Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15664
Revised: April 9, 2014
Accepted: June 26, 2014
Published online: November 14, 2014
Processing time: 264 Days and 12.5 Hours
Wireless capsule endoscopy (CE) is a technology developed for the endoscopic exploration of the small bowel. The first capsule model was approved by the Food and Drug Administration in 2001, and its first and essential indication was occult gastrointestinal (GI) bleeding. Over subsequent years, this technology has been refined to provide superior resolution, increased battery life, and capabilities to view different parts of the GI tract. Indeed, cases for which CE proved useful have increased significantly over the last few years, with new indications for the small bowel and technical improvements that have expanded its use to other parts of the GI tract, including the esophagus and colon. The main challenges in the development of CE are new devices with the ability to provide therapy, air inflation for a better vision of the small bowel, biopsy sampling systems attached to the capsule and the possibility to guide and move the capsule with an external motion control. In this article we review the current and new indications of CE, and the evolving technological changes shaping this technology, which has a promising potential in the coming future of gastroenterology.
Core tip: In this article we present a review of the actual devices and indications of capsule endoscopy. We deal with current and well established indications and with the novel applications of this technology, which being minimally invasive, has a great perspective for technical improvements and clinical applications. Besides dealing with the new and more controversial indications, we review novel devices, some still under development, which will probably achieve worldwide application in the forthcoming years.
- Citation: Redondo-Cerezo E, Sánchez-Capilla AD, De La Torre-Rubio P, De Teresa J. Wireless capsule endoscopy: Perspectives beyond gastrointestinal bleeding. World J Gastroenterol 2014; 20(42): 15664-15673
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15664.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15664
Before the development of capsule endoscopy (CE) the small bowel could be explored only by invasive procedures (intraoperative enteroscopy) or poorly effective methods, such as small bowel series. The widespread availability of CE, which allows a better mucosal visualization with few complications, has elicited a revolution in small bowel endoscopy, and a significant increase in the indications of CE.
The first CE indication was obscure gastrointestinal (GI) bleeding (OGIB), with two key reports published in 2001 and 2002[1]. Since then, technical improvements and increasing clinical experience have led to many studies that analyzed the efficacy of CE in this setting, and its role in the diagnostic algorithm for OGIB.
However, technical improvements and clinical considerations have broadened the range of applicability for CE, including examination of segments of the GI tract other than the small bowel, including the colon and esophagus, which are within reach of conventional endoscopy, but can benefit from increased safety and comfort with CE.
In this review article we will to evaluate the current and novel applications of CE, with focus on the likely expansion of this established but still promising technology to many other fields (Table 1).
| PillCam SB3 | EndoCapsule | MiroCam | OMOM capsule | CapsoCam SV-1 | |
| Length, mm | 26 | 26 | 24 | 28 | 31 |
| Diameter, mm | 11 | 11 | 11 | 13 | 11 |
| Weight, g | 3.4 | 3.8 | 3.4 | 6 | - |
| Frame rate, frames/s | 2-6 | 2 | 3 | 2 | 12-20 (3-5 per camera) |
| Image sensor | CMOS | CCD | CMOS | CMOS | - |
| Field of view | 156° | 145° | 150° | 140° | 360° |
| Illumination | 6 white LEDs | 6 white LEDs | 6 white LEDs | NA | 16 white LEDs |
| Antenna (body leads), n | 8 | 8 | 9 | 14 | NA |
| RT view | RT viewer | VE-1 viewer | Miro Viewer | Real-timer monitoring | - |
| Recording time, h | 8 | 8 | 11 | 6-8 | 15 |
| Image transmission | RF | RF | HBC | RF | CapsoView |
Actually, there are four different manufacturers for small bowel CE devices that have the following common technical features: (1) the capsule, which contains the camera, with differences in size, vision angle, battery life, etc.; (2) the reception system, which includes an antenna array capable of surrounding a body to receive the transmitted video output, a data recorder, and a battery. Everything is set on a belt attached to the patient that holds the entire device; and (3) the workstation, which is a computer used for processing and evaluation of the downloaded images, contained in the data recorder and transformed into a video datastream.
Improvements in these systems have led to better image quality and battery duration, which have increased the diagnostic yield. The currently available CE devices are described below.
PillCam SB3 (Given Imagin Ltd. Yoqnean, Israel): It was the first CE device approved by the FDA, in August 2001 (M2A), and it was followed soon by its second version, M2A plus, and by the PillCam SB series, its third version (PillCam SB3). This new version has a better resolution and an auto adjustable speed of frame acquisition depending on the capsule’s speed of progression in the small bowel. The associated software (Rapid Reader v8) offers improvements, such as the possibility of visualizing several different frames at the same time, the ability to measure lesion’s size, and a detector of bleeding lesions[2] the capability to apply digital light filters to perform electronic chromoendoscopy (FICE, Fuji Intelligent Chromo Endoscopy) for the better characterization of mucosal abnormalities, and an atlas for real-time comparisons.
EndoCapsule (Olympus Corporation, Allentown, PA): The FDA approved this new CE device in 2007. It is similar to PillCam SB and incorporates a blood indicator that marks suspicious bleeding points along the small bowel. It has an automatic control of reproduction speed, and four simultaneous different frames can be visualized at the same time on the screen.
MiRo (IntroMedic Co., Seoul, South Korea): This device was available in many countries between 2007 and 2009, and was approved by the FDA in 2013. This device has a different system of data transmission through the patient’s own tissues[3], allowing an increased battery life and time for frames acquisition. One trial showed similar diagnostic yield and complete small bowel examinations between EndoCapsule and MiRo in 50 patients[4].
ONOM (Jinshan Science and Technology Company, Chongqin, China): Despite the higher size and weight of this capsule, it has widespread applications because of its cost-effectiveness.
CapsoCam SV1 (CapsoVision Inc. Saratoga, CA): This device offers a novel concept, with 4 cameras, a peak acquisition speed of 5 frames/s and a 360º view of the small bowel. The images are loaded into the capsule, without the requirement of an external receptor; however the capsule has to be recovered by the patient and connected to the workstation. In a multicenter trial[5] with 73 patients that compared this capsule with PillCam SB2, a strong diagnostic concordance was reported; however CapsoCam required a longer video analysis time. Nevertheless, this device offers a better vision of the ampullar area, with good visualization of this particular area reported in 70% patients in a study[6].
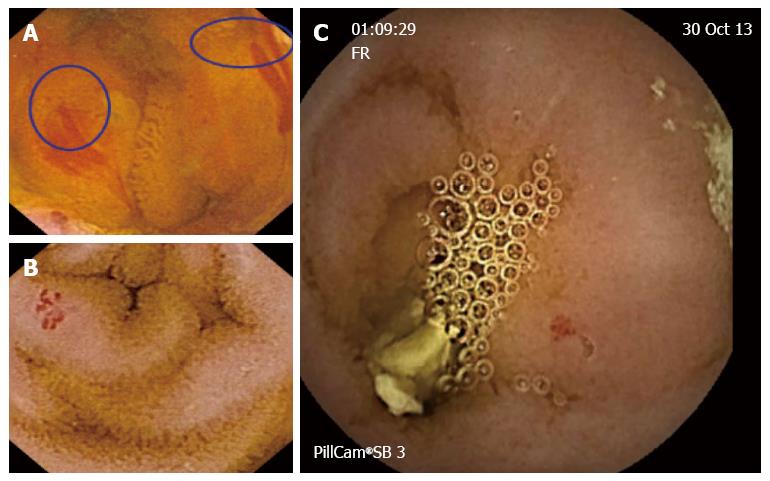
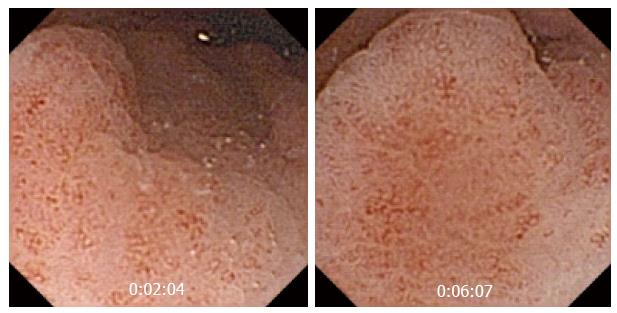
OGIB (Figure 1) (is the first and most common indication for small bowel CE, and shows a better yield. The global diagnostic yield of CE for OGIB ranges between 30% and 70%, which is higher than that of push enteroscopy, double balloon enteroscopy, and small bowel series, with sensitivities of 31%, 23% and 5% respectively. In a 2007 study[7], CE showed its superiority over computed tomography (CT) and angiography in the detection of bleeding lesions, and detected a suspected bleeding source in patients with negative results for two other procedures. CE seems to impact OGIB management and outcomes. In a retrospective study of 75 patients[8], CE diagnosed relevant lesions in 66.7% patients, and 49 (50.7%) of these patients, underwent confirmatory tests and subsequently received specific therapy [surgery, medical therapy, nonsteroidal anti-inflammatory drug (NSAID) withdrawal].
The diagnostic yield of CE for OGIB increases when the procedure is performed in the first 48 h after bleeding onset[9,10]. Other recognized factors related to a higher diagnostic yield include: advanced age, male sex, hospital admission and increased transfusion requirements[11].
The most frequent finding of OGIB is intestinal angiodysplasia (22%). Other causative lesions include: (1) small bowel ulcer (10%); (2) esophago-gastric benign lesions (i.e., esophagitis or gastritis) (11%); (3) blood in the small bowel in the absence an identified lesion (8%); (4) small bowel tumors (7%); and (5) small bowel varices (3%).
Iron deficiency anemia (IDA) is usually determined by blood loss through the GI tract. Therefore, CE is a good method to identify causative lesions, once other common potential bleeding sources located within the reach of upper or lower endoscopy have been ruled out. CE proved its superiority over enteroclysis in a previous study, with a causative lesion identification rate of 57% with the former and 11.8% with the latter[12].
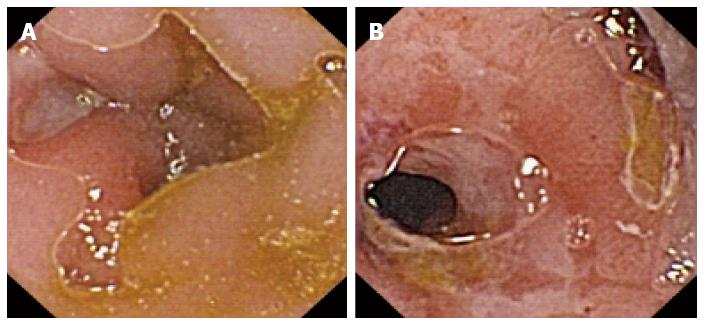
CE plays a role in diagnosing suspected Crohn’s disease (CD) (Figure 2) when the clinical history is compatible with its findings after a normal examination by conventional endoscopy. It also plays a role in small bowel evaluation in patients with indeterminate colitis and disease extension assessment in patients with known CD[13]. The diagnostic yield in this setting is 66%-71% for known CD and 33%-68% for suspected CD[14].
In a 2010 meta-analysis[15], the diagnostic yield for small bowel CD was higher (50%-70%) with CE than with other procedures such as small bowel series (22%), colonoscopy (48%), push enteroscopy (8%), and enteroclysis/CT enterography (31%). In another study[16], CE was compared with magnetic resonance imaging (MRI) enterography and CT enterography, and showed a clearly higher sensitivity and specificity.
Apart from allowing diagnostic confirmation and evaluation of CD extension, CE can be used to appraise disease activity and severity, facilitating therapeutic modifications with the intention to achieve mucosal healing, which has a direct impact on disease prognosis[17].
On the other hand, CE has been shown to identify patients with a higher likelihood of a flare, with some authors observing that the presence of lesions in the jejunum in otherwise asymptomatic patients predicts a higher risk of a clinical exacerbation in the following two years[18]. CE was also proved to be superior to colonoscopy in the detection of postsurgical recurrence of CD (65% vs 25%), with better patient acceptance and tolerability, and it also even allowed the exploration of the neo-ileum that was not accessible by colonoscopy[19].
The main known complication of CE in patients with CD is capsule retention in strictures, which has been observed in up to 5% patients. Therefore, when a stricture is suspected, a patency capsule should be administered before conventional CE. The other option is to select alternative procedures to study the small intestine, such as CT enterography and MRI enterography[20].
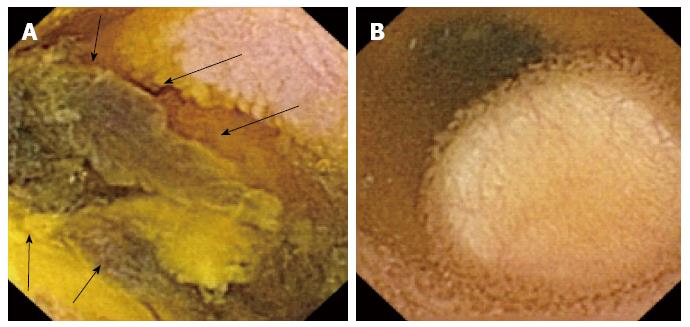
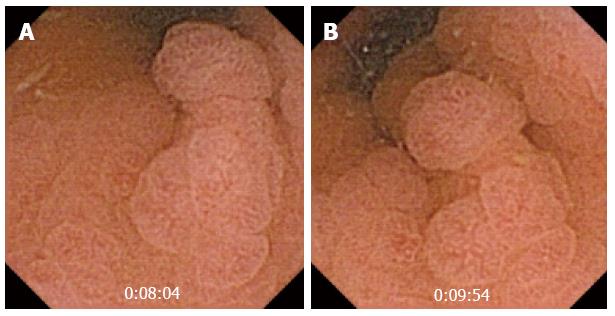
CE is an outstanding method for the detection of small bowel tumors and polyps (Figure 3), and the study of polyps in polyposis syndromes, such as familial adenomatous polyposis (FAP) and Peutz Jeghers syndrome[21] (Figure 4).
The most common presentation of small bowel tumors is OGIB[22]. The most common histopathological type is adenocarcinoma, followed by carcinoid, lymphoma, sarcoma and hamartoma. The most common location is the jejunum (40%-60%), followed by the ileum (25%-40%) and duodenum (15%-25%).
The most commonly occurring benign tumors in the small bowel are inflammatory polyps, lymphangioma, hemangioma, adenoma and lipoma. The most frequent metastatic tumor is melanoma[23], but there also some case reports of metastatic colorectal cancer (CRC) and hepatocellular carcinoma[24].
Vanishing visceral compression that protrudes to the small bowel lumen can confound an inexperienced physician who may misdiagnose it as a subepithelial mass. Some signs, such as well-defined margins, or visualization of the lesion for more than 10 min, increase the likelihood of a true subepithelial mass[25] (Figure 3B).
With regard to small bowel tumors, CE has improved the diagnostic yield of previous procedures[26], allowing an early diagnosis at a lower cost[27]. However, its impact on the management and prognosis of these patients has yet to be proven.
Hereditary polyposis syndromes also affect the small bowel, and the sensitivity and small bowel polyp detection rate are higher with CE than with X-ray series[28], can be considered as an alternative follow-up[29,30]. However, CE tends to underestimate the number of polyps and exhibits poor performance while exploring the ampulla. In a prospective study[31], CE was successful in identifying jejunal or ileal polyps; however it missed the ampullary area in all patients. Therefore, the role of CE in polyposis syndromes has yet to be established.
Other important applications of CE include the diagnosis of patients with suspected B-cell lymphoma; in such cases it can diagnose the condition, assess disease extension, and evaluate the response to chemotherapy[32].
Histology is the gold standard for the diagnosis of celiac disease. Therefore, CE cannot be the primary diagnostic tool in this setting, because of its inability to take biopsies. Nevertheless, CE can identify typical mucosal changes observed in this disease[33], similar to upper endoscopy but with the advantages of the lack of insufflation and the higher image magnification. When compared with histology, CE has a sensitivity of 70% and specificity of 100% for the diagnosis of celiac disease[34]. Therefore, the role of CE is to assess mucosal abnormalities in patients with positive serology but normal histology[35,36], keeping in mind that a normal CE examination does not rule out celiac disease, given its somewhat low negative predictive value (77%)[34].
CE also plays an important role in refractory or complicated celiac disease, allowing the diagnosis of T-cell lymphoma, ulcerative jejunoileitis and adenocarcinoma[37,38].
Graft vs host disease (GVHD) is a severe complication of bone marrow transplantation, and usually requires quick intervention. In most patients, upper endoscopy or colonoscopy with biopsy is required for diagnosis. The role of CE has been evaluated in several studies, with two of them[39,40] reporting relevant findings in regard to acute GVHD. These studies showed a high positive predictive value for CE in patients with suspected GVHD, given that patients with no findings did not develop the disease at the 2 mo follow up.
In conclusion, CE can be as useful as conventional endoscopy and biopsy for the diagnosis of GVHD[41,42].
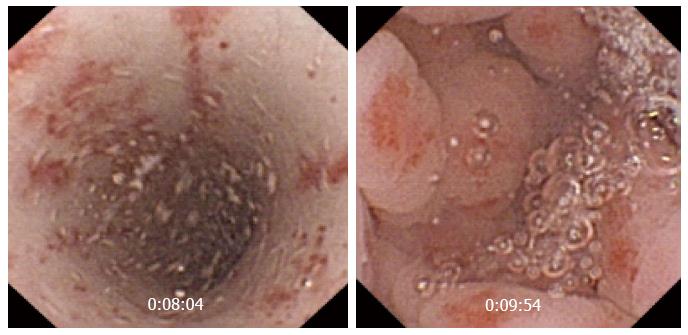
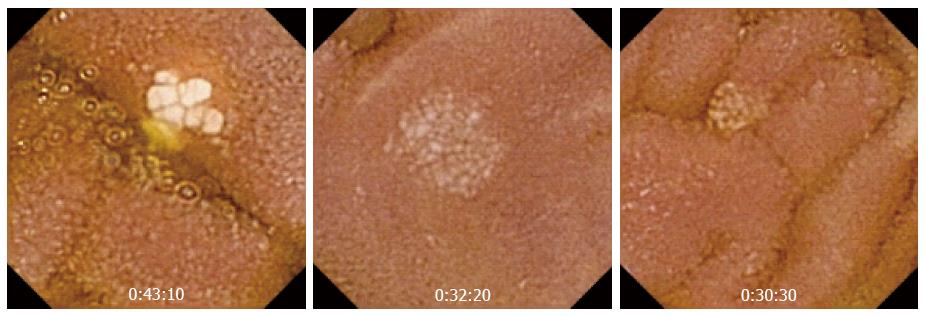
The real clinical impact of CE in this group of patients remains unknown, because up to 44% patients receiving NSAIDs have small bowel lesions. Its role is clearer in patients with OGIB after negative results are obtained in conventional endoscopy. The most common lesions are superficial erosions, petechiae, denudated mucosa, bleeding lesions, and ulcers, etc. Maiden et al[43] showed that CE performed after 2 wk of treatment with NSAIDs and a proton pump inhibitor (PPI) for 40 healthy volunteers detected abnormalities in 65.5% patients, including reddened folds, active bleeding, angiodysplasia, and lymphangiectasia (Figure 5).
CE has shown a low diagnostic yield in patients with abdominal pain (13%) or chronic diarrhea (9%).
From the first few studies, researchers have tried to accurately select cases where CE can demonstrate and improved diagnostic yield. The DEDAP-Plus study[44] comprised 50 patients with abdominal pain and chronic diarrhea. Two independent researchers found relevant findings in 36% and 40% patients, and potentially relevant findings in 14% and 24% patients. In this study patients were classified according to the presence of symptoms or “plus signs” such as weight loss, serum inflammatory markers, chronic anemia, and suspected GI bleeding. Researchers observed an increased diagnostic yield in patients with elevated inflammatory markers (OR = 3.2), with an increase likelihood of CD detection.
In a 2011 multicenter Greek study[45], 72 patients with chronic abdominal pain were evaluated using CE. The global diagnostic yield was 44.4%, ranging from 21.4% in patients with abdominal pain without elevated serum inflammatory markers (CRP, ESR) to 66.7% in patients with altered parameters and 90.1% in patient who also presented with diarrhea. They concluded that elevated serum inflammatory markers are associated with a higher diagnostic yield for CE.
Apart from the abovementioned indications, CE can be useful in other settings with small bowel involvement. Nevertheless, the rarity of those conditions prevents researchers from making general statements on its possible role. Specifically, CE can be useful in diagnosing systemic diseases and vasculitis[46] with small bowel involvement, such as Henoch-Schonlein purpura, Churg-Strauss syndrome and Behçet disease)[47-49].
CE has also been evaluated for use in recipients of small bowel transplantation, for whom ileoscopy is the standard procedure to evaluate rejection. In a 2003 study[50] CE and ileoscopy were used in 5 patients with a prior bowel transplant. CE was better tolerated and provided high quality images of the small bowel in four patients. When the terminal ileum showed no abnormalities with both techniques, CE detected mucosal changes in segments inaccessible by ileoscopy in three patients.
In 2004, Given Imagin Ltd. developed a video capsule (PillCam ESO) for the esophagus, and its third version (PillCam ESO 3) was approved by the FDA in 2011. It has dual cameras that capture 35 frames/s for 30 min. Although its role remains unclear it has been proposed as a minimally invasive procedure for esophageal diseases[50,51] (Figures 6 and 7).
The first PillCam ESO study[52] included 73 patients with gastroesophageal reflux disease and 9 patients with known Barrett’s esophagus who underwent CE followed by standard upper endoscopy. The sensitivity and specificity of CE were 97% and 100% for Barrett’s esophagus and 98% and 100%, respectively for diagnosing esophagitis. However, further cost-effectiveness analyses showed that Barrett’s esophagus screening using PillCam ESO was not cost-effective compared with that using conventional upper endoscopy[53].
On the other hand, some studies point to a role for this capsule as an alternative to the conventional approach in special cases: (1) Patients with gastroesophageal reflux disease[54]; (2) Detection of esophageal varices in patients with cirrhosis. A multicenter study[55] showed its ability to discriminate small and big varices, which may facilitate a specific therapy. Indeed, cost-effectiveness analyses do not support the use of this capsule as a conventional method, which can be reserved for special cases[56]; and (3) In the emergency room, CE shoed better performance compared with a nasogastric tube and similar performance compared with upper endoscopy while determining the presence of an active bleeding, thus demonstrating no therapeutic abilities. Therefore, it is not a true alternative to upper endoscopy in this setting[55-58].
Another important goal for CE developers is the colon. Given Imagin produced the colon capsule (PillCam Colon), and they have now manufactured the second generation of this device. It has dual cameras, enabling it to acquire images from both ends. The angle of view from each imager is 172º. It has been approved in Europe because of its potential role in CCR screening although this remains to be clarified[59-61]. In a 2010 meta-analysis[62] sensitivity and specificity for adenoma and carcinoma detection were 69% and 86%, respectively. A further study[63] observed improved sensitivity and specificity of 88% and 95%, respectively, for polyps measuring ≥ 10 mm, suggesting that CE may be a promising tool for screening, although it needs improvements before becoming an alternative to colonoscopy for CCR screening. This device can also be an alternative when colonoscopy is incomplete, when the patient rejects colonoscopy or when colonoscopy is associated with substantial risks derived from the patient’s condition or comorbidities[64].
In 2012 the European Society of Gastrointestinal Endoscopy (ESGE) introduced guidelines[61] to homogenize clinical practice. Patients with average CRC risk: CE is an alternative for the screening. Patients that a high risk of CRC (alarming symptoms with a family or personal history of CRC): CE is not an alternative, because the probability of finding lesions requiring biopsies or polypectomy is high. Every patient with polyps measuring > 6 mm or with more than three polyps should undergo colonoscopy[65]. Patients without findings on CE should repeat the procedure in 5 years, unless they have poor bowel cleansing. In situations where colonoscopy is not an option, CE can be an alternative, although further studies comparing CE with radiological methods are required.
Finally, CE can be useful for the detection of colonic diverticular disease or mucosal inflammatory changes[63], but no studies have addressed its role in non-neoplastic diseases. There are no objective data to support the use of CE for the diagnosis or follow-up of inflammatory bowel disease. Colon CE has been tested in patients with inflammatory bowel disease, and it exhibited a performance similar to that of colonoscopy[66,67], albeit without the ability to take biopsy samples.
CE has undergone continuous improvements since its first description including better image resolution, an increased number of frames obtained from the explored areas, a longer battery life, and better software for the visualization and management of images.
Apart from the abovementioned CapsoCam SV1, which widened the angle of vision to 360º, there is another device, the Sayaka Capsule (RF Systems Lab Company, Nagano, Japan), which has described in 2005, and has a side camera that rotates, obtaining 30 frames/s. These frames are processed into an extensive series of overlap mosaicing, offering a map of the entire GI tract. The same company designed the Norika capsule, with a lens angled at 75º and a magnetic field based propulsion system.
Capsules with anchoring devices have been developed, allowing for a precise drug delivery into the tract. Various systems are available, such as the one described by Woods[68], with a stopping mechanism that unfolds in 1.8 s and the ability to deliver 1 mL of medication to a target within the small intestines via a 1.5 mm needle.
Two CE systems with remote motion control are under study: (1) External systems, such as magnetic fields that can guide and move the capsule[69-71]; and (2) Internal systems, within the capsule itself, which can move it through the small bowel[71-75].
Peristalsis is a common difficulty faced during exploration of some segments of the small bowel using CE. Inflation in some situations can significantly improve the visualization of these areas. Certain devices are under development, such as the one published by Gorlewicz et al[76], which has, in different compartments, chemical substances that release carbon dioxide when mixed, allowing distension and better small bowel exploration.
CE is a safe and acceptable method for GI tract exploration, and its use is widespread. Although the most common indication for CE is OGIB with suspected origin in the small bowel, there are other situations where it has been used, in other parts of the GI tract that are accessible by standard endoscopy. However, its use should be restricted to patients with risk levels or characteristics that make CE safe and more acceptable.
CE still has two major drawbacks compared with conventional endoscopy: the possibility of external motion control and the inability to treat lesions. Despite this, technological advances in the field may, in the near future, drive CE to become the first choice of modality for the diagnosis, treatment and follow-up of GI tract diseases.
| 1. | Appleyard M, Glukhovsky A, Swain P. Wireless-capsule diagnostic endoscopy for recurrent small-bowel bleeding. N Engl J Med. 2001;344:232-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Buscaglia JM, Giday SA, Kantsevoy SV, Clarke JO, Magno P, Yong E, Mullin GE. Performance characteristics of the suspected blood indicator feature in capsule endoscopy according to indication for study. Clin Gastroenterol Hepatol. 2008;6:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 3. | Bang S, Park JY, Jeong S, Kim YH, Shim HB, Kim TS, Lee DH, Song SY. First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: electric-field propagation. Gastrointest Endosc. 2009;69:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Dolak W, Kulnigg-Dabsch S, Evstatiev R, Gasche C, Trauner M, Püspök A. A randomized head-to-head study of small-bowel imaging comparing MiroCam and EndoCapsule. Endoscopy. 2012;44:1012-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 5. | Pioche M, Vanbiervliet G, Jacob P, Duburque C, Gincul R, Filoche B, Daudet J, Filippi J, Saurin JC; French Society of Digestive Endoscopy (SFED). Prospective randomized comparison between axial- and lateral-viewing capsule endoscopy systems in patients with obscure digestive bleeding. Endoscopy. 2014;46:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Friedrich K, Gehrke S, Stremmel W, Sieg A. First clinical trial of a newly developed capsule endoscope with panoramic side view for small bowel: a pilot study. J Gastroenterol Hepatol. 2013;28:1496-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 7. | Saperas E, Dot J, Videla S, Alvarez-Castells A, Perez-Lafuente M, Armengol JR, Malagelada JR. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 8. | Redondo-Cerezo E, Pérez-Vigara G, Pérez-Sola A, Gómez-Ruiz CJ, Chicano MV, Sánchez-Manjavacas N, Morillas J, Pérez-García JI, García-Cano J. Diagnostic yield and impact of capsule endoscopy on management of patients with gastrointestinal bleeding of obscure origin. Dig Dis Sci. 2007;52:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Lepileur L, Dray X, Antonietti M, Iwanicki-Caron I, Grigioni S, Chaput U, Di-Fiore A, Alhameedi R, Marteau P, Ducrotté P. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol. 2012;10:1376-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Apostolopoulos P, Liatsos C, Gralnek IM, Kalantzis C, Giannakoulopoulou E, Alexandrakis G, Tsibouris P, Kalafatis E, Kalantzis N. Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest Endosc. 2007;66:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Koulaouzidis A, Rondonotti E, Giannakou A, Plevris JN. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012;76:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 12. | Apostolopoulos P, Liatsos C, Gralnek IM, Giannakoulopoulou E, Alexandrakis G, Kalantzis C, Gabriel P, Kalantzis N. The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy. 2006;38:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 14. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-128; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (36)] |
| 15. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 16. | Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (36)] |
| 17. | Maunoury V, Savoye G, Bourreille A, Bouhnik Y, Jarry M, Sacher-Huvelin S, Ben Soussan E, Lerebours E, Galmiche JP, Colombel JF. Value of wireless capsule endoscopy in patients with indeterminate colitis (inflammatory bowel disease type unclassified). Inflamm Bowel Dis. 2007;13:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Flamant M, Trang C, Maillard O, Sacher-Huvelin S, Le Rhun M, Galmiche JP, Bourreille A. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Pons Beltrán V, Nos P, Bastida G, Beltrán B, Argüello L, Aguas M, Rubín A, Pertejo V, Sala T. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Kornbluth A, Colombel JF, Leighton JA, Loftus E. ICCE consensus for inflammatory bowel disease. Endoscopy. 2005;37:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Akin E, Demirezer Bolat A, Buyukasik S, Algin O, Selvi E, Ersoy O. Comparison between Capsule Endoscopy and Magnetic Resonance Enterography for the Detection of Polyps of the Small Intestine in Patients with Familial Adenomatous Polyposis. Gastroenterol Res Pract. 2012;2012:215028. [PubMed] |
| 22. | Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer. 2006;107:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Prakoso E, Selby WS. Capsule endoscopy in patients with malignant melanoma. Am J Gastroenterol. 2007;102:1204-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Spada C, Riccioni ME, Familiari P, Marchese M, Bizzotto A, Costamagna G. Video capsule endoscopy in small-bowel tumours: a single centre experience. Scand J Gastroenterol. 2008;43:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Girelli CM, Porta P, Colombo E, Lesinigo E, Bernasconi G. Development of a novel index to discriminate bulge from mass on small-bowel capsule endoscopy. Gastrointest Endosc. 2011;74:1067-1074; quiz 1115.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Rondonotti E, Pennazio M, Toth E, Menchen P, Riccioni ME, De Palma GD, Scotto F, De Looze D, Pachofsky T, Tacheci I, Havelund T, Couto G, Trifan A, Kofokotsios A, Cannizzaro R, Perez-Quadrado E, de Franchis R; European Capsule Endoscopy Group; Italian Club for Capsule Endoscopy (CICE); Iberian Group for Capsule Endoscopy. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Schwartz GD, Barkin JS. Small-bowel tumors detected by wireless capsule endoscopy. Dig Dis Sci. 2007;52:1026-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Mata A, Llach J, Castells A, Rovira JM, Pellisé M, Ginès A, Fernández-Esparrach G, Andreu M, Bordas JM, Piqué JM. A prospective trial comparing wireless capsule endoscopy and barium contrast series for small-bowel surveillance in hereditary GI polyposis syndromes. Gastrointest Endosc. 2005;61:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Schulmann K, Hollerbach S, Kraus K, Willert J, Vogel T, Möslein G, Pox C, Reiser M, Reinacher-Schick A, Schmiegel W. Feasibility and diagnostic utility of video capsule endoscopy for the detection of small bowel polyps in patients with hereditary polyposis syndromes. Am J Gastroenterol. 2005;100:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Burke CA, Santisi J, Church J, Levinthal G. The utility of capsule endoscopy small bowel surveillance in patients with polyposis. Am J Gastroenterol. 2005;100:1498-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Katsinelos P, Kountouras J, Chatzimavroudis G, Zavos C, Pilpilidis I, Fasoulas K, Paroutoglou G. Wireless capsule endoscopy in detecting small-intestinal polyps in familial adenomatous polyposis. World J Gastroenterol. 2009;15:6075-6079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 32. | Flieger D, Keller R, May A, Ell C, Fischbach W. Capsule endoscopy in gastrointestinal lymphomas. Endoscopy. 2005;37:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Ianiro G, Gasbarrini A, Cammarota G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J Gastroenterol. 2013;19:8562-8570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 34. | Petroniene R, Dubcenco E, Baker JP, Ottaway CA, Tang SJ, Zanati SA, Streutker CJ, Gardiner GW, Warren RE, Jeejeebhoy KN. Given capsule endoscopy in celiac disease: evaluation of diagnostic accuracy and interobserver agreement. Am J Gastroenterol. 2005;100:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Tennyson CA, Green PH. The role of capsule endoscopy in patients with nonresponsive celiac disease. Gastrointest Endosc. 2011;74:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Murray JA, Rubio-Tapia A, Van Dyke CT, Brogan DL, Knipschield MA, Lahr B, Rumalla A, Zinsmeister AR, Gostout CJ. Mucosal atrophy in celiac disease: extent of involvement, correlation with clinical presentation, and response to treatment. Clin Gastroenterol Hepatol. 2008;6:186-93; quiz 125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Culliford A, Daly J, Diamond B, Rubin M, Green PH. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc. 2005;62:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Daum S, Wahnschaffe U, Glasenapp R, Borchert M, Ullrich R, Zeitz M, Faiss S. Capsule endoscopy in refractory celiac disease. Endoscopy. 2007;39:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Neumann S, Schoppmeyer K, Lange T, Wiedmann M, Golsong J, Tannapfel A, Mossner J, Niederwieser D, Caca K. Wireless capsule endoscopy for diagnosis of acute intestinal graft-versus-host disease. Gastrointest Endosc. 2007;65:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Yakoub-Agha I, Maunoury V, Wacrenier A, Couignoux S, Depil S, Desreumaux P, Bauters F, Colombel JF, Jouet JP. Impact of Small Bowel Exploration Using Video-Capsule Endoscopy in the Management of Acute Gastrointestinal Graft-versus-Host Disease. Transplantation. 2004;78:1697-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Shapira M, Adler SN, Jacob H, Resnick IB, Slavin S, Or R. New insights into the pathophysiology of gastrointestinal graft-versus-host disease using capsule endoscopy. Haematologica. 2005;90:1003-1004. [PubMed] |
| 42. | Eisen GM. Using capsule endoscopy to diagnose graft-versus-host disease: seeing is believing? Gastrointest Endosc. 2007;65:410-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 360] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 44. | May A, Manner H, Schneider M, Ipsen A, Ell C. Prospective multicenter trial of capsule endoscopy in patients with chronic abdominal pain, diarrhea and other signs and symptoms (CEDAP-Plus Study). Endoscopy. 2007;39:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Katsinelos P, Fasoulas K, Beltsis A, Chatzimavroudis G, Paroutoglou G, Maris T, Mimidis K, Koufokotsios A, Terzoudis S, Atmatzidis S. Diagnostic yield and clinical impact of wireless capsule endoscopy in patients with chronic abdominal pain with or without diarrhea: a Greek multicenter study. Eur J Intern Med. 2011;22:e63-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Müller-Ladner U. Vasculitides of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2001;15:59-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Fylyk S, Fylyk SN, Safatle-Riberio AV, Neves F, Goncalves CR, Sipahi AM, Ishiok AS, Sakai P, Galvao-Neto M. Small intestine involvement in Behcet’s disease: A capsule endoscopy approach. 6 th ICCE Repor. 2007;19. |
| 48. | Sánchez R, Aparicio JR, Baeza T, Calero Y. Capsule endoscopy diagnosis of intestinal involvement in a patient with Churg-Strauss syndrome. Gastrointest Endosc. 2006;63:1082-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Skogestad E. Capsule endoscopy in Henoch-Schonlein purpura. Endoscopy. 2005;37:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | de Franchis R, Rondonotti E, Abbiati C, Beccari G, Merighi A, Pinna A, Villa E. Capsule enteroscopy in small bowel transplantation. Dig Liver Dis. 2003;35:728-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Eliakim R, Sharma VK, Yassin K, Adler SN, Jacob H, Cave DR, Sachdev R, Mitty RD, Hartmann D, Schilling D. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy versus conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. J Clin Gastroenterol. 2005;39:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Rubenstein JH, Inadomi JM, Brill JV, Eisen GM. Cost utility of screening for Barrett‘s esophagus with esophageal capsule endoscopy versus conventional upper endoscopy. Clin Gastroenterol Hepatol. 2007;5:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Gerson L, Lin OS. Cost-benefit analysis of capsule endoscopy compared with standard upper endoscopy for the detection of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2007;5:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Galmiche JP, Sacher-Huvelin S, Coron E, Cholet F, Soussan EB, Sébille V, Filoche B, d’Abrigeon G, Antonietti M, Robaszkiewicz M. Screening for esophagitis and Barrett’s esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol. 2008;103:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | de Franchis R, Eisen GM, Laine L, Fernandez-Urien I, Herrerias JM, Brown RD, Fisher L, Vargas HE, Vargo J, Thompson J. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Spiegel BM, Esrailian E, Eisen G. The budget impact of endoscopic screening for esophageal varices in cirrhosis. Gastrointest Endosc. 2007;66:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Meltzer AC, Ali MA, Kresiberg RB, Patel G, Smith JP, Pines JM, Fleischer DE. Video capsule endoscopy in the emergency department: a prospective study of acute upper gastrointestinal hemorrhage. Ann Emerg Med. 2013;61:438-443.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Gralnek IM, Ching JY, Maza I, Wu JC, Rainer TH, Israelit S, Klein A, Chan FK, Ephrath H, Eliakim R. Capsule endoscopy in acute upper gastrointestinal hemorrhage: a prospective cohort study. Endoscopy. 2013;45:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 60. | Spada C, Hassan C, Riccioni ME, Costamagna G. False positive at colon capsule endoscopy or false negative at conventional colonoscopy? Endoscopy. 2010;42:427-48; author reply 428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK, Benamouzig R, de Franchis R, Delvaux M, Devière J, Eliakim R, Fraser C, Hagenmuller F, Herrerias JM, Keuchel M, Macrae F, Munoz-Navas M, Ponchon T, Quintero E, Riccioni ME, Rondonotti E, Marmo R, Sung JJ, Tajiri H, Toth E, Triantafyllou K, Van Gossum A, Costamagna G; European Society of Gastrointestinal Endoscopy. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 62. | Rokkas T, Papaxoinis K, Triantafyllou K, Ladas SD. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest Endosc. 2010;71:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581-589.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 64. | Triantafyllou K, Tsibouris P, Kalantzis C, Papaxoinis K, Kalli T, Kalantzis N, Ladas SD. PillCam Colon capsule endoscopy does not always complement incomplete colonoscopy. Gastrointest Endosc. 2009;69:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Lisi D, Hassan C, Crespi M; AMOD Study Group. Participation in colorectal cancer screening with FOBT and colonoscopy: an Italian, multicentre, randomized population study. Dig Liver Dis. 2010;42:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Sung JJY. Evaluation of PillCam colon capsule endoscopy in the assessment of colonic inflammatory lesions in ulcerative colitis: An interim analysis. ICCE. 2008;1:4-5. |
| 67. | Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Fléjou JF, Herfarth H, Hommes DW, Kupcinskas L, Lakatos PL, Mantzaris GJ, Schreiber S, Villanacci V, Warren BF; European Crohn’s and Colitis Organisation (ECCO). European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis. 2008;2:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 379] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 68. | Woods SP, Constandinou TG. Wireless capsule endoscope for targeted drug delivery: mechanics and design considerations. IEEE Trans Biomed Eng. 2013;60:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (3)] |
| 69. | Gao M, Hu C, Chen Z, Zhang H, Liu S. Design and fabrication of a magnetic propulsion system for self-propelled capsule endoscope. IEEE Trans Biomed Eng. 2010;57:2891-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 70. | Gao M; Olympus. Development of capsule endoscopes and peripheral technologies for further expansion and progress in endoscope applications, 2004. Available from: http://www.olympus-global.com/en/news/2004b/nr041130capsle.cfm. |
| 71. | Sendoh M, Ishiyama K, Arai KI. Fabrication of magnetic acurator for use in a capsule endoscope. IEEE ASME Trans Mechatron. 2003;39:3232-3234. [DOI] [Full Text] |
| 72. | Yang S, Park K, Kim J, Kim TS, Cho IJ, Yoon ES. Autonomous locomotion of capsule endoscope in gastrointestinal tract. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6659-6663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Kim B, Lee MG, Lee YP, Kim Y, Lee G. An earthworm-like micro robot using shape memory alloy actuator. Sens Actuators A Phys. 2006;125:429-437. [DOI] [Full Text] |
| 74. | Kim B, Lee S, Park JH, Park JO. Design and fabrication of a locomotive mechanism for capsule-type endoscopes using shape memory alloys (SMAs). IEEE ASME Trans Mechatron. 2005;10:77-86. [DOI] [Full Text] |
| 75. | Kim HM, Yang S, Kim J, Park S, Cho JH, Park JY, Kim TS, Yoon ES, Song SY, Bang S. Active locomotion of a paddling-based capsule endoscope in an in vitro and in vivo experiment (with videos). Gastrointest Endosc. 2010;72:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Gorlewicz JL, Battaglia S, Smith BF, Ciuti G, Gerding J, Menciassi A, Obstein KL, Valdastri P, Webster RJ. Wireless insufflation of the gastrointestinal tract. IEEE Trans Biomed Eng. 2013;60:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
P- Reviewer: Leitman M, Sakata N S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH