Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15845
Revised: February 22, 2014
Accepted: June 2, 2014
Published online: November 14, 2014
Processing time: 337 Days and 2.6 Hours
AIM: To compare efficacy, patient compliance, acceptability, satisfaction, safety, and adenoma detection rate of sodium phosphate tablets (NaP, CLICOLONTM) to a standard 4 L polyethylene glycol (PEG) solution for bowel cleansing for adults undergoing colonoscopy.
METHODS: In this multicenter, randomized, prospective, investigator-blind study, the relatively young (19-60 years) healthy outpatients without comorbidity were randomly assigned to one of two arms. All colonoscopy were scheduled in the morning. The NaP group was asked to take 4 tablets, 5 times the evening before and 4 tablets, 3 times early on the morning of the colonoscopy. The PEG group was asked to ingest 2 L of solution the evening before and 2 L early in the morning of the procedure. Adequacy of bowel preparation was scored using the Boston bowel preparation scale.
RESULTS: No significant differences were observed between the NaP group (n = 158) and PEG group (n = 162) in bowel cleansing quality (adequate preparation 93.0% vs 92.6%, P = 0.877), patient compliance (P = 0.228), overall adverse events (63.3% vs 69.1%, P = 0.269), or adenoma detection rate (34.8% vs 35.2%, P = 0.944). Patient acceptability, satisfaction, and patient rating of taste were higher in the NaP group than in the PEG group (P < 0.001).
CONCLUSION: NaP tablets, compared with PEG solution, produced equivalent colon cleansing, did not cause more side effects, and had better patient acceptability and satisfaction in the relatively young (age < 60 years) healthy individuals without comorbidity. An oral tablet formulation could make bowel preparation less burdensome, resulting in greater patient participation in screening programs.
Core tip: Sodium phosphate (NaP) tablets were equally efficacious as standard 4 L polyethylene glycol (PEG) solution for bowel cleansing for colonoscopy and did not results in greater side effects. Furthermore, patient acceptance and satisfaction of NaP tablets were superior to 4 L PEG solution. NaP tablets in this trial were safe, well-tolerated, and efficient for bowel preparation in the relatively young (age < 60 years) healthy individuals without comorbidity. A more acceptable oral tablet formation might provide a valuable alternative for individuals who are reluctant to undergo colonoscopy because of aversion to the currently available purgatives.
-
Citation: Jung YS, Lee CK, Kim HJ, Eun CS, Han DS, Park DI. Randomized controlled trial of sodium phosphate tablets
vs polyethylene glycol solution for colonoscopy bowel cleansing. World J Gastroenterol 2014; 20(42): 15845-15851 - URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15845.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15845
Colonoscopy is currently the gold standard for detection of colorectal neoplasms[1]. However, inadequate bowel preparation can reduce detection of polyps that are potentially cancerous[2,3]. Several studies reported that 4%-5% of colorectal cancers can be missed on a single colonoscopic examination[4,5] and one of the main reasons for missed colorectal cancers is incomplete bowel cleaning[6]. Moreover, poor bowel preparation can result in a longer procedure time, shorter intervals of follow-up colonoscopy, and increased economic costs[3]. Consequently, professional societies propose measuring bowel preparation quality as one of the most important quality indicators for colonoscopy[7].
Polyethylene glycol (PEG) is the most commonly used agent for colon cleansing because it does not cause fluid exchange across mucosal membranes, limiting fluid and electrolyte disturbances[8]. However, the need to ingest a large volume of fluid and the unpleasant flavor of PEG reduce patient compliance. Although small-volume preparations using 2 L PEG have been introduced, some patients do not tolerate PEG-based bowel preparation.
Sodium phosphate (NaP) tablets were developed to improve patient acceptability of the bowel preparation regimen. In Western countries and Japan, NaP tablets are reported to be similar or better than PEG solution for patient compliance, acceptability, and safety as well as bowel cleansing efficacy[9-13]. In May 2012, a novel tablet-based NaP formulation (CLICOLONTM tablets; Korea Pharma Co.) was approved by the Korean Ministry of Food and Drug Safety for colon cleansing prior to colonoscopy. This study is the first Korean trial to compare the efficacy of NaP tablets and 4 L PEG solution for bowel preparation in controlled circumstances: with outpatients, split-dosing preparation, low-residual diet, and detailed instructions. In addition, this study compared polyp and adenoma detection rates, patient compliance, acceptability, satisfaction, and safety between the two regimens.
This prospective, investigator-blinded, randomized, and multicenter study was conducted at three university hospitals (Kangbuk Samsung Hospital, Kyung Hee University Hospital, and Hanyang University Hospital) in Korea. Between December 2012 and October 2013, consecutive outpatients aged 19-60 years who were scheduled to undergo routine elective colonoscopy were recruited to this study. The study protocol was approved by the Institutional Review Boards at the participating medical centers. All patients who agreed to participate in the study signed a written informed consent form.
Adult outpatients undergoing colonoscopy for colorectal cancer screening were candidates for inclusion. We included only the relatively young (aged < 60 years) healthy subjects without comorbidity. Exclusion criteria were: inpatient status; serious medical conditions such as renal, cardiac, liver, or metabolic disease; electrolyte imbalance such as hypernatremia or hyperphosphatemia; stroke or dementia; major psychiatric illness; pregnancy, breast feeding, or risk of becoming pregnant; known allergy to PEG or NaP; prior history of colonic resection; incomplete colonoscopy examination; functional constipation defined by Rome III diagnostic criteria; or taking angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or nonsteroidal anti-inflammatory drugs (NSAIDs).
After patient enrollment, clinical research coordinators at each participating center randomized patients using a computer-generated random sequence and gave each patient oral and written instructions on one of two bowel preparations: NaP tablet (CLICOLON tablets, South Korea Pharma Co., Seoul; dibasic sodium phosphate anhydrous 398 mg, monobasic sodium phosphate monohydrate 1102 mg) or 4 L PEG solution (Taejoon Pharm Inc., Seoul; 236 g polyethylene glycol, 22.7 g Na2SO4, 6.74 g NaHCO3, 5.86 g NaCl, and 2.97 g KCl). Agents for bowel cleansing were dispensed by clinical research coordinators. All participants were scheduled for colonoscopy in the morning (9:00 AM-1:00 PM) to reduce bias related to procedure time. All participants were instructed to eat a low-residual diet for 3 d before scheduled colonoscopy and a clear liquid diet before 6:00 PM on the day before the colonoscopy. The NaP group was asked to take 20 NaP tablets the evening before (from 8:00 PM) and 12 NaP tablets early in the morning of the colonoscopy (beginning 3 to 5 h before procedure), consuming 4 tablets every 15 min with 240 mL water. The PEG group was asked to ingest 2 L the evening before (from 8:00 PM) and 2 L early in the morning of the colonoscopy at 250 mL every 15 min with complete ingestion at least 3 h before the procedure.
Colonoscopies were performed under conscious sedation by experienced colonoscopists (> 1000 cases) who were blinded to the results of preparation randomization. Colonoscopies used conventional videoendoscopes (CF-Q260AI, CF-H260AI; Olympus Medical Systems, Tokyo, Japan). Bowel cleansing adequacy was assessed using the Boston Bowel Preparation Scale[14], which independently evaluates different colonic segments. The colon was divided into 3 segments: right (cecum and ascending colon), transverse, and left (descending, sigmoid colon, and rectum). Each section was scored as 0 to 3:0, unprepared colon segment with mucosa not seen because of solid stool that could not be cleared; 1, portion of colon segment mucosa seen, but other areas of the colon segment not well seen because of staining, residual stool, and/or opaque liquid; 2, minor amount of residual staining, small stool fragments and/or opaque liquid, but mucosa of colon segment seen well; and 3, entire mucosa of colon segment seen well with no residual staining, small stool fragments and/or opaque liquid. Based on this system, we defined inadequate bowel preparation as a score of 0 or 1 on any colon segment and adequate preparation as a score of ≥ 2 for all location[14]. Prior to study commencement, all endoscopists received information about the classification and performed calibration exercises involving 20 colonoscopies.
Before colonoscopy, we collected patient information, including age, gender, body mass index (BMI), functional constipation according to Rome III diagnostic criteria, indications for colonoscopy, and history of previous operation and colonoscopy. Immediately before colonoscopy, patients completed a questionnaire on their preparation experience (amount of purgative ingested, difficulty and taste of the study preparation, any associated adverse effects, and satisfaction level).
Compliance was rated using a 3-grade scale based on consumption of the study preparation: optimal (100%); good (≥ 75%); poor (< 75%). Acceptability was measured based on difficulty of completing ingestion of the cleansing agent (3-point scale: none, some, much). Patient satisfaction was scored on a 10-point visual analog scale (VAS) of 0 (very bad) to 10 (excellent). Cleansing solution taste was graded using a 5-point scale of very bad, bad, neutral, good, very good. Patients were also asked if they had experienced any adverse events (nausea, vomiting, abdominal pain, bloating, anal irritation symptom, or sleep disturbance).
This study was designed to assess the noninferiority of NaP tablets compared to 4 L PEG for successful bowel cleansing. Noninferiority was defined as a one-sided 97.5%CI greater than -15.0% for the difference in successful cleansing between the two treatment arms. The sample size of 150 patients for each study arm (NaP tablets and 4 L PEG) was determined assuming success rates of 70% for colon cleansing in both treatment arms, a 15.0% noninferiority margin, and a significance level of 0.025 powered at 82%. The success rate for colon cleansing was based on prior studies[15,16]. We estimated a dropout rate of 20% and aimed to recruit 360 participants to provide at least 300 evaluable assessments. Student’s t-test was used to compare numerical variables between groups. χ2, Fisher’s exact test, or linear-by-linear association tests were used to compare categorical variables. P values < 0.05 were considered to be statistically significant. The software program SPSS (v. 18, Chicago, Illinois, United States) was used for statistical analyses.
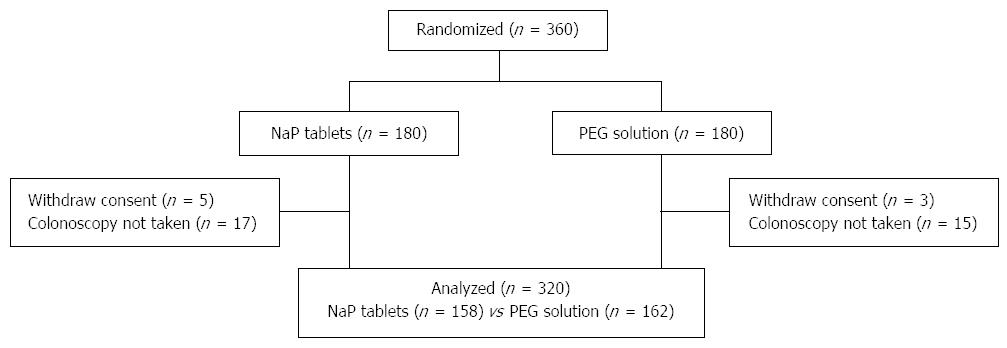
A total of 360 patients were randomized to receive NaP tablets (n = 180) or 4-L PEG (n = 180). Excluded were 40 patients, of whom 8 withdrew consent before examination and 32 did not come to the hospital on the reserved colonoscopy date. Included were 320 patients, of whom 158 received NaP tablets and 162 received 4 L PEG (Figure 1). No significant differences in age, sex, BMI, prior experience with colonoscopy, and surgical history were observed between the two groups. Abdominal pain was more frequent as the indication for colonoscopy in NaP group, whereas family history of CRC was more frequent in PEG group (Table 1).
| NaP tables (n = 158) | PEG solution (n = 162) | P value | |
| Mean age (yr) | 46.5 ± 9.8 | 48.6 ± 10.3 | 0.064 |
| Male | 70 (44.3) | 69 (42.6) | 0.758 |
| BMI | 23.2 ± 3.1 | 23.0 ± 3.0 | 0.628 |
| Experience of colonoscopy | 67 (42.4) | 74 (45.7) | 0.555 |
| Surgical history | 23 (14.6) | 35 (21.6) | 0.102 |
| Indication for colonoscopy | |||
| Screening | 89 (56.3) | 81 (50.0) | 0.257 |
| Bowel habit change | 9 (5.7) | 17 (10.5) | 0.116 |
| Stool caliber change | 8 (5.1) | 6 (3.7) | 0.552 |
| Melena/hematochezia | 13 (8.2) | 16 (9.9) | 0.608 |
| Abdominal pain | 22 (13.9) | 10 (6.2) | 0.021 |
| Anemia | 2 (1.3) | 2 (1.2) | 1.000 |
| Weight loss | 1 (0.6) | 3 (1.9) | 0.623 |
| P/Hx of CRN | 13 (8.2) | 17 (10.5) | 0.487 |
| F/Hx of CRC | 0 | 7 (4.3) | 0.015 |
| For polypectomy | 1 (0.6) | 3 (1.9) | 0.623 |
Bowel cleansing quality in three colon segments is reported by group in Table 2. No significant difference was seen between the two groups in bowel cleansing scores for overall bowel preparation (8.2 vs 8.0, P = 0.221). Similar proportions of patients had adequate bowel preparation in the NaP and PEG groups (93.0% vs 92.6%, P = 0.877).
| NaP tables (n = 158) | PEG solution (n = 162) | P value | |
| Bowel cleansing | |||
| Adequate | 147 (93.0) | 150 (92.6) | 0.877 |
| Inadequate | 11 (7.0) | 12 (7.4) | |
| Boston scale score | |||
| Right colon | 2.6 ± 0.6 | 2.5 ± 0.7 | 0.262 |
| Transverse colon | 2.8 ± 0.5 | 2.7 ± 0.5 | 0.022 |
| Left colon | 2.8 ± 0.5 | 2.8 ± 0.5 | 0.990 |
| Global colon | 8.2 ± 1.3 | 8.0 ± 1.2 | 0.221 |
| Cecal insertion time (min) | 4.5 ± 2.7 | 4.2 ± 2.6 | 0.224 |
| Withdrawal time (min) | 10.5 ± 4.6 | 9.5 ± 3.6 | 0.028 |
| Total colonoscopy time (min) | 15.2 ± 6.4 | 13.7 ± 5.4 | 0.023 |
Withdrawal (10.5 min vs 9.5 min, P = 0.028) and total colonoscopy time (15.2 min vs 13.7 min, P = 0.023) were prolonged in the NaP group compared to the PEG group, but no significant difference was seen in cecal insertion time. We did not consider polypectomy time.
Table 3 shows the detection rates for colorectal polyps and adenomas. The NaP and PEG groups showed no significant differences in the detection rate for polyps (57.6% vs 50.0%, P = 0.173) or adenomas (34.8% vs 35.2%, P = 0.944). No differences were seen in the total number of polyps (1.3 vs 1.2, P = 0.752) or adenomas (0.7 vs 0.6, P = 0.679) per patient regardless of lesion size.
| NaP tablets (n = 158) | PEG solution (n = 162) | P value | |
| Polyps regardless of size | |||
| Participants | 91 (57.6) | 81 (50.0) | 0.173 |
| Polyps/patient | 1.3 ± 1.7 | 1.2 ± 1.8 | 0.752 |
| Polyps diameter ≤ 5 mm | |||
| Participants | 76 (48.1) | 63 (38.9) | 0.096 |
| Polyps/patient | 0.9 ± 1.3 | 0.8 ± 1.5 | 0.770 |
| Polyps diameter > 5 mm | |||
| Participants | 43 (27.2) | 44 (27.2) | 0.991 |
| Polyps/patient | 0.5 ± 1.0 | 0.4 ± 0..8 | 0.508 |
| Adenomas regardless of size | |||
| Participants | 55 (34.8) | 57 (35.2) | 0.944 |
| Polyps/patient | 0.7 ± 1.3 | 0.6 ± 1.1 | 0.679 |
| Adenomas diameter ≤ 5 mm | |||
| Participants | 40 (25.3) | 39 (24.1) | 0.797 |
| Polyps/patient | 0.4 ± 0.9 | 0.4 ± 0.8 | 0.813 |
| Adenomas diameter > 5 mm | |||
| Participants | 29 (18.4) | 33 (20.4) | 0.648 |
| Polyps/patient | 0.3 ± 0.7 | 0.3 ± 0.6 | 0.744 |
We assessed patient acceptability as difficulty of completing ingestion of the cleansing agent (Table 4). More patients in NaP group reported no difficulty completing ingestion of the cleansing agent than in the PEG group (54.4% vs 30.9%, P < 0.001). For cleansing agent taste, fewer patients in the NaP group evaluated the study preparation as “very bad” or “bad” compared with the PEG group (19.0% vs 42.0%, P < 0.001). The mean VAS score indicating patient satisfaction with the bowel preparation regimen was significantly higher in the NaP group than in the PEG group (7.8 vs 6.5, P < 0.001). However, patient compliance was not significantly different between groups, based on consumption of the cleansing agent (optimal preparation 95.6% vs 91.4%, P = 0.228).
| NaP tablets | PEG solution | P value | |
| (n = 158) | (n = 162) | ||
| Compliance (amount of intake of cleansing agent) | 0.228 | ||
| Optimal (100%) | 151 (95.6) | 148 (91.4) | |
| Good (75%-100%) | 6 (3.8) | 14 (8.6) | |
| Poor (< 75%) | 1 (0.6) | 0 | |
| Acceptability (difficulty of the preparation) | < 0.001 | ||
| None | 86 (54.4) | 50 (30.9) | |
| Some | 59 (37.3) | 85 (52.5) | |
| Much | 13 (8.2) | 27 (16.7) | |
| Taste of the preparation | < 0.001 | ||
| Very bad | 4 (2.5) | 14 (8.6) | |
| Bad | 26 (16.5) | 54 (33.3) | |
| Neutral | 82 (51.9) | 75 (46.3) | |
| Good | 38 (24.1) | 14 (8.6) | |
| Very good | 8 (5.1) | 5 (3.1) | |
| Satisfaction level (VAS) | 7.8 ± 2.0 | 6.5 ± 2.4 | < 0.001 |
In the both groups, the most common complaint was nausea and abdominal distension/bloating. The frequency of reported adverse events, including nausea, vomiting, abdominal pain, abdominal distension/bloating, anal irritation symptom, and sleep disturbance was comparable for the two groups (63.3% vs 69.1%, P = 0.269) (Table 5). No serious adverse events occurred and no participant ceased the study because of adverse events.
| Adverse events | NaP tablets (n = 158) | PEG solution (n = 162) | P value |
| Nausea | 53 (33.5) | 53 (32.7) | 0.875 |
| Vomiting | 17 (10.8) | 17 (10.5) | 0.939 |
| Abdominal pain | 11 (7.0) | 14 (8.6) | 0.576 |
| Abdominal distension/bloating | 44 (27.8) | 61 (37.7) | 0.062 |
| Anal irritation symptom | 6 (3.8) | 8 (4.9) | 0.618 |
| Sleep disturbance | 3 (1.9) | 7 (4.3) | 0.336 |
| Total | 100 (63.3) | 112 (69.1) | 0.269 |
NaP tablets were developed to increase patient acceptance of bowel preparation. A tablet formulation of NaP (VisicolTM, Salix Pharmaceuticals, Inc, Morrisville, NC) was approved by the United States Food and Drug Administration in 2001 as a 40-tablet dose (60 g). A concern with the original formulation of Visicol was the appearance in the colon of residue from microcrystalline cellulose, an insoluble binder commonly used in tablet manufacturing that can obscure mucosal visualization. Therefore, the manufacturer developed a residue-free NaP tablet (Osmoprep™, Salix Pharmaceuticals Inc.) with same active ingredient. Several studies confirmed that the 32-tablet residue-free NaP regimen (Osmoprep) is superior to the 40-tablet residue-free NaP and NaP regimen (Visicol) for bowel preparation, based on safety, efficacy, and patient preference[17,18].
CLICOLON tablets, an improved version of Osmoprep tablets, are the first Korean NaP tablets for bowel cleansing. CLICOLON tablets might be more acceptable for swallowing because they are smaller and lighter than Visicol or Osmoprep tablets. In addition, CLICOLON tablets disintegrate more quickly than Visicol or Osmoprep tablets, and thus might have a faster effect. This study was conducted to determine the bowel cleansing efficacy and safety of the newly developed CLICOLON tablets compared with 4 L PEG solution.
In this study, NaP tablets had an equivalent bowel cleansing action as standard 4 L PEG and did not cause greater side effects. Furthermore, NaP tablets were superior to 4 L PEG in patient acceptability, satisfaction, and patient taste ratings. Numerous previous studies compared NaP tablets with PEG solutions for bowel cleansing efficacy and patient tolerance. Aronchick et al[9] compared a PEG solution, an oral NaP solution, and a prototype of the marketed NaP tablet for colonoscopy preparation. According to their results, colon cleansing efficacy was similar for all three purgatives. However, compared with both oral liquid purgatives, significantly fewer patients who used the tablets responded that they would refuse to take the same preparation in the future or would prefer a different bowel preparation. Moreover, no patients using the tablets reported a barely tolerable or unacceptable taste compared with 46% of patients using NaP solution and 14% of patients using PEG solution. Aronchick et al[9] concluded that NaP tablets were preferred over oral NaP solution or PEG solution. Kastenberg et al[10,11] compared NaP tablets (Visicol) with 4 L PEG solution, and reported equivalent colon cleansing, fewer side effects, and better tolerance and acceptability by patients. A recent study conducted in Japan revealed significantly higher preference for and acceptance of NaP tablets than PEG with sodium picosulfate solution[12]. In another Japanese study, NaP tablets were compared with PEG solution and showed equivalent colon cleansing efficacy with a higher detection rate for diminutive polyps[13]. These results and our results suggest that tablet purgatives are preferred by patients and as effective and safe as existing aqueous preparations.
However, several studies have reported adverse events for NaP including electrolyte imbalances such as hypernatremia[19], hyperphosphatemia[20-23], or hypocalcemia[24] and acute phosphate nephropathy[25,26]. Acute phosphate nephropathy, a type of acute renal failure, is a rare but serious adverse event associated with the use of oral NaP tablets for bowel cleansing. NaP regimen should not be used in patients with preexisting renal disease and adequate hydration should be ensured for all patients[27]. Most cases of acute phosphate nephropathy occurred in patients of advanced age or with renal disease or hypertension, or patients using medicines that affect renal perfusion or function (such as ACE inhibitors, ARBs, or NSAIDs)[26,27]. For participant safety, our study excluded people at increased risk of acute phosphate nephropathy such as people more than 60 years old, or patients with comorbidity including renal disease and hypertension. In addition, participants in our study were given detailed instructions from the clinical research coordinator about bowel preparation including to drink adequate amounts of water. No serious adverse events such as acute renal failure were observed in our study. Our study results suggested that NaP tablets were safe for bowel preparation in healthy people under 60 years old, with no comorbidity, who were provided appropriate and detailed instruction on bowel preparation.
Our study had several limitations. First, we included only outpatients without serious comorbidities and patients undergoing morning colonoscopy. Therefore, our results cannot be applied to inpatients with comorbidities or patients undergoing afternoon colonoscopy. Second, interobserver bias might have occurred because several endoscopists performed the colonoscopies and scored bowel preparation quality. However, before study commencement, all endoscopists performed calibration exercises of 20 colonoscopies intended to reduce interobserver bias. Third, renal function and electrolyte level were not monitored after colonoscopy, although they were confirmed to be normal before colonoscopy. However, we excluded patients at risk of renal dysfunction. Furthermore, participants returned to the hospital within 1-2 wk of their colonoscopy to be assessed for purgatives-related complications. Finally, this was an investigator-only blinded study. However, a double-blind or double-dummy design (both patient and investigator) would not be possible because patients needed to take 32 NaP tablets or 4 L PEG solution. This limitation might have affected the patient acceptability and satisfaction results.
In conclusion, NaP tablets were equally efficacious as standard 4 L PEG solution for bowel cleansing for colonoscopy and did not results in greater side effects. Furthermore, patient acceptance and satisfaction of NaP tablets were superior to 4 L PEG solution. NaP tablets in this trial were safe, well-tolerated, and efficient for bowel preparation in the relatively young (aged < 60) healthy individuals without comorbidity. A more acceptable oral tablet formation might provide a valuable alternative for individuals who are reluctant to undergo colonoscopy because of aversion to the currently available purgatives.
The need to ingest a large volume of fluid and the unpleasant flavor of polyethylene glycol (PEG) reduce patient compliance. Sodium phosphate (NaP) tablets were developed to improve patient acceptability of the bowel preparation regimen. In Western countries and Japan, NaP tablets are reported to be similar or better than PEG solution for patient compliance, acceptability, and safety as well as bowel cleansing efficacy.
NaP tablets were developed to increase patient acceptance of bowel preparation. CLICOLON tablets, an improved version of Osmoprep tablets, are the first Korean NaP tablet for bowel cleansing. In this study, the authors demonstrate that CLICOLON tablets, compared with PEG solution, produced equivalent colon cleansing, did not cause more side effects, and had better patient acceptability and satisfaction in the relatively young (aged < 60) healthy individuals without comorbidity.
This study is the first Korean trial to compare the efficacy of NaP tablets and 4 L PEG solution for bowel preparation in controlled circumstances. In this study, NaP tablets had an equivalent bowel cleansing action as standard 4 L PEG and did not cause greater side effects in the relatively young (aged < 60) healthy individuals without comorbidity. Furthermore, NaP tablets were superior to 4 L PEG in patient acceptability, satisfaction, and patient taste ratings.
A more acceptable oral tablet formation might provide a valuable alternative for individuals who are reluctant to undergo colonoscopy because of aversion to the currently available purgatives.
PEG is the most commonly used agent for colon cleansing. However, the need to ingest a large volume of fluid and the unpleasant flavor of PEG reduce patient compliance. NaP tablets were developed to improve patient acceptability of the bowel preparation regimen. CLICOLON tablets are the first Korean NaP tablets for bowel cleansing.
The authors wanted to compare the bowel cleansing efficacy of newer formulation of NaP tablets (CLICOLON) manufactured in South Korea claimed to be smaller, lighter and disintegrate more quickly than United States Food and Drug Administration approved Osmoprep tablets, to PEG solution using a none inferiority randomized study design. Authors found equal efficacy between the two regimens with regards to bowel cleansing but NaP tablets to be better tolerated and preferred.
| 1. | Labianca R, Merelli B. Screening and diagnosis for colorectal cancer: present and future. Tumori. 2010;96:889-901. [PubMed] |
| 2. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 566] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 707] [Article Influence: 33.7] [Reference Citation Analysis (2)] |
| 4. | Gorski TF, Rosen L, Riether R, Stasik J, Khubchandani I. Colorectal cancer after surveillance colonoscopy: false-negative examination or fast growth? Dis Colon Rectum. 1999;42:877-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Haseman JH, Lemmel GT, Rahmani EY, Rex DK. Failure of colonoscopy to detect colorectal cancer: evaluation of 47 cases in 20 hospitals. Gastrointest Endosc. 1997;45:451-455. [PubMed] |
| 6. | Faiss S. The missed colorectal cancer problem. Dig Dis. 2011;29 Suppl 1:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980;78:991-995. [PubMed] |
| 9. | Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 328] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Kastenberg D, Chasen R, Choudhary C, Riff D, Steinberg S, Weiss E, Wruble L. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc. 2001;54:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Kastenberg D, Barish C, Burack H, Dalke DD, Duckor S, Putnam W, Valenzuela G. Tolerability and patient acceptance of sodium phosphate tablets compared with 4-L PEG solution in colon cleansing: combined results of 2 identically designed, randomized, controlled, parallel group, multicenter phase 3 trials. J Clin Gastroenterol. 2007;41:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Hosoe N, Nakashita M, Imaeda H, Sujino T, Bessho R, Ichikawa R, Inoue N, Kanai T, Hibi T, Ogata H. Comparison of patient acceptance of sodium phosphate versus polyethylene glycol plus sodium picosulfate for colon cleansing in Japanese. J Gastroenterol Hepatol. 2012;27:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kambe H, Yamaji Y, Sugimoto T, Yamada A, Watabe H, Yoshida H, Omata M, Koike K. A randomized controlled trial of sodium phosphate tablets and polyethylene glycol solution for polyp detection. J Dig Dis. 2012;13:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 989] [Article Influence: 58.2] [Reference Citation Analysis (1)] |
| 15. | Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol. 2003;98:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Valiante F, Pontone S, Hassan C, Bellumat A, De Bona M, Zullo A, de Francesco V, De Boni M. A randomized controlled trial evaluating a new 2-L PEG solution plus ascorbic acid vs 4-L PEG for bowel cleansing prior to colonoscopy. Dig Liver Dis. 2012;44:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 17. | Wruble L, Demicco M, Medoff J, Safdi A, Bernstein J, Dalke D, Rose M, Karlstadt RG, Ettinger N, Zhang B. Residue-free sodium phosphate tablets (OsmoPrep) versus Visicol for colon cleansing: a randomized, investigator-blinded trial. Gastrointest Endosc. 2007;65:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Rex DK, Schwartz H, Goldstein M, Popp J, Katz S, Barish C, Karlstadt RG, Rose M, Walker K, Lottes S. Safety and colon-cleansing efficacy of a new residue-free formulation of sodium phosphate tablets. Am J Gastroenterol. 2006;101:2594-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Aradhye S, Brensilver JM. Sodium phosphate-induced hypernatremia in an elderly patient: a complex pathophysiologic state. Am J Kidney Dis. 1991;18:609-611. [PubMed] |
| 20. | Vukasin P, Weston LA, Beart RW. Oral Fleet Phospho-Soda laxative-induced hyperphosphatemia and hypocalcemic tetany in an adult: report of a case. Dis Colon Rectum. 1997;40:497-499. [PubMed] |
| 21. | Escalante CP, Weiser MA, Finkel K. Hyperphosphatemia associated with phosphorus-containing laxatives in a patient with chronic renal insufficiency. South Med J. 1997;90:240-242. [PubMed] |
| 22. | Filho AJ, Lassman MN. Severe hyperphosphatemia induced by a phosphate-containing oral laxative. Ann Pharmacother. 1996;30:141-143. [PubMed] |
| 23. | Fine A, Patterson J. Severe hyperphosphatemia following phosphate administration for bowel preparation in patients with renal failure: two cases and a review of the literature. Am J Kidney Dis. 1997;29:103-105. [PubMed] |
| 24. | Boivin MA, Kahn SR. Symptomatic hypocalcemia from oral sodium phosphate: a report of two cases. Am J Gastroenterol. 1998;93:2577-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Belsey J, Epstein O, Heresbach D. Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment Pharmacol Ther. 2009;29:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389-3396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 27. | Russmann S, Lamerato L, Marfatia A, Motsko SP, Pezzullo JC, Olds G, Jones JK. Risk of impaired renal function after colonoscopy: a cohort study in patients receiving either oral sodium phosphate or polyethylene glycol. Am J Gastroenterol. 2007;102:2655-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
P- Reviewer: Madhoun MF, Paoluzi OA, Shehata MMM S- Editor: Ma YJ L- Editor: A E- Editor: Ma S