Published online Nov 28, 2014. doi: 10.3748/wjg.v20.i44.16409
Revised: August 27, 2014
Accepted: October 14, 2014
Published online: November 28, 2014
Processing time: 202 Days and 5.6 Hours
Chronic hepatitis C virus (HCV) infection is the leading cause of death from liver disease and the leading indication for liver transplantation (LT) in the United States and Western Europe. LT represents the best therapeutic alternative for patients with advanced chronic liver disease caused by HCV or those who develop hepatocarcinoma. Reinfection by HCV of the graft is universal and occurs in 95% of transplant patients. This reinfection can compromise graft function and patient survival. In a few cases, the histological recurrence is minimal and non-progressive; however, in most patients it follows a more rapid course than in immunocompetent persons, and frequently evolves into cirrhosis with graft loss. In fact, the five-year and ten-year survival of patients transplanted because of HCV are 75% and 68%, respectively, compared with 85% and 78% in patients transplanted for other reasons. There is also a pattern of recurrence that is very severe, but rare (< 10%), called fibrosing cholestatic hepatitis, which often involves rapid graft loss. Patients who present a negative HCV viremia after antiviral treatment have better survival. Many studies published over recent years have shown that antiviral treatment of post-transplant HCV hepatitis carried out during the late phase is the best option for improving the prognosis of these patients. Until 2011, PEGylated interferon plus ribavirin was the standard of care, resulting in a sustained virological response in around 30% of recipients. The addition of protease inhibitors, such as boceprevir or telaprevir, to the standard of care, or the use of other direct-acting antiviral drugs may involve therapeutic changes in the context of HCV recurrence. This may result a better prognosis for these patients, particularly those with severe recurrence or factors predicting rapid progression of fibrosis. However, the use of these agents in LT still requires clarification in terms of safety and efficacy.
Core tip: Chronic hepatitis C virus (HCV) infection is the reason for about 50% of liver transplants in the western world. Reinfection of the graft is universal and can compromise graft function and patient survival. The development of an efficient antiviral therapeutic strategy has been the focus of clinical research in recent years, including when, how much and at what point this treatment should be applied. The introduction of new drugs for the treatment of chronic HCV hepatitis may involve therapeutic changes and, perhaps, a better prognosis for these patients, particularly those with severe recurrence or factors predicting rapid progression of fibrosis.
- Citation: Jiménez-Pérez M, González-Grande R, Rando-Muñoz FJ. Management of recurrent hepatitis C virus after liver transplantation. World J Gastroenterol 2014; 20(44): 16409-16417
- URL: https://www.wjgnet.com/1007-9327/full/v20/i44/16409.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i44.16409
Chronic hepatitis C virus (HCV) infection is the leading cause of death from liver disease and the leading indication for liver transplantation (LT) in the United States and Western Europe[1,2]. LT represents the best therapeutic alternative for patients with advanced chronic liver disease because of HCV or those who develop hepatocarcinoma.
HCV reinfection of the graft is universal and occurs in 95% of transplant patients. This reinfection can compromise graft function and patient survival. In a few cases, the histological recurrence is minimal and non-progressive; however, in most patients, it follows a more rapid course than in immunocompetent persons, and frequently evolves to cirrhosis with graft loss. The five-year and ten-year survival of patients transplanted because of HCV is 75% and 68%, respectively, compared with 85% and 78% in patients transplanted for other reasons[3]. There is also a pattern of recurrence that is very severe, but rare (< 10%), called fibrosing cholestatic hepatitis, which often involves rapid graft loss[4]. Those patients who present a negative HCV viremia after antiviral treatment have better survival[5].
Many studies published over recent years have shown that antiviral treatment of post-transplant HCV hepatitis carried out during the late phase is the best option for improving the prognosis of these patients. Until 2011, PEGylated interferon plus ribavirin was the standard of care, resulting in a sustained virological response (SVR) in around 30% of recipients[6].
The addition of protease inhibitors (PI), such as boceprevir or telaprevir, to the standard of care or the use of other direct-acting antiviral (DAA) drugs may involve therapeutic changes in the context of HCV recurrence. This may result in a better prognosis for these patients, particularly those with severe recurrence or factors predicting rapid progression of fibrosis[7]. However, the use of these agents in LT still requires clarification in terms of safety and efficacy.
Viral infection recurs in almost all cases and occurs immediately after the graft reperfusion phase. The diagnosis of viral recurrence is purely virological and is established by detection in serum of HCV RNA using polymerase chain reaction (PCR) techniques. The levels of viremia are generally far higher than those existing before the transplant[8]. However, the diagnosis of relapse of hepatitis or disease in the graft is based on histological findings.
Pathophysiologically, two patterns of recurrence can be distinguished: (1) a pattern of chronic HCV hepatitis similar to that seen in non-transplanted patients, but with a faster course, reaching states of advanced fibrosis or cirrhosis in a shorter time (9-12 years vs 20-50 years); and (2) fibrosing cholestatic hepatitis, which is less common (3%-5%) but very severe, and generally appears in the context of intense immunosuppression. It can present as an initial manifestation of disease relapse or, less commonly, in the context of recurrent chronic hepatitis. Fibrosing cholestatic hepatitis is characterized by marked jaundice with cholestasis and high titers of viremia. This form usually progresses rapidly to acute liver failure, with graft loss soon after.
Histological confirmation is necessary to establish the diagnosis of HCV recurrence, as well as enabling assessment of the degree of activity and a periodic follow-up of histological disease progression. This not only provides information about the prognosis, but also establishes the differential diagnosis with other complications, such as rejection, biliary disease or vascular problems[4,9-11].
A new non-invasive technique, hepatic elastography, has become available recently, which appears to correlate well with the stage of fibrosis. This technique can detect an important degree of fibrosis (F ≥ 2) from the sixth month after transplantation, and has an excellent diagnostic capacity at 12 mo post-transplantation[12].
The histological involvement of the graft and the natural history of recurrence both vary, with different presenting forms. Post-transplant reinfection with HCV is associated with greater aggressiveness than in immunocompetent patients[13,14].
At around the fifth month after transplantation, acute hepatitis occurs, which is generally asymptomatic in 50% of patients. Histologically it presents characteristics of lobular hepatitis with varying degrees of inflammatory infiltrate in the portal space, mainly of lymphocytes and macrovesicular steatosis, similar to the histological pattern found in acute hepatitis in immunocompetent patients.
Of those patients who experience relapse of their HCV infection after LT, 20% have histological lesions compatible with mild chronic hepatitis 5 years post-transplantation. The others experience a more important chronic evolution. The progression to hepatic cirrhosis occurs in 30% of these patients after 5 to 7 years post-transplant, and is much faster than in immunocompetent persons[15].
The progression of fibrosis is much more accelerated in those patients who receive their transplants because of HCV infection and who have a recurrence of the disease: up to five times faster than in immunocompetent persons. Accordingly, the cirrhosis evolves earlier, with an average of 10 years compared with 20-30 years for immunocompetent persons with chronic HCV infection[15,16].
Once cirrhosis is reached, 40%-50% of transplanted patients will experience their first decompensation within one year. Survival after this first episode of decompensation is 50%[14,16].
The course of post-transplant hepatitis C is determined by the interaction of different factors that affect the severity and timing of HCV recurrence.
Pre-transplant factors - donor and host related: Certain pre-transplant factors in the recipient are associated with worse evolution, including female sex, older age, and the presence of diabetes or metabolic syndrome[17-21]. HCV has a reciprocal relation with insulin resistance, in both transplanted and non-transplanted persons: HCV predisposes to insulin resistance, but insulin resistance itself contributes to increasing the morbidity and mortality associated with HCV infection. Other pre-transplant factors depend on the virus; for example, the genotypes HCV 1b and 4, which are factors predicting a poor response to standard antiviral therapy, or a high pre-transplant viral load (especially above 1 million IU/mL)[22]. The absence of response to antiviral therapy and coinfection with HIV are associated with a worse prognosis[23].
Other factors related with the donor and the peri-operative period can also affect the severity and the time to relapse of post-transplant HCV infection, such as an older donor age (> 50 years), a high degree of steatosis in the donor liver, a prolonged ischemia time, a non-heart beating donor, a living donor, preservation lesion, a partial split graft or anti-HCV positive donors, all of which have been associated with a worse evolution[24-27].
Recent studies appear to show that polymorphisms in the interleukin 28 B gene (IL-28B), in both the donor and the recipient, may influence not only the response to antiviral therapy, but also the evolution of hepatitis C from post-transplant HCV reinfection, with a worse evolution in those with the genotypes CT and TT (of the polymorphism rs12979860) compared with the genotype CC[28,29].
Post-transplant metabolic syndrome: Patients who received LT because of HCV who develop metabolic syndrome (50% during the first year) present a greater risk for fibrosis if they experience a recurrence of their HCV in the graft. This is why it is necessary to start preventive measures, as well as maintain a strict control of the post-transplant metabolic complications, particularly diabetes[18,30,31].
Immunosuppression: Immunosuppression is one of the factors that can cause recurrence of HCV, although no direct relation has been found with any particular therapeutic regimen[11]. Immunosuppression is associated with greater replication of HCV, especially during the early post-transplant period. It also results in a reduced activation of the immune cellular system, vital for the defense against the virus, and a weakened response in cases of severe recurrence, as in fibrosing cholestatic hepatitis[32,33].
Steroids: Steroid administration, particularly high doses in the form of a bolus to control severe rejection, is associated with greater severity of HCV recurrence[11,34]. This explains why some authors defend the use of steroid-free regimens, which also reduces the incidence of metabolic complications, especially hyperglycemia[35,36]. However, most transplant groups still use steroids in recipients who have HCV, although with optimized doses. Rather than a rapid taper, a slow taper is preferred in general practice[34].
Calcineurin inhibitors: The course of post-transplant HCV is not related to the type of calcineurin inhibitor given. Data are contradictory, and though no concrete recommendation has yet been established, a valid option is to begin tacrolimus immediately after transplantation, converting to cyclosporine if treatment for HCV is required[37-39].
Other immunosuppressive agents: No evidence-based recommendations exist concerning the influence of other immunosuppressive drugs, such as azathioprine, mycophenolate or mTOR inhibitors.
In conclusion, the principal aim is to optimize the treatment, avoiding over-immunosuppression[34]. On the other hand, in the era of the new direct acting antiviral (DAA) drugs against HCV, the choice of immunosuppressive agent will be affected not only by these considerations, but also by potential drug interactions.
The main goal of antiviral treatment is the permanent eradication of HCV and the achievement of a SVR. Additionally, antiviral therapy can also provide stabilization of disease progression and prevention of graft loss even in the absence of a virological response[40].
The treatment of HCV recurrence is similar to that for non-transplanted patients, including the use of new antiviral agents. However, there is no overall agreement on patient selection or the treatment regimen, though it may be similar to that used in immunocompetent persons[41].
Up to 50% of patients require treatment modification, with 25% even requiring withdrawal, as a result of side effects, mainly a marked reduction in hemoglobin (60%-80%), alterations in mood (10%) and asthenia (60%-70%). Acute rejection occurs in around 6% of treated patients, triggered mainly by PEGylated interferon. Less than 1% experience chronic rejection, which occurs most commonly in recipients who have a better response to antiviral treatment. In this situation, the rejection has been attributed to improved hepatic function with the resulting change in metabolism of the immunosuppressive drugs, which could determine a reduction in their blood levels[41,42]. Accordingly, close vigilance and monitoring of the immunosuppression are necessary during treatment, as well as a histological study in the event of unexplained laboratory findings.
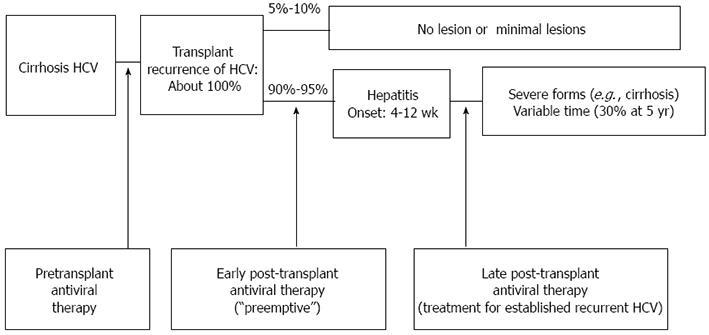
Overall, different points of therapeutic intervention have been used to attempt to prevent or eradicate HCV infection in liver transplant patients (Figure 1).
The aim of pre-transplant antiviral therapy is to inhibit viral replication before transplantation, and lower HCV viremia and its recurrence after LT. Nevertheless, many patients with HCV on the transplant waiting list have advanced disease precludes them from antiviral treatment. Thus, this treatment is only indicated in 50% of cases, with just 40% of these able to have the optimal dose and duration, which is associated with lower rates of viral response. Nonetheless, this treatment results in 30% of recipients reaching the transplantation process with no detectable viral load, a state that minimizes the risk of recurrence, although it does not completely prevent the reappearance of the virus in the graft[43]. Accordingly, before transplantation, all patients should be treated if they have no liver decompensation, are in Child-Pugh A and have a MELD < 18, provided there is no contraindication[44,45]. This poor tolerance to treatment in cirrhotic patients has led to the concept of the low accelerating dose regimen (LADR), which contemplates the introduction of antiviral treatment at minimal doses, increasing the dose every 2 wk depending on tolerance, attempting to reach the full dose[44].
Two treatment strategies can be adopted once the patient has received the transplant.
Pre-emptive therapy: The aim of this strategy is to eliminate HCV before the appearance of hepatic lesions. The potential advantage of treating recipients at an early stage, usually with from the first month, is the absence of severe graft involvement or fibrosis. However, during this stage, the patients are still recovering from the surgery, are receiving multiple drugs and high doses of immunosuppressors, and have a greater risk of rejection, so that postponing antiviral therapy is recommended[46-49]. Although this treatment is effective in 1%-13% of cases, 35% of the patients who have this option require drug withdrawal because of intolerance or side effects[49]. Recipients with a history of an aggressive infection or who are coinfected may be candidates for early treatment, provided the presence of rejection is excluded.
Treatment delayed until after the recurrence of the HCV: The most widely used strategy involves initiating antiviral therapy once the histological consequences of HCV recurrence are detected by a histopathological study of the graft. In this later state the recipient receives fewer immunosuppressive agents and usually has a better clinical and analytical status, which permits antiviral treatment to be optimized and is efficient in 20%-40% of cases[5,42,50,51]. Even so, 28% of recipients require early withdrawal of the treatment and 73% require the dose of the antivirals to be minimized. This reduced exposure to the treatment, together with a greater viral replication and unfavorable genotypes, explain the reduced treatment response compared with non-transplanted patients[6]. Thus, treatment strategies should be individualized, considering patient comorbidity (renal failure, hyperglycemia), graft function or a history of rejection, and the characteristics of the HCV[52].
Factors predicting antiviral response in a patient who undergoes LT because of HCV infection are similar to those seen in immunocompetent patient. Factors associated with a worse response include advanced donor age, advanced fibrosis, the presence of genotype 1, a high initial viral load and the presence of metabolic syndrome. Obtaining a rapid viral response (4 wk after starting antiviral therapy) and an early viral response at 12 wk of treatment predict a sustained viral response, as seen with HCV treatment in non-transplant patients[53,54].
Polymorphisms in interleukin (IL-28B) related with response to antiviral therapy in immunocompetent patients[55] are also related to response in transplant patients, with similar results. The CC genotype is associated with higher SVR rates[56]. Interestingly, a donor with the CC genotype may partially restore sensitivity to treatment in an unfavorable IL28B genotype recipient. This could explain the lack of association between pre- and post-transplant treatment outcome[57]. Based on these findings, a lack of response to antiviral treatment before a transplant should not prevent an attempt to re-treat HCV in the same patient, particularly if the donor genotype is different to that of the recipient[28].
Another important factor associated with a greater sustained viral response concerns treatment adherence; at least 80% compliance should be aimed for. The role of baseline immunosuppression on viral response is still under debate. The only prospective, randomized study reported to date did not find significant differences in SVR in patients treated with cyclosporine vs those treated with tacrolimus[58].
The PHOENIX study observed an SVR in 22% of patients treated with an early regimen as opposed to 21% of patients who started treatment after confirmation of the recurrence in the graft, with the former experiencing a higher incidence of adverse reactions and treatment withdrawal[59].
On the other hand, the role of maintenance therapy in virological non-responders has not been adequately assessed, especially in those who achieve clinical or histological benefit on hemodynamic, histological or elastography study, or in patients who normalize transaminases during treatment[60].
In the United States, 30% of all liver retransplants occur because of recurrence of HCV[1]. The International Liver Transplantation Society Expert Panel indicated that the age of the recipients and the donors, a bilirubin ≥ 10 mg/dL, renal dysfunction and early recurrence of HCV-related cirrhosis after transplant are all associated with a worse prognosis after retransplantation[61]. The development of fibrosing cholestatic hepatitis also has an unfavorable prognosis after retransplantation[62].
Models predicting survival after retransplantation have been validated. These include the Markmann score[63] and the Rosen score[64], which are the most accepted and enable prediction of prognosis in the retransplant patient, thus improving associated survival. Generally speaking, a retransplant is indicated in recipients with an estimated 1-year survival of at least 55%, which includes patients with a Rosen score < 20.5[62].
Although retransplantation is generally associated with worse survival, it is not clear whether HCV-positive patients have significantly worse results. Whatever the case, it is important to personalize each case and just select those patients with favorable clinical characteristics.
The advent of new drugs for the treatment of HCV infection, as well as polymerase and protease inhibitors, will considerably change the management of HCV infection because of their high antiviral power[65,66]. Around 50% of non-transplant patients who are difficult to treat because of the presence of factors predicting a lack of response experience a greater sustained viral response[52]. However, little information is available about the use of these drugs in liver transplant patients.
The DAAs telaprevir and boceprevir, approved in 2011 for the treatment of genotype 1 HCV, increase the SVR in both naive and previously treated patients[67]. However, their use in cirrhotic patients is limited, and they have not been approved for patients with decompensated cirrhosis, a situation common to many patients on waiting lists. These patients can only receive these DAAs as off-label therapy in selected cases and with great caution given the limited information available. The concentration of boceprevir in patients with advanced cirrhosis (Child-Pugh C) is between 45% and 62%[68] higher, whereas the concentration of telaprevir may be reduced by 46% in patients in Child-Pugh B[69]; no recommendations currently existing for adjusting the dose in these cases. The lead-in of PEG-IFN and RBV could prove useful to check the tolerability in these patients before adding a protease inhibitor.
Although few studies are available, the use of triple therapy in patients on the liver transplant waiting list is associated with high rates of early viral response; however, in up to 25% of cases, early withdrawal is necessary because of secondary effects and 10% of patients experience decompensation of their disease[70,71]. Accordingly, there is currently no general recommendation for the use of triple therapy in patients on the liver transplant waiting list, although it can be contemplated in select non-decompensated cirrhotic patients and under close control.
In transplanted patients, the increase in efficacy, applicability and tolerance of this therapy, and the possible interactions with other drugs, remain unknown and more studies are required.
Although the evidence available for the efficacy of triple therapy in transplanted patients is scarce, it has shown increased rates of rapid and early viral response with DAAs. The main limitation of triple therapy, however, is interaction with immunosuppressive drugs. Telaprevir and boceprevir are inhibitors of cytochrome P450 3A, responsible for the metabolism of both cyclosporine and tacrolimus. Studies in healthy volunteers showed that boceprevir and telaprevir increase the area under the curve of cyclosporine and tacrolimus by 2.7- and 17-fold, respectively[72]. Some authors recommend reconversion to cyclosporine in all possible patients before starting triple therapy. The levels of other immunosuppressors, such as everolimus or sirolimus, may also rise because of the same mechanism.
Only a few, small studies have assessed real-life experience with DAAs in post-transplant recurrence. The preliminary data, obtained from the experience of single centers[73-77], show an increase in early viral response compared with double therapy, although there is a need to reduce the dose of cyclosporine, particularly tacrolimus, and a greater rate of secondary effects.
Coilly et al[7] undertook the first multicenter study comprising 37 patients with recurrence of HCV. The end-of-treatment virological response rate was 72% in the boceprevir group and 40% in the telaprevir group. The cyclosporine dose was reduced 1.8-fold with boceprevir and 3.4-fold with telaprevir. The use of tacrolimus necessitated reducing the dose 5-fold with bocepevir and 23-fold with telaprevir.
Another multicenter study[78], this time involving 60 patients treated with triple therapy (35 with telaprevir and 25 with boceprevir), showed early SVR rates that were better than those with double therapy. Most patients needed a reduction in their immunosuppressive drugs from the first day of antiviral therapy, with strict control of the blood levels. The results concerning efficacy coincided with those of the individual study reported initially.
The secondary effects of triple therapy also constitute a limitation to its use in transplant patients, who frequently have a reduction in the doses of PEGylated interferon and rivabirin, high rates of early withdrawal from treatment and the requirement for colony-stimulating factors.
The main secondary effect is medullary toxicity, with a greater incidence of cytopenia than in non-transplant patients, particularly anemia, requiring a reduction in the dose of ribavirin and the addition of erythropoietin, in up to 95% of cases according to some series[73]. The requirement for transfusions is also more common. Other secondary effects reported include skin symptoms, anorectal syndrome and dysgeusia.
Both the multicenter studies mentioned above reported the presence of severe infections, with sepsis being the main cause of death in some series[7,78].
Rejection, described with the standard treatment in relation to interferon, may be more common in patients treated with triple therapy, particularly at the end of this treatment, because of the recovery of cytochrome p450 activity and therefore of the metabolism of the immunosuppressors, with a sudden and severe reduction in plasma levels.
Thus DAAs open up a hopeful new era in the treatment of post-transplant HCV relapse, especially that caused by the increase in viral response rates, which could even warrant the consideration of anticipatory treatment in this group of patients. Nonetheless, further studies and evidence are required, from both clinical trials and real-life experience, particularly concerning tolerability and safety. Precisely because of this limitation, the future perspectives, such second generation DAAs and interferon-free treatment, are also promising.
Hepatitis C recurrence continues to present a major challenge in LT. Despite recent advances, the results in patients with HCV infection are not satisfactory, mainly because of recurrence of the primary disease and a lack of availability of an efficient prophylactic therapy. Likewise, antiviral therapy still presents important limitations, particularly its poor tolerance, which hinders its use at full doses or for a sufficient duration to achieve an adequate response. The most recommended attitude is to attempt antiviral therapy before the transplant, particularly for those patients with maintained liver function, in an attempt to avoid disease progression; however, if this is not possible, at least reach transplantation with a negative viremia. Once recurrence is established, the principles of management include optimal donor selection, early identification of HCV recurrence, diligent and aggressive use of antiviral therapy, and close attention to immunosuppression management. Strict monitoring of the progression of the fibrosis by serial biopsies and/or elastography will enable early identification of those patients who might benefit from antiviral therapy to delay the advance of the disease and thus avoid the need for a retransplant. The introduction of DAAs is provides hope for the development in the near future of new protocols with novel antiviral drugs for LT that are safer and more effective.
| 1. | Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2010 Annual data report. Available from: http://optn.transplant.hrsa.gov/data/annualreport.asp. Accessed Feb, 2014. |
| 2. | Guillouche P, Féray C. Systematic review: anti-viral therapy of recurrent hepatitis C after liver transplantation. Aliment Pharmacol Ther. 2011;33:163-174. [PubMed] [DOI] [Full Text] |
| 3. | Neumann UP, Berg T, Bahra M, Puhl G, Guckelberger O, Langrehr JM, Neuhaus P. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004;77:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Roche B, Samuel D. Hepatitis C virus: Up to the Minute. Liver Transpl. 2010;16 Suppl 2:S26-S35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, Lampasi F, Tartaglione MT, Marsilia GM, Calise F. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 6. | Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 7. | Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S, Pageaux GP, Si-Ahmed SN, Guillaud O, Antonini TM. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (10)] |
| 8. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [PubMed] [DOI] [Full Text] |
| 9. | Berenguer M, Rayón JM, Prieto M, Aguilera V, Nicolás D, Ortiz V, Carrasco D, López-Andujar R, Mir J, Berenguer J. Are posttransplantation protocol liver biopsies useful in the long term? Liver Transpl. 2001;7:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Berenguer M, Aguilera V, Prieto M, Carrasco D, Rayón M, San Juan F, Landaverde C, Mir J, Berenguer J. Delayed onset of severe hepatitis C-related liver damage following liver transplantation: a matter of concern? Liver Transpl. 2003;9:1152-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Samuel D, Forns X, Berenguer M, Trautwein C, Burroughs A, Rizzetto M, Trepo C. Report of the monothematic EASL conference on liver transplantation for viral hepatitis (Paris, France, January 12-14, 2006). J Hepatol. 2006;45:127-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Carrión JA, Torres F, Crespo G, Miquel R, García-Valdecasas JC, Navasa M, Forns X. Liver stiffness identifies two different patterns of fibrosis progression in patients with hepatitis C virus recurrence after liver transplantation. Hepatology. 2010;51:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 13. | Berenguer M, Prieto M, Rayón JM, Mora J, Pastor M, Ortiz V, Carrasco D, San Juan F, Burgueño MD, Mir J. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 406] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, Córdoba J, Herola A, Ascher N, Mir J. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 830] [Article Influence: 34.6] [Reference Citation Analysis (2)] |
| 16. | Firpi RJ, Clark V, Soldevila-Pico C, Morelli G, Cabrera R, Levy C, Machicao VI, Chaoru C, Nelson DR. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Firpi RJ, Abdelmalek MF, Soldevila-Pico C, Cabrera R, Shuster JJ, Theriaque D, Reed AI, Hemming AW, Liu C, Crawford JM. One-year protocol liver biopsy can stratify fibrosis progression in liver transplant recipients with recurrent hepatitis C infection. Liver Transpl. 2004;10:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Gallegos-Orozco JF, Yosephy A, Noble B, Aqel BA, Byrne TJ, Carey EJ, Douglas DD, Mulligan D, Moss A, de Petris G. Natural history of post-liver transplantation hepatitis C: A review of factors that may influence its course. Liver Transpl. 2009;15:1872-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Pérez MJ, García DM, Taybi BJ, Daga JA, Rey JM, Grande RG, Lombardo JD, López JM. Cardiovascular risk factors after liver transplantation: analysis of related factors. Transplant Proc. 2011;43:739-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Jiménez-Pérez M, García DM, Grande RG, Daga JA, Pulido LB, Aguilar MD, Bravo MA, López JM, de la Mata Garcia MM. Analysis of the recurrence of hepatitis C virus after liver transplantation: results of the Andalusian liver registry. Transplant Proc. 2013;45:276-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Roche B, Samuel D. Risk factors for hepatitis C recurrence after liver transplantation. J Viral Hepat. 2007;14 Suppl 1:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? J Hepatol. 2005;42:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Perez-Daga JA, Santoyo J, Suárez MA, Fernández-Aguilar JA, Ramírez C, Rodríguez-Cañete A, Aranda JM, Sánchez-Pérez B, Montiel C, Palomo D. Influence of degree of hepatic steatosis on graft function and postoperative complications of liver transplantation. Transplant Proc. 2006;38:2468-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Machicao VI, Bonatti H, Krishna M, Aqel BA, Lukens FJ, Nguyen JH, Rosser BG, Satyanarayana R, Grewal HP, Hewitt WR. Donor age affects fibrosis progression and graft survival after liver transplantation for hepatitis C. Transplantation. 2004;77:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Pine JK, Aldouri A, Young AL, Davies MH, Attia M, Toogood GJ, Pollard SG, Lodge JP, Prasad KR. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl. 2009;15:1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Humar A, Horn K, Kalis A, Glessing B, Payne WD, Lake J. Living donor and split-liver transplants in hepatitis C recipients: does liver regeneration increase the risk for recurrence? Am J Transplant. 2005;5:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Charlton MR, Thompson A, Veldt BJ, Watt K, Tillmann H, Poterucha JJ, Heimbach JK, Goldstein D, McHutchison J. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011;53:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Brocato M, Trotter JF, Forman L, Riccardi S, Burton JR, Everson GT, Smith M, Susskind BM, Spritz R, Kam I. Genetion variation of IL-28B associated with severity of HCV recurrence following liver transplantation. AASLD 2010. Abstract poster 1158. Hepatology. 2010;52 suppl 4:875A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Hanouneh IA, Feldstein AE, McCullough AJ, Miller C, Aucejo F, Yerian L, Lopez R, Zein NN. The significance of metabolic syndrome in the setting of recurrent hepatitis C after liver transplantation. Liver Transpl. 2008;14:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | González-Grande R, Jiménez-Perez M, Sáez-Goméz AB, Rodrigo-López JM. Metabolic syndrome after liver transplantation. Liver transplantation-Technical issues and complications. Rijeka (Croatia): InTech 2012; 349-360. |
| 32. | McCaughan GW. Immunosuppression for HCV following liver transplantation: enough is just enough. Transplantation. 2004;78:1413-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Samonakis DN, Triantos CK, Thalheimer U, Quaglia A, Leandro G, Teixeira R, Papatheodoridis GV, Sabin CA, Rolando N, Davies S. Immunosuppression and donor age with respect to severity of HCV recurrence after liver transplantation. Liver Transpl. 2005;11:386-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 34. | Berenguer M, Aguilera V, Prieto M, San Juan F, Rayón JM, Benlloch S, Berenguer J. Significant improvement in the outcome of HCV-infected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J Hepatol. 2006;44:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Sgourakis G, Radtke A, Fouzas I, Mylona S, Goumas K, Gockel I, Lang H, Karaliotas C. Corticosteroid-free immunosuppression in liver transplantation: a meta-analysis and meta-regression of outcomes. Transpl Int. 2009;22:892-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, Montgomery RA, Cameron AM, Maley WR. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007;13:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Levy G, Grazi GL, Sanjuan F, Wu Y, Mühlbacher F, Samuel D, Friman S, Jones R, Cantisani G, Villamil F. 12-month follow-up analysis of a multicenter, randomized, prospective trial in de novo liver transplant recipients (LIS2T) comparing cyclosporine microemulsion (C2 monitoring) and tacrolimus. Liver Transpl. 2006;12:1464-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | O’Grady JG, Hardy P, Burroughs AK, Elbourne D. Randomized controlled trial of tacrolimus versus microemulsified cyclosporin (TMC) in liver transplantation: poststudy surveillance to 3 years. Am J Transplant. 2007;7:137-141. [PubMed] |
| 40. | Rubín A, Aguilera V, Berenguer M. Liver transplantation and hepatitis C. Clin Res Hepatol Gastroenterol. 2011;35:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Oton E, Barcena R, Moreno-Planas JM, Cuervas-Mons V, Moreno-Zamora A, Barrios C, Garcia-Garzon S, Moreno A, Boullosa-Graña E, Rubio-Gonzalez EE. Hepatitis C recurrence after liver transplantation: Viral and histologic response to full-dose PEG-interferon and ribavirin. Am J Transplant. 2006;6:2348-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Berenguer M, Palau A, Aguilera V, Rayón JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 43. | Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, Merli M, Strazzabosco M, Tisone G. A randomized study on Peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol. 2007;46:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 244] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 45. | Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 227] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Sheiner PA, Boros P, Klion FM, Thung SN, Schluger LK, Lau JY, Mor E, Bodian C, Guy SR, Schwartz ME. The efficacy of prophylactic interferon alfa-2b in preventing recurrent hepatitis C after liver transplantation. Hepatology. 1998;28:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, Riely C, Martin P, Teperman L, Jiao J. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005;41:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 48. | Sugawara Y, Makuuchi M, Matsui Y, Kishi Y, Akamatsu N, Kaneko J, Kokudo N. Preemptive therapy for hepatitis C virus after living-donor liver transplantation. Transplantation. 2004;78:1308-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 49. | Shergill AK, Khalili M, Straley S, Bollinger K, Roberts JP, Ascher NA, Terrault NA. Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. Am J Transplant. 2005;5:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, Bosch J, Forns X. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 51. | Gurusamy KS, Tsochatzis E, Xirouchakis E, Burroughs AK, Davidson BR. Antiviral therapy for recurrent liver graft infection with hepatitis C virus. Cochrane Database Syst Rev. 2010;CD006803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2839] [Article Influence: 946.3] [Reference Citation Analysis (0)] |
| 52. | Aytaman A, Kaufman M, Terrault NA. Management of posttransplant hepatitis C infection. Curr Opin Organ Transplant. 2010;15:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Berenguer M, Palau A, Fernandez A, Benlloch S, Aguilera V, Prieto M, Rayón JM, Berenguer J. Efficacy, predictors of response, and potential risks associated with antiviral therapy in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2006;12:1067-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Jiménez-Pérez M, Sáez-Gómez AB, Pérez-Daga JA, Lozano-Rey JM, de la Cruz-Lombardo J, Rodrigo-López JM. Hepatitis C virus recurrence after liver transplantation: analysis of factors related to sustained viral response. Transplant Proc. 2010;42:666-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, Uchiyama H, Soejima Y, Shirabe K, Matsuura Y. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139:1577-185, 1577-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 56. | Coto-Llerena M, Pérez-Del-Pulgar S, Crespo G, Carrión JA, Martínez SM, Sánchez-Tapias JM, Martorell J, Navasa M, Forns X. Donor and recipient IL28B polymorphisms in HCV-infected patients undergoing antiviral therapy before and after liver transplantation. Am J Transplant. 2011;11:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Feliu A, Carrión JA, Massaguer A, Martínez-Bauer E, García-Retortillo M, González P, Costa J, Sánchez-Tapias JM, Forns X. Sensitivity to antiviral therapy may change after liver transplantation in patients with chronic hepatitis C virus infection. J Viral Hepat. 2006;13:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Firpi RJ, Soldevila-Pico C, Morelli GG, Cabrera R, Levy C, Clark VC, Suman A, Michaels A, Chen C, Nelson DR. The use of cyclosporine for recurrent hepatitis C after liver transplant: a randomized pilot study. Dig Dis Sci. 2010;55:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Bzowej N, Nelson DR, Terrault NA, Everson GT, Teng LL, Prabhakar A, Charlton MR. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011;17:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 60. | Walter T, Scoazec JY, Guillaud O, Hervieu V, Chevallier P, Boillot O, Dumortier J. Long-term antiviral therapy for recurrent hepatitis C after liver transplantation in nonresponders: biochemical, virological, and histological impact. Liver Transpl. 2009;15:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Carrión JA, Navasa M, Forns X. Retransplantation in patients with hepatitis C recurrence after liver transplantation. J Hepatol. 2010;53:962-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Martí J, Charco R, Ferrer J, Calatayud D, Rimola A, Navasa M, Fondevila C, Fuster J, García-Valdecasas JC. Optimization of liver grafts in liver retransplantation: a European single-center experience. Surgery. 2008;144:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Markmann JF, Gornbein J, Markowitz JS, Levy MF, Klintmalm GB, Yersiz H, Morrisey M, Drazan K, Farmer DG, Ghobrial RM. A simple model to estimate survival after retransplantation of the liver. Transplantation. 1999;67:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation. Hepatology. 1999;29:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Kwo PY, Lawitz E, McCone J, Schiff ER, Vierling J, Pound D, Davis M, Galati J, Gordon S, Ravendhran N. Response- guided therapy for boceprevir combination treatment; results from HCV SPRINT-1. Hepatology. 2009;50 Suppl 4:1035A. [DOI] [Full Text] |
| 66. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 795] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 67. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1865] [Article Influence: 124.3] [Reference Citation Analysis (4)] |
| 68. | Product information. Victrelis (boceprevir). Whitehouse Station: NMC, Inc; 2011; Available from: http://www.accessdata.fda.gov/drugsatfda_docs. Accessed Feb, 2014. |
| 69. | Product information. Incivek (telaprevir). Cambridge, MA: Vertex Pharmaceuticals Incorporated; 2011; Available from: http://www.accessdata.fda.gov/drugsatfda_docs. Accessed Feb, 2014. |
| 70. | Hezode C, Dorival C, Zoulim F, Poynard T, Mathurin P, Pol S, Larrey D, Cacoub P, de Ledinghen V, Bourlière M. Safety of Telaprevir or Boceprevir in Combination with Peginterferon alfa/Ribavirin, in Cirrhotic Non Responders. First Results of the French Early Access Program (ANRS CO20-CUPIC). J Hepatol. 2012;56 Suppl 2:S4. [DOI] [Full Text] |
| 71. | Hsu SH, Yeh ML, Wang SN. New insights in recurrent HCV infection after liver transplantation. Clin Dev Immunol. 2013;2013:890517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Hulskotte E, Gupta S, Xuan F, van Zutven M, O’Mara E, Feng HP, Wagner J, Butterton J. Pharmacokinetic interaction between the hepatitis C virus protease inhibitor boceprevir and cyclosporine and tacrolimus in healthy volunteers. Hepatology. 2012;56:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 73. | Coilly A, Roche B, Botta-Fridlun D, Leroy V, Pageaux PG, Si-Ahmed SN, Antonini TM, Samuel D, Duclos-Vallee JC. Efficacy ad safety of protease inhibitors for several hepatitis C recurrence after liver transplantation: a first multicenter experience. J Hepatol. 2012;56 suppl 2:S21. [DOI] [Full Text] |
| 74. | Kwo P, Ghabril M, Lacerda M, Vinayek R, Tector AJ, Fridell J, Vianna R. Use of telaprevir plus peginterferon(ribavirin for null responders postOL with advanced fibrosis/cholestatic hepatitis C. J Hepatol. 2012;56 Suppl 2:S86. |
| 75. | Burton JR, Everson GT. Initial experience with telaprevir for treating hepatitis C virus in liver recipients: virologic response, safety, and tolerability. Am J Transplant. 2012;12 Suppl 3:188. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Kawaoka T, Takahashi S, Tatsukawa Y, Hiramatsu A, Hiraga N, Miki D, Tsuge M, Imamura M, Kawakami Y, Aikata H. Two patients treated with pegylated interferon/ribavirin/telaprevir triple therapy for recurrent hepatitis C after living donor liver transplantation. Hepatol Res. 2014;44:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Werner CR, Egetemeyr DP, Lauer UM, Nadalin S, Königsrainer A, Malek NP, Berg CP. Feasibility of telaprevir-based triple therapy in liver transplant patients with hepatitis C virus: SVR 24 results. PLoS One. 2013;8:e80528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, Yataco ML, Satyanarayana R, Rosser BG, Vargas HE. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
P- Reviewer: Fourtounas C, Matsumori A, Yamagiwa S S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Zhang DN