Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15763
Revised: April 24, 2014
Accepted: June 12, 2014
Published online: November 14, 2014
Processing time: 236 Days and 17 Hours
AIM: To investigate the effects of gastric lavage with 2000 mL of saline in laparoscopic and endoscopic cooperative surgery.
METHODS: Twenty two patients who were diagnosed with a gastric gastrointestinal stromal tumor were enrolled. In former term, irrigations of the stomach were conducted whenever it was necessary, not systematically (Non systemic lavage group). In latter term, the stomach was thoroughly cleaned with 2000 mL of saline using an endoscope with a water jet, and Duodenal balloon occlusion was conducted to prevent refluxed bile and pancreatic juice (Systemic lavage+balloon occlusion group). The gastric wall was sprayed with 20 mL of distilled water, and 20 mL of gastric juice was collected in a sterile tube and submitted for culture. 20 mL of ascites was also collected from the laparoscopic ports and submitted for culture. We compared WBC, CRP, BT between two groups, and verify the reduction effect of bacterial counts in Systemic lavage+balloon occlusion group.
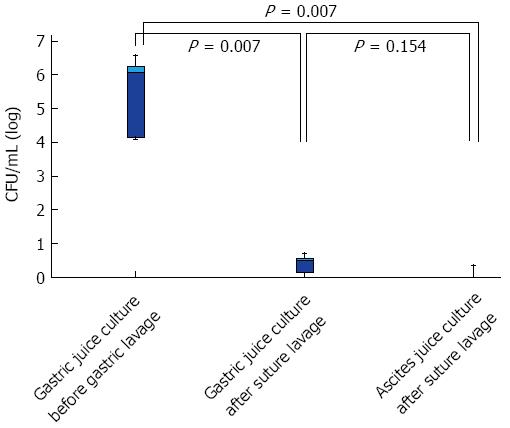
RESULTS: WBC count before, 1 d after, and 3 d after laparoscopic and endoscopic cooperative surgery (LECS) were 5060 (95%CI: 4250-9640), 12140 (6050-14110), and 6910 (5320-12520) in Non systemic lavage group, 4400 (3660-7620), 8910 (6480-10980), and 5950 (4840-7860) in Systemic lavage+balloon occlusion group. Significant differences between two groups at the day after LECS (P = 0.029) and the 3 d after LECS (P = 0.042). CRP levels in Non systemic lavage group and in Systemic lavage+balloon occlusion group were significantly different at the day after LECS (P = 0.005) and the 3 d after LECS (P = 0.028). BTs (°C) in Non systemic lavage group and in Systemic lavage+balloon occlusion group were also significantly different at the day after LECS (P = 0.004) and the 3 d after LECS (P = 0.006). In a logarithmic comparison, bacterial load before gastric lavage, after lavage, and ascites culture were 6.08 (95%CI: 4.04-6.97), 0.48 (0-0.85), and 0.21 (0-0.56). The bacterial counts before and after gastric lavage were significantly suppressed (P = 0.007), but no significant difference between gastric juice culture after lavage and ascites (P = 0.154).
CONCLUSION: Pre-LECS lavage with 2000 mL of saline exhibited a bacteria-reducing effect equivalent to disinfectants and obtained favorable results in terms of clinical symptoms and data.
Core tip: Although laparoscopic and endoscopic cooperative surgery (LECS) is a safer minimally invasive surgery that utilizes the advantages of both flexible endoscopy and laparoscopy, LECS invariably involves exposure to bacteria in the oral cavity because the flexible endoscope is passed through the oral cavity into the stomach. Nevertheless, there was no report how to disinfect the digestive tract more effectively. In this study, we established the systematic disinfection procedures of digestive tract lavage, endoscope disinfection and clean procedure of endoscopist and assistants in preparation for more minimal invasive flexible endoscopic surgery in near future.
- Citation: Mori H, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, Ayaki M, Yachida T, Tani J, Miyoshi H, Morishita A, Masaki T. Reduction effect of bacterial counts by preoperative saline lavage of the stomach in performing laparoscopic and endoscopic cooperative surgery. World J Gastroenterol 2014; 20(42): 15763-15770
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15763
Recent years have seen an increase in the number of reported cases of laparoscopic and endoscopic cooperative surgery (LECS), a minimally invasive surgery that utilizes the advantages of both flexible endoscopy and laparoscopy to treat gastrointestinal stromal tumors (GIST) and other gastric submucosal tumors not requiring lymph node dissection. LECS is now covered by insurance in Japan. With the ingenuities of endoscopists and surgeons, various techniques have been reported for LECS[1-3]. In approaches to a target organ through a skin incision, as with surgery, indigenous bacteria, Mycobacterium tuberculosis, filamentous fungi, spore-forming bacteria, viruses, etc., become causative organisms of infection; thus, surgical disinfection has been performed to prevent contamination of the surgical field[4], reducing the resident bacteria from 10[4,5] to approximately 10[2,3].
LECS invariably involves exposure to bacteria in the oral cavity because the flexible endoscope is passed through the oral cavity into the stomach; however, no study has reported methods of disinfection or lavage that systematically reach inside the stomach from the oral cavity. LECS entails exposure of the flexible endoscope from the digestive lumen to inside the abdominal cavity; however, whether iodine disinfection, as in surgery, lavage with saline, or other disinfection methods is effective is unreported and unknown.
Natural orifice transluminal endoscopic surgery (NOTES) is a procedure that involves perforation of the stomach wall. In animal experiments, a transgastric route in NOTES is also a means for predicting intraperitoneal infection upon endoscopic submucosal dissection (ESD) perforation[5]. The present study is a comparative trial that aimed to verify the effects of gastric lavage with 2000 mL of saline in LECS on the gastric and intraperitoneal bacterial loads.
A comparative study between the former term and the latter term was conducted with twenty two patients who were diagnosed with a gastric GIST between September 2009 and December 2013 at Kagawa University Hospital and Ehime Rosai Hospital underwent LECS. In former term, between September 2009 and August 2011, twelve patients were performed LECS without systematic disinfection of flexible endoscope and endoscopist before LECS. All patients with observed bacterial infection with Helicobacter pylori (H. pylori) were preoperatively eradicated. A dose of 30 mg of the proton pump inhibitor (PPI) lansoprazole was administered once daily from the day before surgery. In this former term, we didn’t sterilize endoscopes using during LECS with ethylene oxide (EtO) gas. We used only 2% glutaraldehyde washing to disinfect flexible endoscope which was recommended by Japanese Gastroenterological Society (JGES) officially. Irrigations of the stomach were conducted whenever it was necessary, not systematically (Non systemic lavage group).
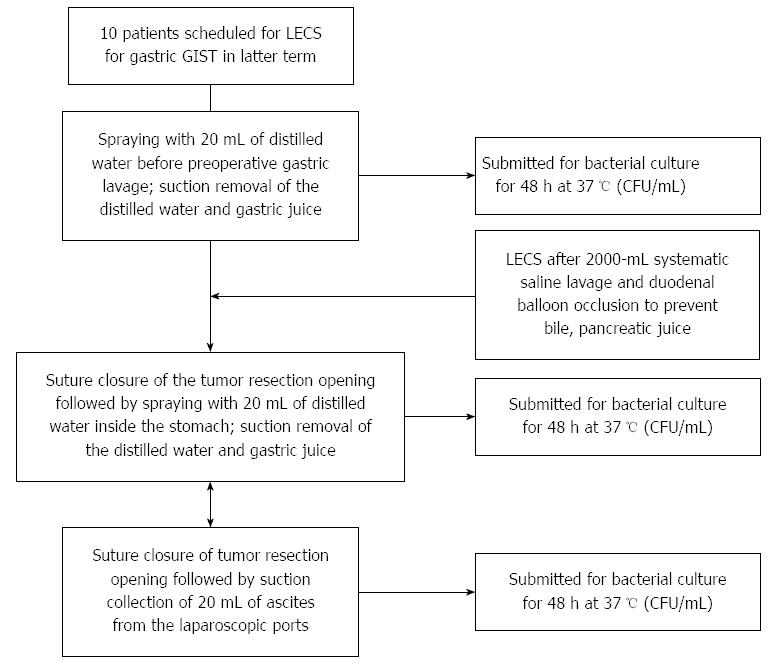
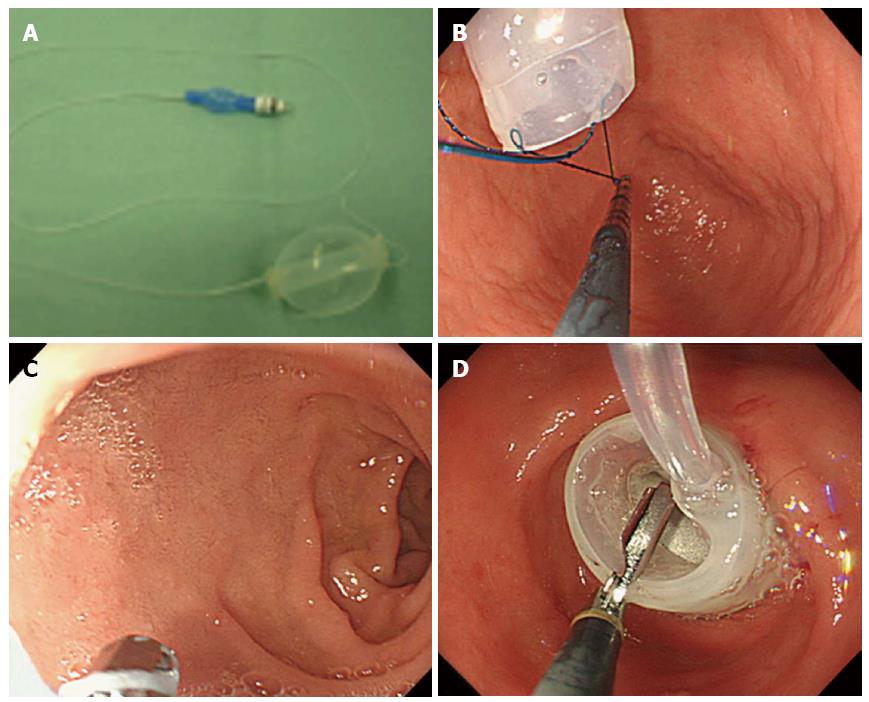
Figure 1 shows a flowchart of irrigation and pre- and post- LECS gastric juice sampling for bacterial culture in latter term between September 2011 and December 2013. A dose of 30 mg of the proton pump inhibitor (PPI) lansoprazole was administered once daily from the day before surgery. The patinets gargled and rinsed their mouths five times with iodine the day before surgery; on the day of the LECS, they similarly gargled and rinsed their mouths 3 h before and 30 min before surgery. In addition to the iodine mouth gargle, the patients performed a throat gargle with 0.45% povidone iodine solution diluted with purified water, which is used for throat gargling in routine clinical practice. The inside of the stomach was evenly sprayed with 20 mL of distilled water before the start of the LECS, and 20 mL of gastric juice was collected in a sterile tube and submitted for stomach bacterial culture before gastric lavage. The inside of the stomach was thoroughly cleaned with 2000 mL of saline using an endoscope with a water jet (GIF Q260J, Olympus, Tokyo, Japan). Duodenal balloon occlusion was conducted as follows: A nylon loop was made at the tip of the balloon to facilitate firm grasping. The balloon was passed through to the bulbus duodeni. The balloon was inserted into the descending part of the duodenum and pulled up to the bulbus. The balloon was inflated and fixed in place by injecting it with 60-70 mL of air to prevent refluxed bile and pancreatic juice (Figure 2).
During insertion from the esophagus to the stomach through an overtube, the digestive tract was cleaned carefully with 2000 mL of saline, and then all of the lavage solution was suctioned out. Next, during the removal of the endoscopes from the stomach, lavage was performed along the esophagus and overtube. The face, mouthpiece, and areas around the mouth were also disinfected with iodine. To begin sterile operation, the gas-sterilized endoscope was replaced by the endoscopic surgeon and assistants after washing their hands (Systemic lavage + balloon occlusion group).
The patients were placed in a supine position, and faces oriented slightly leftward. A 15-mm incision was made in the umbilicus through a laparoscopic port for camera port insertion. Two 12-mm ports were inserted in the upper left and right umbilicus, and a 5-mm port was inserted in the upper left abdomen in an inverted trapezoidal shape. The stomach periphery was observed, and where necessary, the greater omentum was also treated. For cardia and posterior wall lesions, the lesser omentum was dissected to open omental bursa, and gauze was inserted to protect the dorsal organs. Two oral endoscopes sterilized with EtO gas were used. Endoscope insufflation with CO2 was used all cases. A safety margin of approximately 8-10 mm around the tumor was marked for the incision with Dual Knife (Olympus, Tokyo, Japan). Mucosal local injection was made with equal amount hyaluronic acid and Glyceol with 0.5 mL of indigo carmine. An incision was made through the deepest layers of the submucosa using ESD, leaving only the muscle layer. After full-thickness resection of the gastric wall, the tumors were excised transorally with the flexible endoscopes with assistive laparoscopy.
After collection of the resected tumor, without lavage, the gastric wall was sprayed with 20 mL of distilled water, and 20 mL of gastric juice was collected in a sterile tube and submitted for culture. In addition, 20 mL of ascites was collected from the laparoscopic ports and submitted for culture without lavage. These samples were cultured in the Brain Heart Infusion pure culture media (37 °C, 48 h). In addition, after we took samples from formed colonies, we cultured these samples in blood agar media (37 °C, 24 h). We detected bacterial strains using the Gram Positive and Gram negative detect card: VITEK 2® (BIOMERIEUX Co., Tokyo, Japan). Cultured bacterial loads were converted to logarithmic representation and compared.
All LECS were performed only by Dr. H. Mori and all the procedure of LECS were not changed since we previously reported[6]. We changed only the pre-operative disinfection methods such as non-systemic lavage and systemic lavage.
Endoscopes: The endoscopes used were GIF types Q260J and H260Z (Olympus). All the endoscopes were sterilized with EtO gas. Operations using a flexible endoscope were performed aseptically.
Incision knife: Dual Knife (KD-650L, Olympus) and IT knife 2 (KD-611L, Olympus) Hemostatic forceps: Coagrasper (FD-410LR, OLYMPUS, Tokyo, Japan) Tip attachment: Elastic Touch Attachment (TOP co., Tokyo, Japan) Overtube: Split Barrel (TOP co, Tokyo, Japan) CO2 insufflation device: UCR (OLYMPUS, Tokyo, Japan).
The present clinical study was conducted with preapproval by the institutional ethics committee of Kagawa University Hospital, Kagawa, Japan, and was enrolled in the university hospital medical information network (No. 000008691).
Temporal change in the bacterial load in the gastric juice and ascites cultures before and after LECS, during which gastric systematic saline lavage and duodenal balloon occlusion was performed or not.
White blood cell (WBC) and C-reactive protein (CRP) values before LECS and on postoperative days 1 and 3, with gastric saline lavage or not. Change in body temperature (BT) before LECS and on postoperative days 1 and 3, with gastric saline lavage or not.
Patient baseline statistics were analyzed using the unpaired t-test and chi square test. All values were presented as median (95%CI). P < 0.05 was considered statistically significant. The multidata from the comparison of the WBC, CRP, and BT values were analyzed using the unpaired t-test at a significant level of 5%. Data and statistical analyses were performed using GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, CA, United States).
Table 1 shows patient baseline such as the ages, sexes, excision sites, excision diameter, operation time, and length of hospital stay, and there were no significant differences between two groups.
| Non systemic lavage group (n = 12) | Systemic lavage + balloon occlusion group (n = 10) | P value | |
| Age (yr), (range) (mean ± SD) | 52-78 (69.7 ± 8.8) | 48-76 (66.3 ± 9.2) | 0.3261 |
| Sex; Male/Female | 3/9 | 2/8 | 0.6992 |
| Location; U/M/L | 6/5/1 | 5/5/0 | 0.1662 |
| Operation time (min) | 128-249 (146.3 ± 83.8) | 112-216 (131.6 ± 63.6) | 0.0871 |
| Resected specimen (mm) | 28-43 (38.7 ± 14.0) | 26-40 (32.9 ± 16.3) | 0.7931 |
| Hospitalization duration (d) | 10 (7-13) | 9 (8-12 ) | 0.8241 |
Table 2 shows median changes and 95%CI in the WBC count, CRP and BT of both groups, the day before LECS, the day after LECS, and 3 d after LECS.
| Non systemic lavage group (n = 12) | Systemic lavage + balloon occlusion group (n = 10) | P value (unpaired t-test) | ||
| WBC (/μL) median (95%CI) | Day before LECS | 5060 (4250-9640) | 4400 (3660-7620) | 0.125 |
| Day after LECS | 12140 (6050-14110) | 8910 (6480-10980) | 0.029 | |
| LECS day 3 | 6910 (5320-12520) | 5950 (4840-7860) | 0.042 | |
| CRP (mg/mL) median (95%CI) | Day before LECS | 0.08 (0.02-0.52) | 0.03 (0.01-1.10) | 0.158 |
| Day after LECS | 5.49 (3.56-7.51) | 2.21 (0.39-5.17) | 0.005 | |
| LECS day 3 | 5.35 (0.67-20.1) | 2.17 (0.14-3.90) | 0.028 | |
| BT (°C) median (95%CI) | Day before LECS | 36.4 (36.1-37.0) | 37.1 (36.5-37.4) | 0.071 |
| Day after LECS | 38.1 (37.6-38.5) | 37.6 (36.8-38.6) | 0.004 | |
| LECS day 3 | 37.6 (36.0-38.0) | 37.1 (36.3-37.4) | 0.006 |
The median changes in the WBC count (/μL) before, 1 d after, and 3 d after LECS were 5060 (95%CI: 4250-9640), 12140 (6050-14110), and 6910 (5320-12520), respectively in Non systemic lavage group, on the other hand, the WBC count (/μL) before, 1 d after, and 3 d after LECS were 4400 (95%CI: 3660-7620), 8910 (6480-10980), and 5950 (4840-7860), respectively in Systemic lavage+ balloon occlusion group. There were significant differences between two groups at the day after LECS (P = 0.029) and the 3 d after LECS (P = 0.042). The median changes in the CRP levels (mg/mL) the day before, the day after, and 3 d after LECS were 0.08 (95%CI: 0.02-0.52), 5.49 (3.56-7.51), and 5.35 (0.67-20.1) in Non systemic lavage group, on the other hand, the CRP levels (mg/mL) before, 1 d after, and 3 d after LECS were 0.03 (95%CI: 0.01-1.10), 2.21 (0.39-5.17), and 2.17 (0.14-3.90), respectively in Systemic lavage+ balloon occlusion group. There were significant differences between two groups at the day after LECS (P = 0.005) and the 3 d after LECS (P = 0.028). The median changes in the BTs (°C) the day before, the day after, and 3 d after LECS were 36.4 (95%CI: 36.1-37.0), 38.1 (37.6-38.5), and 37.6 (36.0-38.0) in Non systemic lavage group, on the other hand, the median changes in the BTs (°C) before, 1 d after, and 3 d after LECS were 37.1 (95%CI: 36.5-37.4), 37.6 (36.8-38.6), and 37.1 (36.3-37.4), respectively in Systemic lavage+ balloon occlusion group. There were significant differences between two groups at the day after LECS (P = 0.004) and the 3 d after LECS (P = 0.006).
Table 3 shows the actual results of bacterial cultures. In a logarithmic comparison of the changes in bacterial load in the gastric juice culture before gastric lavage, gastric juice culture after wound suture closure, and ascites culture from the ports after wound suture closure, the median values obtained were 6.08 (95%CI: 4.04-6.97), 0.48 (0-0.85), and 0.21 (0-0.56), respectively. The bacterial counts in the gastric juice culture before gastric lavage and after suture closure were significantly suppressed (P = 0.007). The bacterial counts in the gastric juice culture before gastric lavage and the ascites culture after suture closure were also significantly reduced (P = 0.007), but no significant difference was observed in the bacterial counts in the gastric juice culture and ascites culture after suture closure (P = 0.154; Figure 3).
| Case No. | Gastric juice culture before gastric lavage (CFU/mL) | Gastric juice culture after suture closure (CFU/mL) | Ascites culture after suture closure (CFU/mL) |
| 1 | 1.5 × 106 | 1 | 7 |
| 2 | 2.1 × 105 | 7 | 1 |
| 3 | 1.3 × 104 | 1 | 1 |
| 4 | 1.5 × 104 | 3 | 3 |
| 5 | 1.1 × 104 | 2 | 1 |
| 6 | 9.4 × 106 | 4 | 1 |
| 7 | 1.2 × 106 | 3 | 1 |
| 8 | 2.5 × 105 | 5 | 2 |
| 9 | 1.8 × 104 | 1 | 1 |
| 10 | 1.0 × 106 | 3 | 1 |
There was no report with regard to relationships between systematic lavage from oral cavity to stomach before LECS and the preoperative and postoperative changes in the gastric juice and ascites bacterial loads, the clinical data, and the incidence of fever. Before and after LECS for GISTs < 40 mm, which is the limit size retrieved from the oral cavity, gastric lavage clearly lowered the gastric bacterial load, and the bacterial counts in the gastric juice and ascites cultures after suture closure were significantly suppressed equivalently in 48 h of culture at 37 °C. The bacteria detected in the cultures included Streptococcus salivarius, S. mitis, S. mitior, S. mutans, Porphyromonas gingivalis, Bacterionema matruchotii, and Propionbacterium acnes, which are normal and representative resident flora of the oral cavity. The oral cavity showed no substantial change in the predominant species in terms of the distribution of these resident flora. We detected Gram - positive coccus such as Streptococcus salivarius, Streptococcus mitis, Streptococcus mitior by Gram staining which are normal and representative resident flora of the oral cavity, and there were any differences in bacterial type detected in gastric juice and ascites cultures.
Regarding the risk of infection, the pull technique can induce fistula infection or peritonitis by the resident flora of the oral cavity, which has occurred during per oral gastrostomy (PEG), but with the push technique, complications of fistula infection and peritonitis are rare. Therefore, the risk for infection with the resident flora of the oral cavity cannot be ignored[7]. This suggests that although the bacteria in the oral cavity can cause infection, bacteria in the stomach cannot cause intraperitoneal infection in the presence of the strongly acidic gastric juices. Normal gastric juice has a pH of ≤ 3, and almost all bacteria cannot grow inside the stomach; however, gastric migration of resident flora of the oral cavity via PPI administration and feeding tubes has been reported to cause gastric bacterial infection and growth[8]. With regard to LECS, considering the exposure of gastric juice which damages abdominal organs, PPI is administered to suppress the gastric acid. This indicates that the resident flora of the oral cavity are transferred to the inside of the stomach via the endoscope insertion, leading to an increase in the population of the resident flora of the oral cavity in the stomach, which have surgical site infection to some extent. In the present study, for the actual bacterial loads, gastric lavage with 2000 mL of saline caused the suppression of the bacterial count in case 6, who had the greatest bacterial count before gastric lavage, from a large number at 9.4 million/mL of gastric juice to as low as 4 CFU/mL of gastric juice and 1 CFU/mL of ascites with 2000-mL saline lavage. With these resident flora of the oral cavity, growth can be adequately suppressed with the bacterial count-suppressing effects of lavage and with antibiotic administration, but patients infected with H. pylori, a gram-negative bacterium with a toxic outer membrane and high pathogenicity[9], are thought to all require preoperative eradication before bacterial exposure of the abdominal cavity.
In other reports, 500-mL saline lavage and irrigation with 200 mL of 5% Betadine obtained by diluting povidone iodine (Betadine) with purified water was reported in pig experiments to have reduced 15-17 × 103 CFU/mL bacterial load in gastric juice culture before lavage to as low as 0-3 CFU/mL, making it possible to suppress adhesion and abscess formation after NOTES[10]. Gastric lavage with a povidone iodine solution via a transgastric route in humans has been reported to be effective, but no research has systematically addressed infection. Reports indicate that no evident adhesion or abscess formation occurred with gastric lavage with povidone iodine solution or 500-mL saline, and whether either has a reliable effect in reducing bacterial load is unknown[11,12].
We previously reported a prospective randomized trial of the effects of reducing gastric bacteria with or without gastric lavage (a clean group with gastric lavage and a regular group without gastric lavage) in 50 cases of ESD for early gastric cancer. In that study, the clean group had a median gastric juice bacterial load of 6.50 (95%CI: 3.88-8.11) before gastric lavage and 1.69 (0.84-3.68) after ESD completion after gastric lavage, indicating that the bacterial load in the gastric juice culture was significantly reduced by the 2000-mL saline gastric lavage, a result similar to that of the present study.
However, with ESD, the WBC counts before surgery and days 1 and 2 after surgery in the clean group were not significantly elevated (H = 4.99, P = 0.08), with a preoperative median of 5140 (95%CI: 3240-7590) and a postoperative median of 6460 (3241-7900) on day 1 and 5530 (3243-7500) on day 2; moreover, the CRP levels were not significantly different among the three groups (H = 7.06, P = 0.06), with a preoperative median of 0.09 (95%CI: 0.01-0.87) and a postoperative median of 0.27 (0.01-1.34) on day 1 and 0.37 (0.01-2.50) on day 2[13], a result unlike the significant increase in WBC count and CRP level in the present study. One factor for this could be surgical stress, which reportedly causes a significant elevation in WBC count and CRP level during open surgery, which results in a large surgical wound. In reports on open and laparoscopic cholecystectomies[14,15], ESD does not involve abdominal wall destruction or a full-thickness resection that includes the muscle layer. By contrast, LECS does cause some surgical stress due to the full-thickness resection with the abdominal wall wounds caused by inserting four ports. The bacterial count after LECS was also almost zero, indicating that the slight increases in the WBC count and CRP level were not associated with fever, which explains the difference between ESD and LECS in terms of surgical stress.
Reports on gastric lavage also indicated that 500-mL saline significantly reduced the bacterial load in gastric juice cultures with respect to the placement of biologic mesh used in the treatment of abdominal wall herniation by transgastric NOTES in a pig experiment[16,17].
The povidone iodine solution exhibited a bactericidal effect because of the oxidizing property of iodine, but simultaneously caused tissue damage to normal living cells. Gastric lavage with 5% povidone iodine solution undeniably causes gastric mucosal damage, although there is no report to support this. A 2000 mL saline gastric lavage yielded results similar to those after gastric lavage with iodine. While the extent of gastric mucosal damage caused by ≥ 5% concentrations of iodine is unknown, gastric lavage using 2000 mL of saline appears valid in terms of safety.
In conclusion, preoperative lavage with 2000 mL of saline for LECS exhibited a bacteria-reducing effect equivalent to disinfection using disinfectants and obtained favorable results in terms of clinical symptoms and data. Moreover, LECS seems to be a sterile operation with the use of flexible endoscopes, which allows for a systematic implementation of LECS.
Laparoscopic and endoscopic cooperative surgery (LECS) entails exposure of the flexible endoscope through the oral cavity to inside the abdominal cavity; however, there was no report how to disinfect the digestive tract more effectively.
In open surgery, to approach a target organ through a skin incision, as indigenous bacteria, Mycobacterium tuberculosis, filamentous fungi, spore-forming bacteria, viruses, etc., become causative organisms of infection, surgical disinfection has been performed to prevent contamination of the surgical field. LECS invariably involves exposure to bacteria in the oral cavity because the flexible endoscope is passed through the oral cavity into the stomach.
Natural orifice transluminal endoscopic surgery (NOTES) is a procedure that involves perforation of the stomach wall. In animal experiments, a transgastric route in NOTES is also a means for predicting intraperitoneal infection. The present study is a comparative trial that aimed to verify the effects of gastric lavage with 2000 mL of saline in LECS on the gastric and intraperitoneal bacterial loads.
Gastric lavage with 2000 mL of saline in LECS is best way to reduce the bacterial counts and to prevent post-operative surgical site infection from the result of gastric juice and ascites bacterial culture as well as clinical data such as WBC, CRP and BT.
LECS is a minimally invasive surgery that utilizes the advantages of both flexible endoscopy and laparoscopy.
The authors examined and established the systematic disinfection procedures of digestive tract lavage, endoscope disinfection and clean procedure of endoscopist and assistants in preparation for more minimal invasive flexible endoscopic surgery in near future.
| 1. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [PubMed] |
| 2. | Hoteya S, Haruta S, Shinohara H, Yamada A, Furuhata T, Yamashita S, Kikuchi D, Mitani T, Ogawa O, Matsui A. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. 2014;26:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol. 2013;19:5720-5726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51:1-45, quiz CE1-CE4. [PubMed] |
| 5. | Guarner-Argente C, Beltrán M, Martínez-Pallí G, Navarro-Ripoll R, Martínez-Zamora MÀ, Córdova H, Comas J, de Miguel CR, Rodríguez-D’Jesús A, Almela M. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Mori H, Kobara H, Kobayashi M, Muramatsu A, Nomura T, Hagiike M, Izuishi K, Suzuki Y, Masaki T. Establishment of pure NOTES procedure using a conventional flexible endoscope: review of six cases of gastric gastrointestinal stromal tumors. Endoscopy. 2011;43:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Akkersdijk WL, van Bergeijk JD, van Egmond T, Mulder CJ, van Berge Henegouwen GP, van der Werken C, van Erpecum KJ. Percutaneous endoscopic gastrostomy (PEG): comparison of push and pull methods and evaluation of antibiotic prophylaxis. Endoscopy. 1995;27:313-316. [PubMed] |
| 8. | Heyland D, Bradley C, Mandell LA. Effect of acidified enteral feedings on gastric colonization in the critically ill patient. Crit Care Med. 1992;20:1388-1394. [PubMed] |
| 9. | Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 502] [Article Influence: 38.6] [Reference Citation Analysis (2)] |
| 10. | Zheng YZ, Wang D, Gu JJ, Zhou MM, Yu Kong X, Xin Deng S, Ju Su X, Yin J, Gong YF, Wu RP. An experimental study of betadine irrigation for preventing infection during the natural orifice transluminal endoscopic surgery (NOTES) procedure. J Dig Dis. 2011;12:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Rao GV, Reddy DN, Banerjee R. NOTES: human experience. Gastrointest Endosc Clin N Am. 2008;18:361-370; x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Steele K, Schweitzer MA, Lyn-Sue J, Kantsevoy SV. Flexible transgastric peritoneoscopy and liver biopsy: a feasibility study in human beings (with videos). Gastrointest Endosc. 2008;68:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Mori H, Kobara H, Rafiq K, Nishiyama N, Fujihara S, Oryu M, Masaki T. Effects of gastric irrigation on bacterial counts before endoscopic submucosal dissection: a randomized case control prospective study. PLoS One. 2013;8:e65377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Landman J, Olweny E, Sundaram CP, Chen C, Rehman J, Lee DI, Shalhav A, Portis A, McDougall EM, Clayman RV. Prospective comparison of the immunological and stress response following laparoscopic and open surgery for localized renal cell carcinoma. J Urol. 2004;171:1456-1460. [PubMed] |
| 15. | Luo K, Li JS, Li LT, Wang KH, Shun JM. Operative stress response and energy metabolism after laparoscopic cholecystectomy compared to open surgery. World J Gastroenterol. 2003;9:847-850. [PubMed] |
| 16. | Miedema BW, Bachman SL, Sporn E, Astudillo JA, Thaler K. Transgastric placement of biologic mesh to the anterior abdominal wall. Surg Endosc. 2009;23:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
P- Reviewer: Liu ZW, Park WS S- Editor: Qi Y L- Editor: A E- Editor: Liu XM