosuppression are closely related to infection in the late stage of SAP[28].
In addition to lymphocyte-related defective defense systems in AP, mononuclear cell dysfunction may occur, which is characterized by a significant decrease in the expression of human leukocyte antigen (HLA-DR) and the synthesis of pro-inflammatory cytokines (e.g., TNF-α)[16]. The expression of low-density HLA-DR in mononuclear cells indicates an impaired antigen-presenting function. Mentula et al[29,30] reported that reduced expression of HLA-DR in monocytes in early SAP most likely predicted the occurrence of a secondary infection and fatal complications in AP. HLA-DR expression can be detected by flow cytometry within 30-60 min; therefore, it can be used as a routine test to identify the immune status of AP patients and to predict the outcomes of AP[13].
Furthermore, intestinal immune function can be impaired in early AP, manifesting as damage to the integrity of intestinal mucosal barriers, decreased levels of immune cells and the absence of secretory immunoglobulin A (sIgA) in the intestine. These events may in turn cause translocations of intestinal bacteria and endotoxins, as well as infectious complications[31].
SIRS, MARS and CARS are traditionally thought to appear successively in AP. MARS is regarded as a transient dynamic balance in the transition from SIRS to CARS. However, increasing evidence has revealed that there is no distinct boundary between SIRS and CARS and that CARS also occurs in the early stages of sepsis or SAP[14,28]. Therefore, MARS cannot be a transition period from SIRS to CARS; instead, it may constitute an independent reaction in tandem with an excessive inflammatory response and immune suppression in early SAP[4,14-16].
In AP, the overactivity of pro-inflammatory cells and factors may aggravate local inflammation in the pancreas, facilitating the development of SIRS. Anti-inflammatory factors will not work effectively when their levels are below those of the pro-inflammatory factors[32,33]. Theoretically, the modulation of immunocytes and inflammatory mediators may be able to prevent overactive immune reactions and to alleviate inflammatory injuries; this hypothesis has been gradually confirmed by accumulating animal experiments and clinical trials over the past 20 years. Blocking these inflammatory pathways (Figure 1), moderately and at the appropriate time, may be an effective treatment for AP. However, the inflammatory reaction involves numerous factors and cells. Thus, treatments targeting only certain factors or cells may be unable to terminate the entire overactive inflammatory reaction on their own.
NF-κB, a vital transcription factor, is activated early in the course of AP and regulates the transcription of many genes involved in inflammatory responses, such as TNF-α, IL-1β and IL-6[18] (Figure 1). It has been found that the inhibition of NF-κB activity with amobarbital, a NF-κB essential modifier binding domain peptide, or pyrrolidine dithiocarbamate (NF-κB activity inhibitor) attenuated AP-associated injuries in the pancreas and lungs. Currently, these results are only based on animal studies[34-36].
TNF-α is a monocyte-derived pro-inflammatory cytokine. In AP, TNF-α was found to contribute to pancreatic acinar cell death and to the expression of other pro-inflammatory factors, such as IL-1 and IL-6[37,38] (Figure 1). Animal studies have shown that TNF-α blockade with anti-TNF-α antibodies or soluble TNF-α receptors was able to decrease the severity of AP and reduce the associated mortality rate by prohibiting the effect of TNF-α[39-43]. In addition, several case reports have demonstrated that infliximab (monoclonal anti-TNF-α antibody) was effective in AP-complicated acute Kawasaki or active Crohn’s disease and had a preventive effect on AP in a patient with recurrent acute exacerbation of chronic pancreatitis[44,45].
Various pro-inflammatory interleukins, such as IL-1β, IL-6, IL-8 and IL-18, are involved in the inflammatory responses associated with AP[46-51]. Animal studies have suggested that the inhibition of pro-inflammatory interleukins with antibodies (e.g., anti-IL-6 receptor antibody or anti-IL-8 antibody), receptor antagonists (IL-1 receptor antagonist) or biosynthesis inhibitors (IL-1-converting enzyme inhibitor) ameliorated pancreatic and lung injuries in AP and reduced the associated mortality rate[46,52-56].
During the overactive inflammatory reactions of AP, the anti-inflammatory cytokine IL-10 is unable to inhibit the inflammatory reactions effectively, as its serum level is far below that of the pro-inflammatory factors, such as TNF-α[32,33]. In animal experiments associated with AP, exogenous IL-10 or supplementation with its effective fragment (IT9302), IL-10 gene transfer and insulin-like growth factor administration were each reported to result in the downregulation of serum TNF-α levels, the alleviation of pancreatic injury and a decrease in death rates by increasing the circulating levels of IL-10 or its effective fragment[57-63]. In clinical trials, there were conflicting results regarding the ability of IL-10 to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis[64-66]. Different inclusion criteria and follow-up times may be responsible for the controversy. Nevertheless, persistent elevation in circulating IL-10 levels has been found to be one of the causes of immunosuppression in some AP patients[67].
Although the effectiveness of anti-inflammatory therapies targeting IL has been confirmed by many animal experiments, only treatment with IL-10 has been investigated through clinical trials. Larger randomized clinical trials (RCTs) are required to assess the safety and efficacy of these therapies.
Platelet activating factor (PAF), a biologically active phospholipid that is released from macrophages, endothelial cells and platelets, has been shown to induce neutrophil-platelet aggregation and to increase vascular permeability[68] (Figure 1). Lexipafant, a PAF antagonist, is able to inhibit the inflammatory response and to ameliorate the severity of pancreatitis-associated intestinal and lung damage in experimental models of AP[69-73]. Several large-scale RCTs have also indicated that lexipafant led to significant decreases in mortality and the incidence of systemic complications of AP[74-76] (Table 1). The recommended administration of lexipafant is intravenous infusion at a dose of 100 mg/d for 7 d within 72 h after AP onset[74,75]. However, a phase III clinical trial indicated that lexipafant had no effect on new organ failure or death rates in AP[76].
| Ref. | Study design | Severity of AP | No. | Interval | Dosage and administration | Major variables | Outcomes |
| Kingsnorth et al[74], 1995 | Multi-center | Mix | 83 | < 48 h | 60 mg/d, i.v., × 3 d | Organ failure | Positive |
| double-blind | OFS | ||||||
| RCT | IL6, IL8 | ||||||
| McKay et al[75], 1997 | Multi-center | APACHEII > 5, | 50 | < 72 h | 100 mg/d, i.v., × 7 d | OFS | Positive |
| double-blind | Glasgowscore ≥ 3, and | Systemic complications | |||||
| RCT | C-reactive protein ≥ 120 mg/L | Mortality | |||||
| Johnson et al[76], 2001 | Multi-center double-blind RCT | APACHEII > 6 | 290 | < 72 h | 100 mg/d, i.v., × 7 d | Systemic sepsis Pseudocysts New organ failure. Mortality | Positive in systemic sepsis and pseudocysts |
Endothelins (ETs: including ET-1, ET-2 and ET-3) and their receptors (ETA and ETB) mediate the vasoconstriction response and maintain vascular tension[77]. It has been found that elevated plasma endothelin-1 levels are associated with low perfusion and pancreatic necrosis in AP[78,79]. Currently, it remains controversial whether ET receptor antagonists have beneficial effects on pancreatic perfusion and the mortality of AP[80-86]. However, studies have involved only animal experiments, and no relevant clinical studies have been completed to date.
Somatostatin (SST), a multifunctional neuropeptide, is mainly released from sensory nerve endings and gastrointestinal neuroendocrine cells. Accumulating evidence has suggested that SST causes a significant anti-inflammatory effect in AP, in addition to its potential roles in inhibiting exocrine pancreatic function and regulating the tone of the sphincter of Oddi[6]. Our previous animal experiments demonstrated that SST could relieve inflammatory injuries by blocking the TLR4-myeloid differentiation factor 88 (MyD88)-dependent and -independent pathways (Figure 1), as well as by inhibiting the activity of intestinal mucosal mast cells[21,87,88]. Over the past two years, our clinical studies have indicated that plasma SST levels decreased within 48 h after AP onset, along with increased plasma levels of IL-6 and TNF-α[89,90]. Thus, an early replacement of exogenous SST or its analogue, octreotide, may be beneficial for patients with AP. Our recent prospective RCTs have shown that octreotide administration attenuated SAP to some extent and prevented the development of SAP in obese patients and other patients with predicted SAP by raising their plasma SST levels and decreasing their circulating levels of TNF-α and IL-6[89-91]. However, the effect of SST or octreotide on AP remains controversial[92-100]. Different timings, doses or durations of octreotide administration may contribute to these disputed results (Table 2). More research is needed to fully understand the roles of SST and octreotide in AP and to identify the optimal treatment timing and dosage.
| Ref. | Study design | Severity of AP | No. | interval | Dosage and administration | Major variables | Outcomes |
| Wang et al[89], 2013 | Prospective RCT | P-SAP; SAP | P-SAP: 236 | < 48 h | 50 μg/h, continued i.v., × 3 d + 25 μg/h, continued i.v., × 4 d, or 25 μg/h, continued i.v., × 7 d | APACHE II, SIRS score, and MOF score Local complication | Positive at higher dosage |
| SAP: 136 | IL6, TNF-α | ||||||
| Yang et al[90], 2012 | Multi-center | MAP | 161 | < 48 h | 50 μg/h, continued i.v., × 3 d | APACHE II and MOF score | Positive |
| RCT | Local complication | ||||||
| IL6, TNF-α | |||||||
| Nikou et al[91], 2004 | Prospective RCT | MAP | 36 | < 12 h | 200 or 500 μg, i.h., 3 times/d × 5 d | IL-6, C-reactive protein | Positive IL-6 outcome at higher dosages |
| Beechey-Newman[93], 1993 | Prospective case-control study | MAP | 19 | N | 250 μg, i.h., then 0.5 μg/kg per hour, continued i.v., × 10 d | Biochemical and physiological parameters | Positive in serum calcium, albumin, hematocrit, hemoglobin, PaO2 |
| Binder et al[94], 1994 | Prospective trial | Mix | 8 | N | 100, 200 or 500 μg, i.h., 3 times/d × 10 d | Complications | Positive at two higher dosages |
| Paran et al[95], 1995 | Multi-center RCT | MAP | 38 | N | 100 μg, i.h., 3 times/d × 14 d | Organ dysfunction | Positive in organ dysfunction and length of hospital stay |
| Local complications | |||||||
| Length of hospital stay | |||||||
| Mortality | |||||||
| Fiedler et al[96], 1996 | Prospective | SAP | 39 | N | 100 μg, i.v., 3 times/d × 10 d | Organ dysfunction | Positive |
| case-control study | Mortality | ||||||
| McKay et al[97], 1997 | Multi-center RCT | Mix | 58 | N | 40 μg/h, continued, i.v., × 5 d | Complications | Negative |
| Mortality | |||||||
| Karakoyunlar et al[98], 1999 | Prospective controlled study | Mix | 43 | N | 250 μg/h, continued i.v., × 2 d | Biochemical, physiological and | Positive outcomes for serum amylase levels, pancreatic edema and earlier return to oral intake |
| radiological changes | |||||||
| Mortality | |||||||
| Paran et al[99], 2000 | Case-controlled study | Mix | 50 | N | 100 μg, i.h., 3 times/d, × 14 d | Organ dysfunction | Positive in organ dysfunction, length of hospital stay and mortality |
| Local complications | |||||||
| Length of hospital stay | |||||||
| Mortality | |||||||
| Nikou et al[100], 2001 | Prospective RCT | Mix | 120 | N | 100, 200 or 300 μg, i.h., | Duration of pain | Little benefit only at two higher dosages |
| 3 times/d, × 7 d | Organ dysfunction | ||||||
| Local complications |
In AP, immune cells (macrophages, monocytes, neutrophils and lymphocytes) migrate to the sites of inflammation and release pro-inflammatory cytokines and chemokines with the aid of chemokines and adhesion molecules, which recruit even more immune cells, aggravating the inflammation[13]. There is increasing evidence that inhibiting the activation and migration of immune cells may have a therapeutic effect on excessive inflammatory responses[20,101-103].
Macrophages are one of the major classes of immune cells involved in the pathogenesis of AP. They mainly present as M1 macrophages to release pro-inflammatory cytokines and to aggravate pancreatic and systemic inflammation[101,102]. In an in vitro study, IL-4 and IL-13 were able to convert M1 macrophages into M2 macrophages, which have an anti-inflammatory role. However, these cytokines failed to show similar results in vivo[101]. In addition, hemin and gadolinium chloride were able to attenuate the inflammation and AP-associated organ injuries in rats by inhibiting the pro-inflammatory effects of macrophages[102,103]. Although there is no related clinical trial, the results from the above animal studies may provide a new direction for the development of immunomodulatory therapy for AP[102,103].
Chemokines and their receptors contribute to the migration of leukocytes to areas of injury and the development of inflammation[104]. Chemokines can be broadly divided into CXC and CC subgroups. In the CXC subgroup, the first two cysteine residues (C) out of four are separated by another amino acid (X), whereas in the CC subgroup, the first two cysteine residues are adjacent[104,105]. Many animal experiments on AP have revealed that the blockage of chemokine synthesis (monocyte chemotactic protein-1 or fractalkine) or neutralization of chemokines with antibodies (anti-cytokine-induced neutrophil chemoattractant antibodies or anti-CC receptor 5 ligand antibodies) was able to relieve inflammatory reactions, increasing the survival rates[104-109]. In addition, the inhibition of combined chemokines (CXC or CC) and their receptors was also found to have a similar therapeutic effect on AP[110-112]. However, those results have not been confirmed by clinical trials.
Adhesion molecules are glycoprotein molecules that are located on the cell surface and are involved in binding to other cells or the extracellular matrix. Intercellular adhesion molecule-1 (ICAM-1) is one of the adhesion molecules expressed on endothelial cells; it mediates the adhesion and migration of immune cells, facilitates leukocyte infiltration and exacerbates systemic inflammatory reactions[3,83,113-118]. Studies using animal models of AP have shown that ICAM-1 antibodies attenuate inflammatory cell infiltration and pancreatic and lung injuries[83,114-116,118]. Unfortunately, there are no relevant clinical research data currently available.
In the late stages of AP, a decreased number of lymphocytes can lead to impaired cellular and humoral immune function, including the inability to release cytokines and reduced ratios of CD4+/CD8+ T cells and Th1/Th2 helper T cells[28,119]. These changes, together with monocyte dysfunction, will most likely render the host susceptible to infection and death due to pathogenic invasion. Therefore, immunostimulation targeting CD4+ cells, Th1 cells and monocytes may be effective for treating or preventing infectious complications of SAP. Monitoring immune cells and cytokines could be useful for predicting the prognosis of AP[119,120].
It has been found that a reduction in the circulating CD4+ T cell levels and the CD4+/CD8+ ratio may be partly responsible for the development of immunosuppression in SAP[121]. The application of thymosin alpha 1 showed a protective effect against SAP in rats, improving their survival rate by restoring serum CD4+ T cell levels and the CD4+/CD8+ ratio[122]. Moreover, an imbalance between Th1 and Th2 cells was also found to be associated with the pathogenesis of immunosuppression in SAP, which included deficiencies in the number and function of Th1 cells and a large number and hyperfunctionality of Th2 cells[123,124]. Many studies have revealed that the granulocyte-macrophage colony-stimulating factor (GM-CSF) and/or interferon-γ (IFN-γ) have been able to restore the balance between Th1 and Th2 to some extent, based on in vitro and in vivo experiments associated with AP[122,125,126]. However, clinical studies supporting these findings remain sparse.
Additionally, monocyte dysfunction may result in AP-associated immunosuppression, including impaired antigen presentation capacity (marked by reduced HLA-DR expression) and insufficient synthesis of pro-inflammatory cytokines[127]. The administration of IFN-γ or GM-CSF was reported to raise the HLA-DR expression levels on monocytes and to enhance their capacity for TNF-α production in septic patients[128,129]. The only related RCT in the past five years also showed that the subcutaneous injection of GM-CSF (4 mg/kg per day) for 8 d was safe and effective for restoring monocytic immunocompetence and shortening the course of sepsis-associated immunosuppression[130]. GM-CSF or IFN-γ administration in vitro was able to upregulate HLA-DR expression and the TNF-α production of monocytes from SAP patients[127]. It has been suggested that combination therapy of GM-CSF and IFN-γ is able to completely reverse monocyte dysfunction[127]. To date, GM-CSF and IFN-γ have been widely used in the treatment of sepsis. More research is needed before GM-CSF and IFN-γ can be used in patients with AP-associated immunosuppression.
Intestinal immunosuppression may occur in early SAP (within 24 h after AP onset) and is characterized by a significant decrease in sIgA secretion and in the number of CD4+ T lymphocytes in the intestinal mucosa, which is one of the most important causes for bacterial and endotoxin translocation[31]. Restoring intestinal immune function as early as possible may represent a promising treatment to prevent infectious complications in SAP. In animal experiments on SAP, oral supplementation with arginine, glutamine and probiotics increased the number of CD4+ T lymphocytes and sIgA levels in the intestine and circulation[31,131]. Furthermore, clinical trials have reached similar conclusions. Early enteral nutrition (within 48 h after admission) has been suggested to cause an increase in serum IgG levels and HLA-DR expression in T lymphocytes, thus reducing the incidence of multiple organ dysfunction syndrome, SIRS and pancreatic infection[132].
For patients with AP-associated immunosuppression, proper immunostimulation may alleviate the disease and prevent serious complications[130,132]. However, those results are mainly based on animal or in vitro studies. More large-scale RCTs are needed to evaluate the safety and efficacy of immunostimulatory therapies for AP-associated immunosuppression.
As mentioned above, different treatment regimens should be administered to AP patients according to their different immune statuses. However, it is currently unclear how the immune status of patients can be monitored to identify the optimal treatment timing. Genetic testing for gene polymorphisms in certain inflammatory mediators can be used to screen high-risk AP patients for severe infection, thus preventing severe infections[23,133,134].
Clinical trials have shown that peripheral blood lymphocyte levels are significantly reduced to approximately 67% of the lower limit of normal within 24-72 h after a SAP attack[28,135]. This reduction may be associated with the depletion of lymphocytes, lymphocyte apoptosis or gut associated lymphocyte homing[13,24-27,136]. During the same period, peripheral blood CD4+ T cell levels, the ratio of CD4+/CD8+ T cells and the ratio of Th1/Th2 helper T cells all significantly decreased[27,28,123,124,137]. Moreover, the antigen-presenting function of monocytes was also impaired (characterized by low HLA-DR expression) within 24-72 h after SAP onset[27,30,138]. It has been reported that a continuous decline of the above indicators from the 7th to the 14th d in the course of AP could predict a higher risk of infectious complications[27,28,30,123,124,138-140].
Many of the findings in the literature have revealed that the serum levels of pro-inflammatory cytokines (e.g., IL-8, TNF-α, IL-6, IL-2, IL-12 and IFN-γ)[67,123,124,137,138,141-143] and anti-inflammatory cytokines (e.g., IL-10 and IL-4)[67,123,124,138,141] were significantly elevated within 12-72 h after a SAP attack; thereafter, the shared pattern diverged. Retrospective studies in the past two years have suggested that the serum TNF-α, IL-6, IL-12 and IFN-γ levels gradually decreased in the 3rd-5th d after the onset of SAP[67,137]. In addition, the serum IL-10 and IL-4 levels of SAP patients with infections showed a continuous upward trend from the 3rd d to the 14th d after a SAP attack, whereas the anti-inflammatory cytokine levels of patients without infections declined continuously[67]. Despite certain shortcomings, serum cytokine levels remain one of the most important rapid indicators of a patient’s immune status[120].
Monitoring the levels of immune cells and cytokines could enable the early detection of the immune suppression state over the course of SAP, and their appropriate modulation could be expected to reduce the incidence of infection and death.
In addition, clinical research completed during the past year showed that the overexpression of the factor-associated suicide (Fas) ligand on T lymphocytes in SAP patients resulted in the excessive apoptosis of T lymphocytes and a sharp drop in CD4+ T cells, thus inducing immune suppression and sepsis[27]. Fas expression on T lymphocytes in SAP patients was transiently reduced within 48 h after AP onset but continuously increased within 8 d thereafter[124]. Therefore, for AP patients with continuously high expression of Fas for 10 d after AP onset, appropriate immune stimulation may improve the immune suppression state.
The interventional window for AP mentioned in the previous studies refers to the timing of anti-inflammatory therapy. Many researchers have found that this window usually arose between 12-18 h and 2-3 d after the onset of AP[16]. However, accumulated evidence has indicated that the timing for both anti-inflammatory and immune stimulation therapies should be considered. Thus, according to the above data, the duration of anti-inflammatory therapy may be shorter than expected (< 24 h), as immunosuppression can occur within 24 h after SAP onset[27,28,30,67,123,124,137-140], whereas appropriate immune stimulation therapy should be administered during the period between the 3rd and 14th d in the course of AP[27,28,30,67,123,124,137-140]. Nevertheless, the optimal interventional window of AP needs to be defined by further prospective studies.
Many factors are involved in the pathogenesis and progression of AP. The studies available on monotherapies for a certain factor did not yield the desired results. Thus, increasingly more investigators have begun to focus on multi-drug combination therapies for AP. Their studies have confirmed that a multi-drug combination is more effective than monotherapy. These therapies have comprised either the combination of various types of anti-inflammatory drugs[144,145] or a combined drug treatment for immunosuppression[127]. Currently, there are no published reports on the combination of anti-inflammatory and immune-stimulating therapies for AP, although this topic holds great promise as a new approach to AP immunotherapy.
Genetic polymorphisms of certain inflammatory mediators were found to be closely related to infections in AP patients. It has been reported that AP patients with TLR4 Asp299Gly mutations were prone to necrosis and infection of the pancreas[23] and that SAP patients carrying the IL-10-1082G[133], TNF2 and TNFB2 alleles[134] were highly susceptible to septic shock. Therefore, the detection of these genotypes could be helpful in screening high-risk AP populations for severe infections. These findings suggest that we may be able to develop more successful interventions for those patients in the future, potentially preventing more severe infections.
In summary, immunomodulatory therapy will hopefully improve the outcomes of AP. In the course of AP, it is necessary to monitor the indicators related to immune cells and inflammatory mediators and to develop more successful and individualized interventions for AP patients using immunomodulatory therapies. During the early stages of AP, treatment should include the inhibition of the overactive inflammatory responses while avoiding excessive immunosuppression to maintain normal immune function and to reduce the incidence of infectious complications and organ failure. Furthermore, the duration of anti-inflammatory therapies may be shorter than previously expected (< 24 h), whereas appropriate immune stimulation therapy should be administered between the 3rd and14th d of the AP disease course. The combined therapy of anti-inflammatory and immune-stimulating drugs may represent an alternative to anti-inflammatory drug monotherapies. Finally, the detection of the genotypes of critical inflammatory mediators would facilitate early interventions in AP populations at high-risk for severe infections to improve their prognosis.
| 1. | Pezzilli R, Ceciliato R, Barakat B, Corinaldesi R. Immune-manipulation of the inflammatory response in acute pancreatitis. What can be expected? JOP. 2004;5:115-121. [PubMed] |
| 2. | Spanier B, Bruno MJ, Dijkgraaf MG. Incidence and mortality of acute and chronic pancreatitis in the Netherlands: a nationwide record-linked cohort study for the years 1995-2005. World J Gastroenterol. 2013;19:3018-3026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 3. | Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (1)] |
| 4. | Mayerle J, Dummer A, Sendler M, Malla SR, van den Brandt C, Teller S, Aghdassi A, Nitsche C, Lerch MM. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology. 2002;2:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Li J, Wang R, Tang C. Somatostatin and octreotide on the treatment of acute pancreatitis - basic and clinical studies for three decades. Curr Pharm Des. 2011;17:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Pezzilli R, Miglioli M. Multicentre comparative study of two schedules of gabexate mesilate in the treatment of acute pancreatitis. Italian Acute Pancreatitis Study Group. Dig Liver Dis. 2001;33:49-57. [PubMed] |
| 8. | Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, Imrie CW, Johnson CD, Knaebel HP, Laterre PF. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Isenmann R, Rünzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, Malfertheiner P, Goebell H, Beger HG. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997-1004. [PubMed] |
| 10. | Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1428] [Article Influence: 109.8] [Reference Citation Analysis (3)] |
| 11. | Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 480] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 12. | Gao YJ, Li YQ, Wang Q, Li SL, Li GQ, Ma J, Zeng XZ, Huang LY, Yuan SA, Liu CA. Analysis of clinical features of acute pancreatitis in Shandong Province, China. J Gastroenterol Hepatol. 2007;22:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 14. | Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K, Holzmann B. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology. 2012;217:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Osuchowski MF, Craciun F, Weixelbaumer KM, Duffy ER, Remick DG. Sepsis chronically in MARS: systemic cytokine responses are always mixed regardless of the outcome, magnitude, or phase of sepsis. J Immunol. 2012;189:4648-4656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Kylänpää L, Rakonczay Z, O’Reilly DA. The clinical course of acute pancreatitis and the inflammatory mediators that drive it. Int J Inflam. 2012;2012:360685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988;3:105-112. [PubMed] |
| 18. | Rakonczay Z, Hegyi P, Takács T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259-267. [PubMed] |
| 19. | Pezzilli R, Billi P, Gullo L, Beltrandi E, Maldini M, Mancini R, Incorvaia L, Miglioli M. Behavior of serum soluble interleukin-2 receptor, soluble CD8 and soluble CD4 in the early phases of acute pancreatitis. Digestion. 1994;55:268-273. [PubMed] |
| 20. | Gea-Sorlí S, Closa D. In vitro, but not in vivo, reversibility of peritoneal macrophages activation during experimental acute pancreatitis. BMC Immunol. 2009;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Wu H, Liu L, Tan Q, Wang C, Guo M, Xie Y, Tang C. Somatostatin limits intestinal ischemia-reperfusion injury in macaques via suppression of TLR4-NF-kappaB cytokine pathway. J Gastrointest Surg. 2009;13:983-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Gao HK, Zhou ZG, Li Y, Chen YQ. Toll-like receptor 4 Asp299Gly polymorphism is associated with an increased risk of pancreatic necrotic infection in acute pancreatitis: a study in the Chinese population. Pancreas. 2007;34:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Chen HW, Hsu C, Lue SI, Yang RC. Attenuation of sepsis-induced apoptosis by heat shock pretreatment in rats. Cell Stress Chaperones. 2000;5:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496-501. [PubMed] |
| 26. | Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Qin Y, Pinhu L, You Y, Sooranna S, Huang Z, Zhou X, Yin Y, Song S. The role of Fas expression on the occurrence of immunosuppression in severe acute pancreatitis. Dig Dis Sci. 2013;58:3300-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Immunosuppression in patients with severe acute pancreatitis. J Gastroenterol. 2006;41:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Mentula P, Kylänpää-Bäck ML, Kemppainen E, Takala A, Jansson SE, Kautiainen H, Puolakkainen P, Haapiainen R, Repo H. Decreased HLA (human leucocyte antigen)-DR expression on peripheral blood monocytes predicts the development of organ failure in patients with acute pancreatitis. Clin Sci (Lond). 2003;105:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Mentula P, Kylänpää ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, Haapiainen R, Repo H. Plasma anti-inflammatory cytokines and monocyte human leucocyte antigen-DR expression in patients with acute pancreatitis. Scand J Gastroenterol. 2004;39:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Qiao SF, Lu TJ, Sun JB, Li F. Alterations of intestinal immune function and regulatory effects of L-arginine in experimental severe acute pancreatitis rats. World J Gastroenterol. 2005;11:6216-6218. [PubMed] |
| 32. | Ohmoto K, Yamamoto S. Serum interleukin-6 and interleukin-10 in patients with acute pancreatitis: clinical implications. Hepatogastroenterology. 2005;52:990-994. [PubMed] |
| 33. | Zhang XP, Chen HQ, Liu F, Zhang J. Advances in researches on the immune dysregulation and therapy of severe acute pancreatitis. J Zhejiang Univ Sci B. 2009;10:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Dunn JA, Li C, Ha T, Kao RL, Browder W. Therapeutic modification of nuclear factor kappa B binding activity and tumor necrosis factor-alpha gene expression during acute biliary pancreatitis. Am Surg. 1997;63:1036-1043; discussion discussion 1043-1044. [PubMed] |
| 35. | Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg. 2002;195:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Długosz JW, Andrzejewska A, Nowak K, Wróblewski E, Dabrowski A. The cumulative effect of nuclear factor-kappaB (NF-kappaB) inhibition and endothelins in early cerulein-induced acute pancreatitis in rats. Rocz Akad Med Bialymst. 2005;50:230-236. [PubMed] |
| 37. | Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. TNF-alpha as a therapeutic target in acute pancreatitis--lessons from experimental models. ScientificWorldJournal. 2007;7:431-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Guice KS, Oldham KT, Remick DG, Kunkel SL, Ward PA. Anti-tumor necrosis factor antibody augments edema formation in caerulein-induced acute pancreatitis. J Surg Res. 1991;51:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Oruc N, Ozutemiz AO, Yukselen V, Nart D, Celik HA, Yuce G, Batur Y. Infliximab: a new therapeutic agent in acute pancreatitis? Pancreas. 2004;28:e1-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Hughes CB, Gaber LW, Mohey el-Din AB, Grewal HP, Kotb M, Mann L, Gaber AO. Inhibition of TNF alpha improves survival in an experimental model of acute pancreatitis. Am Surg. 1996;62:8-13. [PubMed] |
| 41. | Hughes CB, Grewal HP, Gaber LW, Kotb M, El-din AB, Mann L, Gaber AO. Anti-TNFalpha therapy improves survival and ameliorates the pathophysiologic sequelae in acute pancreatitis in the rat. Am J Surg. 1996;171:274-280. [PubMed] |
| 42. | Aydin S, Isik AT, Unal B, Comert B, Ozyurt M, Deveci S, Ozgur G, Cengiz O, Tasci I, Mas MR. Effects of infliximab on bacterial translocation in experimental acute necrotizing pancreatitis. Indian J Med Res. 2012;135:656-661. [PubMed] |
| 43. | Norman JG, Fink GW, Messina J, Carter G, Franz MG. Timing of tumor necrosis factor antagonism is critical in determining outcome in murine lethal acute pancreatitis. Surgery. 1996;120:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Jimenez-Fernandez SG, Tremoulet AH. Infliximab treatment of pancreatitis complicating acute kawasaki disease. Pediatr Infect Dis J. 2012;31:1087-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Triantafillidis JK, Cheracakis P, Hereti IA, Argyros N, Karra E. Acute idiopathic pancreatitis complicating active Crohn’s disease: favorable response to infliximab treatment. Am J Gastroenterol. 2000;95:3334-3336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Norman J, Franz M, Messina J, Riker A, Fabri PJ, Rosemurgy AS, Gower WR. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Shimada M, Andoh A, Hata K, Tasaki K, Araki Y, Fujiyama Y, Bamba T. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;168:861-868. [PubMed] |
| 48. | Digalakis MK, Katsoulis IE, Biliri K, Themeli-Digalaki K. Serum profiles of C-reactive protein, interleukin-8, and tumor necrosis factor-alpha in patients with acute pancreatitis. HPB Surg. 2009;2009:878490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Endo S, Inoue Y, Fujino Y, Wakabayashi G, Inada K, Sato S. Interleukin 18 levels reflect the severity of acute pancreatitis. Res Commun Mol Pathol Pharmacol. 2001;110:285-291. [PubMed] |
| 50. | Pastor CM, Morel DR, Vonlaufen A, Schiffer E, Lescuyer P, Frossard JL. Delayed production of IL-18 in lungs and pancreas of rats with acute pancreatitis. Pancreatology. 2010;10:752-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Perejaslov A, Chooklin S, Bihalskyy I. Implication of interleukin 18 and intercellular adhesion molecule (ICAM)-1 in acute pancreatitis. Hepatogastroenterology. 2008;55:1806-1813. [PubMed] |
| 52. | Tanaka N, Murata A, Uda K, Toda H, Kato T, Hayashida H, Matsuura N, Mori T. Interleukin-1 receptor antagonist modifies the changes in vital organs induced by acute necrotizing pancreatitis in a rat experimental model. Crit Care Med. 1995;23:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Paszkowski AS, Rau B, Mayer JM, Möller P, Beger HG. Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann Surg. 2002;235:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Zhang XH, Zhu RM, Xu WA, Wan HJ, Lu H. Therapeutic effects of caspase-1 inhibitors on acute lung injury in experimental severe acute pancreatitis. World J Gastroenterol. 2007;13:623-627. [PubMed] |
| 55. | Chao KC, Chao KF, Chuang CC, Liu SH. Blockade of interleukin 6 accelerates acinar cell apoptosis and attenuates experimental acute pancreatitis in vivo. Br J Surg. 2006;93:332-338. [PubMed] |
| 56. | Osman MO, Kristensen JU, Jacobsen NO, Lausten SB, Deleuran B, Deleuran M, Gesser B, Matsushima K, Larsen CG, Jensen SL. A monoclonal anti-interleukin 8 antibody (WS-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits. Gut. 1998;43:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Keceli M, Kucuk C, Sozuer E, Kerek M, Ince O, Arar M. The effect of interleukin-10 on acute pancreatitis induced by cerulein in a rat experimental model. J Invest Surg. 2005;18:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 229] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 60. | Chen ZQ, Tang YQ, Zhang Y, Jiang ZH, Mao EQ, Zou WG, Lei RQ, Han TQ, Zhang SD. Adenoviral transfer of human interleukin-10 gene in lethal pancreatitis. World J Gastroenterol. 2004;10:3021-3025. [PubMed] |
| 61. | Warzecha Z, Dembinski A, Ceranowicz P, Konturek SJ, Tomaszewska R, Stachura J, Konturek PC. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J Physiol Pharmacol. 2003;54:575-590. [PubMed] |
| 62. | Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284-288; discussion 289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Osman MO, Jacobsen NO, Kristensen JU, Deleuran B, Gesser B, Larsen CG, Jensen SL. IT 9302, a synthetic interleukin-10 agonist, diminishes acute lung injury in rabbits with acute necrotizing pancreatitis. Surgery. 1998;124:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Devière J, Le Moine O, Van Laethem JL, Eisendrath P, Ghilain A, Severs N, Cohard M. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology. 2001;120:498-505. [PubMed] |
| 65. | Dumot JA, Conwell DL, Zuccaro G, Vargo JJ, Shay SS, Easley KA, Ponsky JL. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. Am J Gastroenterol. 2001;96:2098-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Sherman S, Cheng CL, Costamagna G, Binmoeller KF, Puespoek A, Aithal GP, Kozarek RA, Chen YK, Van Steenbergen W, Tenner S. Efficacy of recombinant human interleukin-10 in prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in subjects with increased risk. Pancreas. 2009;38:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Shen Y, Cui N, Miao B, Zhao E. Immune dysregulation in patients with severe acute pancreatitis. Inflammation. 2011;34:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Soybir G, Köksoy F, Ekiz F, Yalçin O, Fincan K, Haklar G, Yüksel M. The effects of free oxygen radical scavenger and platelet-activating factor antagonist agents in experimental acute pancreatitis. Pancreas. 1999;19:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Leveau P, Wang X, Sun Z, Börjesson A, Andersson E, Andersson R. Severity of pancreatitis-associated gut barrier dysfunction is reduced following treatment with the PAF inhibitor lexipafant. Biochem Pharmacol. 2005;69:1325-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Wang X, Sun Z, Börjesson A, Andersson R. Inhibition of platelet-activating factor, intercellular adhesion molecule 1 and platelet endothelial cell adhesion molecule 1 reduces experimental pancreatitis-associated gut endothelial barrier dysfunction. Br J Surg. 1999;86:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Liu Q, Djuricin G, Rossi H, Bewsey K, Nathan C, Gattuso P, Weinstein RA, Prinz RA. The effect of lexipafant on bacterial translocation in acute necrotizing pancreatitis in rats. Am Surg. 1999;65:611-616; discussion 617. [PubMed] |
| 72. | Wang X, Sun Z, Börjesson A, Haraldsen P, Aldman M, Deng X, Leveau P, Andersson R. Treatment with lexipafant ameliorates the severity of pancreatic microvascular endothelial barrier dysfunction in rats with acute hemorrhagic pancreatitis. Int J Pancreatol. 1999;25:45-52. [PubMed] |
| 73. | Galloway SW, Kingsnorth AN. Lung injury in the microembolic model of acute pancreatitis and amelioration by lexipafant (BB-882), a platelet-activating factor antagonist. Pancreas. 1996;13:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Kingsnorth AN, Galloway SW, Formela LJ. Randomized, double-blind phase II trial of Lexipafant, a platelet-activating factor antagonist, in human acute pancreatitis. Br J Surg. 1995;82:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | McKay CJ, Curran F, Sharples C, Baxter JN, Imrie CW. Prospective placebo-controlled randomized trial of lexipafant in predicted severe acute pancreatitis. Br J Surg. 1997;84:1239-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 76. | Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62-69. [PubMed] |
| 77. | Piechota A, Polańczyk A, Goraca A. Role of endothelin-1 receptor blockers on hemodynamic parameters and oxidative stress. Pharmacol Rep. 2010;62:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Chen CY, Lu CL, Chang FY, Lu RH, Ng WW, Lee SD. Endothelin-1 is a candidate mediating intestinal dysmotility in patients with acute pancreatitis. Dig Dis Sci. 1999;44:922-926. [PubMed] |
| 79. | Liu XH, Kimura T, Ishikawa H, Yamaguchi H, Furukawa M, Nakano I, Kinjoh M, Nawata H. Effect of endothelin-1 on the development of hemorrhagic pancreatitis in rats. Scand J Gastroenterol. 1995;30:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ, Foitzik T. Effect of endothelin and endothelin receptor blockade on capillary permeability in experimental pancreatitis. Gut. 2000;46:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Todd KE, Lewis MP, Gloor B, Lane JS, Ashley SW, Reber HA. An ETa/ETb endothelin antagonist ameliorates systemic inflammation in a murine model of acute hemorrhagic pancreatitis. Surgery. 1997;122:443-449; discussion 449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Foitzik T, Eibl G, Hotz HG, Faulhaber J, Kirchengast M, Buhr HJ. Endothelin receptor blockade in severe acute pancreatitis leads to systemic enhancement of microcirculation, stabilization of capillary permeability, and improved survival rates. Surgery. 2000;128:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Uhlmann D, Lauer H, Serr F, Ludwig S, Tannapfel A, Fiedler M, Hauss J, Witzigmann H. Pathophysiological role of platelets in acute experimental pancreatitis: influence of endothelin A receptor blockade. Cell Tissue Res. 2007;327:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Andrzejewska A, Dlugosz JW, Augustynowicz A. Effect of endothelin-1 receptor antagonists on histological and ultrastructural changes in the pancreas and trypsinogen activation in the early course of caerulein-induced acute pancreatitis in rats. World J Gastroenterol. 2005;11:1115-1121. [PubMed] |
| 85. | Długosz JW, Nowak K, Andrzejewska A, Wróblewski E, Dabrowski A. The effect of endothelin-1, endothelin-2 and endothelin-3 in early cerulein-induced acute pancreatitis in rats. Rocz Akad Med Bialymst. 2004;49:85-92. [PubMed] |
| 86. | Martignoni ME, Ceyhan GO, Ayuni E, Kondo Y, Zimmermann A, Büchler MW, Friess H. Endothelin receptor antagonists are not beneficial in the therapy of acute experimental pancreatitis. Langenbecks Arch Surg. 2004;389:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Zhou C, Li J, Wang H, Tang C. Decreased somatostatin is related to the hypersensitivity of intestinal epithelia to LPS via upregulated TLR4-TBK1 pathway in rats chronically exposed to ethanol. Alcohol. 2009;43:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Tang C, Lan C, Wang C, Liu R. Amelioration of the development of multiple organ dysfunction syndrome by somatostatin via suppression of intestinal mucosal mast cells. Shock. 2005;23:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Wang R, Yang F, Wu H, Wang Y, Huang Z, Hu B, Zhang M, Tang C. High-dose versus low-dose octreotide in the treatment of acute pancreatitis: a randomized controlled trial. Peptides. 2013;40:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Yang F, Wu H, Li Y, Li Z, Wang C, Yang J, Hu B, Huang Z, Ji R, Zhan X. Prevention of severe acute pancreatitis with octreotide in obese patients: a prospective multi-center randomized controlled trial. Pancreas. 2012;41:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Nikou GC, Giamarellos-Bourboulis EJ, Grecka P, Toumpanakis Ch, Giannikopoulos G, Katsilambros N. Effect of octreotide administration on serum interleukin-6 (IL-6) levels of patients with acute edematous pancreatitis. Hepatogastroenterology. 2004;51:599-602. [PubMed] |
| 92. | Cavallini G, Frulloni L. Somatostatin and octreotide in acute pancreatitis: the never-ending story. Dig Liver Dis. 2001;33:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Beechey-Newman N. Controlled trial of high-dose octreotide in treatment of acute pancreatitis. Evidence of improvement in disease severity. Dig Dis Sci. 1993;38:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Binder M, Uhl W, Friess H, Malfertheiner P, Büchler MW. Octreotide in the treatment of acute pancreatitis: results of a unicenter prospective trial with three different octreotide dosages. Digestion. 1994;55 Suppl 1:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 95. | Paran H, Neufeld D, Mayo A, Shwartz I, Singer P, Kaplan O, Skornik Y, Klausner J, Freund U. Preliminary report of a prospective randomized study of octreotide in the treatment of severe acute pancreatitis. J Am Coll Surg. 1995;181:121-124. [PubMed] |
| 96. | Fiedler F, Jauernig G, Keim V, Richter A, Bender HJ. Octreotide treatment in patients with necrotizing pancreatitis and pulmonary failure. Intensive Care Med. 1996;22:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | McKay C, Baxter J, Imrie C. A randomized, controlled trial of octreotide in the management of patients with acute pancreatitis. Int J Pancreatol. 1997;21:13-19. [PubMed] |
| 98. | Karakoyunlar O, Sivrel E, Tanir N, Deneçli AG. High dose octreotide in the management of acute pancreatitis. Hepatogastroenterology. 1999;46:1968-1972. [PubMed] |
| 99. | Paran H, Mayo A, Paran D, Neufeld D, Shwartz I, Zissin R, Singer P, Kaplan O, Skornik Y, Freund U. Octreotide treatment in patients with severe acute pancreatitis. Dig Dis Sci. 2000;45:2247-2251. [PubMed] |
| 100. | Nikou GC, Arnaoutis TP, Giamarellos-Bourboulis EJ, Samolada O, Vafiadis-Zouboulis I, Katsilambros N, Arvanitakis C. The significance of the dosage adjustment of octreotide in the treatment of acute pancreatitis of moderate severity. Hepatogastroenterology. 2001;48:1754-1757. [PubMed] |
| 101. | Gloor B, Blinman TA, Rigberg DA, Todd KE, Lane JS, Hines OJ, Reber HA. Kupffer cell blockade reduces hepatic and systemic cytokine levels and lung injury in hemorrhagic pancreatitis in rats. Pancreas. 2000;21:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Gea-Sorlí S, Closa D. Role of macrophages in the progression of acute pancreatitis. World J Gastrointest Pharmacol Ther. 2010;1:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 103. | Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, Slavin J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 105. | Moreno C, Nicaise C, Gustot T, Quertinmont E, Nagy N, Parmentier M, Louis H, Devière J. Chemokine receptor CCR5 deficiency exacerbates cerulein-induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1089-G1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1259-G1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Zhou GX, Zhu XJ, Ding XL, Zhang H, Chen JP, Qiang H, Zhang HF, Wei Q. Protective effects of MCP-1 inhibitor on a rat model of severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9:201-207. [PubMed] |
| 108. | Ishibashi T, Zhao H, Kawabe K, Oono T, Egashira K, Suzuki K, Nawata H, Takayanagi R, Ito T. Blocking of monocyte chemoattractant protein-1 (MCP-1) activity attenuates the severity of acute pancreatitis in rats. J Gastroenterol. 2008;43:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Huang L, Ma J, Tang Y, Chen P, Zhang S, Zhang Y, Yuan YZ. siRNA-based targeting of fractalkine overexpression suppresses inflammation development in a severe acute pancreatitis rat model. Int J Mol Med. 2012;30:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Bhatia M, Hegde A. Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul Pept. 2007;138:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Gerard C, Frossard JL, Bhatia M, Saluja A, Gerard NP, Lu B, Steer M. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest. 1997;100:2022-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 157] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | He M, Horuk R, Bhatia M. Treatment with BX471, a nonpeptide CCR1 antagonist, protects mice against acute pancreatitis-associated lung injury by modulating neutrophil recruitment. Pancreas. 2007;34:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4705] [Cited by in RCA: 4735] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 114. | Lundberg AH, Fukatsu K, Gaber L, Callicutt S, Kotb M, Wilcox H, Kudsk K, Gaber AO. Blocking pulmonary ICAM-1 expression ameliorates lung injury in established diet-induced pancreatitis. Ann Surg. 2001;233:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 115. | Werner J, Z’graggen K, Fernández-del Castillo C, Lewandrowski KB, Compton CC, Warshaw AL. Specific therapy for local and systemic complications of acute pancreatitis with monoclonal antibodies against ICAM-1. Ann Surg. 1999;229:834-840; discussion 841-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Rau B, Bauer A, Wang A, Gansauge F, Weidenbach H, Nevalainen T, Poch B, Beger HG, Nussler AK. Modulation of endogenous nitric oxide synthase in experimental acute pancreatitis: role of anti-ICAM-1 and oxygen free radical scavengers. Ann Surg. 2001;233:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Sun W, Watanabe Y, Wang ZQ. Expression and significance of ICAM-1 and its counter receptors LFA-1 and Mac-1 in experimental acute pancreatitis of rats. World J Gastroenterol. 2006;12:5005-5009. [PubMed] |
| 118. | Rau B, Paszkowski A, Esber S, Gansauge F, Poch B, Beger HG, Möller P. Anti-ICAM-1 antibody modulates late onset of acinar cell apoptosis and early necrosis in taurocholate-induced experimental acute pancreatitis. Pancreas. 2001;23:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Oiva J, Mustonen H, Kylänpää ML, Kyhälä L, Kuuliala K, Siitonen S, Kemppainen E, Puolakkainen P, Repo H. Acute pancreatitis with organ dysfunction associates with abnormal blood lymphocyte signaling: controlled laboratory study. Crit Care. 2010;14:R207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 120. | Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967-1974. [PubMed] |
| 121. | Tang WF, Wang YG, Zhu L, Wan MH, Chen GY, Xia Q, Ren P, Huang X. Effect of somatostatin on immune inflammatory response in patients with severe acute pancreatitis. J Dig Dis. 2007;8:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 122. | Yao W, Zhu Q, Yuan Y, Qiao M, Zhang Y, Zhai Z. Thymosin alpha 1 improves severe acute pancreatitis in rats via regulation of peripheral T cell number and cytokine serum level. J Gastroenterol Hepatol. 2007;22:1866-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Li JP, Yang J, Huang JR, Jiang DL, Zhang F, Liu MF, Qiang Y, Gu YL. Immunosuppression and the infection in patients with early SAP. Front Biosci (Landmark Ed). 2013;18:892-900. [PubMed] |
| 124. | Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, Dabrowski A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol. 2006;12:5344-5351. [PubMed] |
| 125. | Murphey ED, Herndon DN, Sherwood ER. Gamma interferon does not enhance clearance of Pseudomonas aeruginosa but does amplify a proinflammatory response in a murine model of postseptic immunosuppression. Infect Immun. 2004;72:6892-6901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 126. | Flohé SB, Agrawal H, Flohé S, Rani M, Bangen JM, Schade FU. Diversity of interferon gamma and granulocyte-macrophage colony-stimulating factor in restoring immune dysfunction of dendritic cells and macrophages during polymicrobial sepsis. Mol Med. 2008;14:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Kylanpaa ML, Mentula P, Kemppainen E, Puolakkainen P, Aittomaki S, Silvennoinen O, Haapiainen R, Repo H. Monocyte anergy is present in patients with severe acute pancreatitis and is significantly alleviated by granulocyte-macrophage colony-stimulating factor and interferon-gamma in vitro. Pancreas. 2005;31:23-27. [PubMed] |
| 128. | Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678-681. [PubMed] |
| 129. | Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R, Schneider CG, Pothmann W, Brassel AK, Schulte Am Esch J. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003;29:646-651. [PubMed] |
| 130. | Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 131. | Zou XP, Chen M, Wei W, Cao J, Chen L, Tian M. Effects of enteral immunonutrition on the maintenance of gut barrier function and immune function in pigs with severe acute pancreatitis. JPEN J Parenter Enteral Nutr. 2010;34:554-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Sun JK, Mu XW, Li WQ, Tong ZH, Li J, Zheng SY. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol. 2013;19:917-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 133. | Zhang DL, Zheng HM, Yu BJ, Jiang ZW, Li JS. Association of polymorphisms of IL and CD14 genes with acute severe pancreatitis and septic shock. World J Gastroenterol. 2005;11:4409-4413. [PubMed] |
| 134. | Zhang D, Li J, Jiang ZW, Yu B, Tang X. Association of two polymorphisms of tumor necrosis factor gene with acute severe pancreatitis. J Surg Res. 2003;112:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, Matsumoto I, Ajiki T, Fujino Y, Kuroda Y. Lactate dehydrogenase-to-lymphocyte ratio for the prediction of infection in acute necrotizing pancreatitis. Pancreas. 2007;35:378-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 136. | Wittel UA, Rau B, Gansauge F, Gansauge S, Nussler AK, Beger HG, Poch B. Influence of PMN leukocyte-mediated pancreatic damage on the systemic immune response in severe acute pancreatitis in rats. Dig Dis Sci. 2004;49:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 137. | Uehara S, Gothoh K, Handa H, Tomita H, Tomita Y. Immune function in patients with acute pancreatitis. J Gastroenterol Hepatol. 2003;18:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 138. | Ho YP, Chiu CT, Sheen IS, Tseng SC, Lai PC, Ho SY, Chen WT, Lin TN, Lin CY. Tumor necrosis factor-α and interleukin-10 contribute to immunoparalysis in patients with acute pancreatitis. Hum Immunol. 2011;72:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 139. | Richter A, Nebe T, Wendl K, Schuster K, Klaebisch G, Quintel M, Lorenz D, Post S, Trede M. HLA-DR expression in acute pancreatitis. Eur J Surg. 1999;165:947-951. [PubMed] |
| 140. | Satoh A, Miura T, Satoh K, Masamune A, Yamagiwa T, Sakai Y, Shibuya K, Takeda K, Kaku M, Shimosegawa T. Human leukocyte antigen-DR expression on peripheral monocytes as a predictive marker of sepsis during acute pancreatitis. Pancreas. 2002;25:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 142. | Rau B, Steinbach G, Gansauge F, Mayer JM, Grünert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 234] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 143. | Riché FC, Cholley BP, Laisné MJ, Vicaut E, Panis YH, Lajeunie EJ, Boudiaf M, Valleur PD. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003;133:257-262. [PubMed] |
| 144. | Werner J, Hartwig W, Hackert T, Kaiser H, Schmidt J, Gebhard MM, Büchler MW, Klar E. Multidrug strategies are effective in the treatment of severe experimental pancreatitis. Surgery. 2012;151:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 145. | Pereda J, Sabater L, Cassinello N, Gómez-Cambronero L, Closa D, Folch-Puy E, Aparisi L, Calvete J, Cerdá M, Lledó S. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg. 2004;240:108-116. [PubMed] |
P- Reviewer: Capasso R, Ciccocioppo R, Valle Tovo C S- Editor: Nan J L- Editor: Wang TQ E- Editor: Wang CH