Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14185
Revised: February 17, 2014
Accepted: June 14, 2014
Published online: October 21, 2014
Processing time: 283 Days and 21 Hours
Nonalcoholic fatty liver disease (NAFLD) is common in the elderly, in whom it carries a more substantial burden of hepatic (nonalcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma) and extra-hepatic manifestations and complications (cardiovascular disease, extrahepatic neoplasms) than in younger age groups. Therefore, proper identification and management of this condition is a major task for clinical geriatricians and geriatric hepatologists. In this paper, the epidemiology and pathophysiology of this condition are reviewed, and a full discussion of the link between NAFLD and the aspects that are peculiar to elderly individuals is provided; these aspects include frailty, multimorbidity, polypharmacy and dementia. The proper treatment strategy will have to consider the peculiarities of geriatric patients, so a multidisciplinary approach is mandatory. Non-pharmacological treatment (diet and physical exercise) has to be tailored individually considering the physical limitations of most elderly people and the need for an adequate caloric supply. Similarly, the choice of drug treatment must carefully balance the benefits and risks in terms of adverse events and pharmacological interactions in the common context of both multiple health conditions and polypharmacy. In conclusion, further epidemiological and pathophysiological insight is warranted. More accurate understanding of the molecular mechanisms of geriatric NAFLD will help in identifying the most appropriate diagnostic and therapeutic approach for individual elderly patients.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is a common disease in the elderly, in whom it follows a more severe course due to worse hepatic and extrahepatic manifestations. The former include nonalcoholic steatohepatitis and hepatocellular carcinoma; the latter include cardiovascular and metabolic complications. The pathophysiology, diagnosis and principles of treatment that are specifically relevant to elderly patients with NAFLD are critically revised here. Based on these findings, we conclude that a more accurate understanding of the molecular mechanisms of geriatric NAFLD will help in identifying the most appropriate diagnostic and therapeutic approach in the individual elderly patient.
- Citation: Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, Romagnoli D, Loria P. Nonalcoholic fatty liver disease and aging: Epidemiology to management. World J Gastroenterol 2014; 20(39): 14185-14204
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14185.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14185
Increased life expectancy has resulted in a dramatic surge of the median age of the population. Given that such a trend is expected to continue, the increased proportion of elderly and very elderly people in future decades will undoubtedly represent a relevant challenge in terms of health costs and proper resource allocation[1]. With aging, the liver undergoes substantial changes in structure and function that are associated with significant impairment of many hepatic metabolic and detoxification activities[2-10] (Table 1).
| Increased fibrosis with reduced percentage of fat on liver biopsy[3,4]; decreased size mirroring decreased hepatic blood flow[5] |
| Loss of regenerative capacity, i.e. “replicative senescence” involving cross talks of growth hormone, glycogen synthase kinase 3β and cyclin D3 and shortening of telomeric ends of chromosomes[5,6] |
| Increased inflammatory changes, hence the definition of “inflamm-aging” which, in its turn, has a detrimental effect on the regenerative response[7] |
| Defective autophagy[8,9] |
| Decrease in hepatic free radical scavenging system leads to increased oxidative stress resulting mainly from non-enzymatic processes in the liver[5] and is exacerbated by ethanol drinking[10] |
Non-alcoholic fatty liver disease (NAFLD), the spectrum of hepatic disorders embracing uncomplicated fatty liver (i.e., “pure steatosis”) and nonalcoholic steatohepatitis (NASH) is associated with features of metabolic syndrome (MS) and several hepatic and extra-hepatic complications[11,12]. Not uncommon in young adults, in children and teen-agers[13-15], NAFLD occurs more often in males than in females[16] and affects mainly the middle-aged and the elderly given that that risk factors for its development tend to increase in prevalence with advancing age[3,17,18]. Worryingly, older patients show more severe biochemical, hematological and histological changes[3], and the female sex is no longer protective with increasing age[18].
No specific recommendations are provided by the available guidelines regarding the diagnostic conduct to be followed in the elderly[19]. Accordingly, a non-invasive diagnostic approach[20] appears to be more rational in this age group. Finally, physical exercise and dietary restriction can be very effective in carefully selected patients and should be used as part of a multidisciplinary approach to overcome the physical limitations not uncommon in older patients[11].
On these grounds, the present review paper aims to systematically and critically analyze bibliographic data that are specifically relevant to NAFLD in geriatric clinical practice.
Epidemiological data on NAFLD may be derived from studies conducted either in the general population/large cohorts or in selected geriatric cohorts.
Data from studies conducted in three continents are summarized in Table 2[17,18,21-46]. By analyzing such data, the role of gender and age may be discussed in some detail.
| Asia | ||
| Nomura et al[21], 1988 | Cross sectional study on 2574 Japanese healthy residents (1271 males), age 0-70+ yr , FL in 14% | Prevalence of FL in persons under 19 yr old was only 1.2%, and increased with age to a maximum (25.6%) in persons 40-49 yr of age and then decreased |
| Kojima et al[22], 2003 | Cross-sectional and prospective study on 39151 Japanese first-time examinees, 61% males, 12-yr survey period | The prevalence of FL was uniformly high in males and declined in those in the 6th and 7th decades. The prevalence in females tended to rise gradually with age and declined in those in their 7th decade of life |
| Shen et al[23], 2003 | 4009 administrative officers non-drinkers, aged 20-81 yr, 64% males, FL in 12.9% | At multivariate analysis the prevalence of FL was positively correlated to several risk factors, including male gender and age > 50 yr |
| Fan et al[24], 2005 | This cross-sectional study with randomized multistage stratified cluster sampling included 3175 subjects (1218 men) with a mean age of 52 yr. FL was found in 661 (20.82%) subjects | Age-adjusted prevalence of FL was significantly higher in men than women, and LRA showed that male gender was closely related to FL. The prevalence of FL increased with age in both sexes, peaking in women 60-69 yr of age, and in men 40-49 yr of age. Interestingly, the prevalence was higher in males than females under the age of 50, but was lower in males than females among people older than 50 yr |
| Chen et al[25], 2006 | The cross-sectional community study examined 3245 adults in a rural village of Taiwan.The prevalence of NAFLD was 11.5% (372/3245) | Male sex was an independent risk factors for NAFLD in the general population. Age ≥ or 65 yr was inversely related to NAFLD |
| Park et al[26], 2006 | Cross-sectional study was performed with data obtained from 6648 subjects, all of whom were older than 20 yr of age (3530 men and 3118 women). The unadjusted and age-adjusted prevalences of NAFLD were 18.7% (23% in men, 13.7% in women) and 16.1% (21.6% in men, 11.2% in women), respectively | NAFLD prevalence was higher in men than in women. Age was an independent predictor of NAFLD in women only. Menopause status was an independent predictor of NAFLD |
| Zelber-Sagi et al[27], 2006 | A cross-sectional study of a subsample of the Israeli national health survey (n = 352). Three hundred and twenty-six subjects (53.4% male, mean age 50.5 ± 10.3) met the inclusion criteria. The prevalence of primary NAFLD was 30% (25%-35% 95%CI) | Male gender was found to be an independent risk factor for NAFLD which remained significant even after adjustment for obesity and abdominal obesity |
| Zhou et al[28], 2007 | A cross-sectional survey of a random sample of 3543 over 7-yr-old inhabitants in 6 urban and rural areas in China. Among the 3543 subjects, 609 (17.2%) were diagnosed having FLD (18.0% males, 16.7% females, P > 0.05). Among them, the prevalence of confirmed alcoholic liver disease (ALD), suspected ALD and nonalcoholic fatty liver disease (NAFLD) were 0.4%, 1.8%, and 15.0%, respectively. The prevalence rate (23.0%) was significantly higher in urban areas than (12.9%) in rural areas | The prevalence rate was significantly higher in men than in women under the age of 50 yr. However, the opposite phenomenon was found over the age of 50 yr Multivariate and logistic regression analysis indicated that male gender was among the independent risk factors for FLD |
| Eguchi et al[34], 2012 | A cross-sectional study was conducted among 8352 subjects who received health checkups from 2009 to 2010 in three health centers in Japan The overall prevalence of NAFLD was 29.7% | The prevalence of NAFLD is higher in males than that in females at all ages, it is “inverted U-Shaped” in males (peaking at 40-49 yr); it gradually increases in all age groups in women but declines in those ladies in their seventies |
| Al-hamoudi et al[35], 2012 | Prospective study among 1312 patients referred for ultrasonography in Saudi Arabia The prevalence of NAFLD was 16.6% (218/1312) | In a multivariate analysis, younger age and being male were significant predictors of high ALT levels |
| Wang et al[18], 2013 | Chinese case-control study based on all 4226 adults above 60 yr of age from a previously cohort investigated compared to 3145 randomly selected younger controls (< 60 yr) from the same cohort. NAFDL was higher in the elderly (26.7%) than in the non-elderly (22.8%) and similar in the elderly between men and women (26.6% vs 27.0%, P > 0.05) | The prevalence of FLD is higher in the elderly, and is broadly related to the same metabolic risk factors as in the non-elderly. However, female-sex is no longer protective with increasing age, and the prevalence of steatohepatitis with advanced fibrosis is estimated to be considerably higher in the elderly FLD patients than in the younger FLD controls |
| Foster et al[36], 2013 | 3056 participants of the Multi Ethnic Study of Atherosclerosis were included in this study. NAFLD was defined as LS ratio < 1, the prevalence of NAFLD was and 11 % in African Americans | Younger age was among the independent correlates of NAFLD in this population |
| Xu et al[37], 2013 | Cross-sectional study on 6905 nonobese (BMI < 25). Risk factors for the development of NAFLD were assessed in a subsequent prospective study in NAFLD-free individuals at baseline, 494 of whom had developed NAFLD during the 5-yr follow-up. Prevalence of NAFLD was 7.27% | Older age and male gender are associated with both the prevalence and the incidence of novel NAFLD cases in a non obese Chinese population |
| Yan et al[38], 2013 | Random sampling of 3762 adults Chinese residents. Ultrasonography revealed fatty liver in 1486 residents with a prevalence of 39.5% | At univariate analysis, NAFLD cases were statistically younger and more often males |
| Europe | ||
| Lonardo et al[39], 2006 | Hospital cohort study of 449 individuals undergoing both liver and carotid US evaluation for clinical indications | U-shaped curve with NAFLD peak prevalence in the 30-39 and 40-49 age groups. The phenomenon may either reflect a decrease of risk factor for NAFLD, (e.g., obesity) or selectively increased mortality among those with FL. At logistic regression analysis FL is more common in younger male individuals |
| Bedogni et al[40], 2007 | A follow-up study on 144 subjects without and 336 with fatty liver followed for a median 8.5 yr time | Male sex is a predictor of incidence of fatty liver; male sex and age are predictors of death in those with fatty liver |
| Caballeria et al[41], 2010 | multicentre, cross-sectional, populational study recruiting 766 Individuals between 15 and 85 yr of age randomly selected from 25 primary healthcare centres in Spain | On multivariate analysis, male sex, age and other factors were associated with NAFLD |
| Koehler et al[17], 2012 | 2811 participants in the population-based Rotterdam Study. Mean age of participants was 76.4 yr (range 65.3-98.7 yr) | NAFLD is common in the elderly. However, the prevalence of NAFLD decreased with advancing age suggesting a positive selection of the elderly without NAFLD |
| Soresi et al[42], 2013 | Study on the prevalence of NAFLD in metabolic syndrome | Men and women with steatosis were younger than those without steatosis. At multivariate analyses, was associated with higher ALT |
| United States of America | ||
| Browning et al[43], 2004 | An observational study on 2287 subjects from a multiethnic, population-based sample (of white, Black, and Hispanic) | In whites, but not in Hispanics or blacks, the frequency of hepatic steatosis was approximately 2-fold higher in men than in women related to ethanol intake, not to differences in the frequency of obesity or insulin resistance |
| Younossi et al[44], 2012 | A retrospective study on 11613 participants included in the National Health and Nutrition Examination Survey III (NHANES III); 2185 had NAFLD; and, of these, 307 had NASH | NAFLD was independently associated with younger age and female sex; and NASH was independently associated with having a younger age in those United States lean individuals with NAFLD |
| Bambha et al[45], 2012 | 628 adults enrolled either in the observational Database or the PIVENS trial between 2004 and 2008 and thus submitted to liver biopsy | Advancing age and female gender were among the independent predictors of advanced fibrosis at LRA |
| North et al[46], 2012 | A total of 1242 (1064 EA, 178 AA) and 1477 (1150 EA, 327 AA) men and women, respectively, underwent CT examination from which LA and abdominal adipose volume were measured | Mean LA varied significantly by sex, [(men) 57.76 ± 10.03 HU and (women) 60.03 ± 10.91 HU, P = 0.0002], but not by race. Higher LA was associated with older age |
| Foster et al[36], 2013 | 3056 participants of the Multi Ethnic Study of Atherosclerosis were included in this study. NAFLD was defined as LS ratio < 1 | Younger age was among the independent correlates of NAFLD in this population |
Our group previously reported a prevalence of male individuals with NAFLD in published studies[16], supporting a role for endocrine determinants in the development of this condition. Most Asian and European studies strongly confirm such a view (Table 2). In males, NAFLD tends to increase from younger to middle-aged groups of individuals and the prevalence of disease begins to decline at the age of 50 or 60. This has been defined as an “inverted U shaped curve”. Of interest, a study by Nakajima, although conducted in a restricted series of liver biopsies, was nevertheless able to demonstrate that advancing age was inversely correlated with steatosis[47].
Consistent with a protective role of estrogens, during their fertile period, women tend to be spared from NAFLD compared to men. However, although they tend to develop the disease approximately 10 years later than men, post-menopausal women are no longer spared from NAFLD. The prevalence of NAFLD in women tends to decrease after the seventh decade of life based on both epidemiological (Table 2) and clinical studies[48].
While hormonal changes have consistently been proposed to account for the varying prevalence rates of NAFLD in either gender, the explanation of decreased rates of NAFLD in the elderly is deemed to reflect either selectively decreased survival in those with NAFLD[17,39] or decreased fatty changes in advanced NASH[49].
Findings from three studies conducted in selected geriatric cohorts are summarized in Table 3[3,50,51]. Information from such studies needs to be interpreted with caution because the findings may merely reflect bias introduced by restricting the analysis to this relatively unique subset of patients[52]. Accordingly, more extensive, less biased cohort clinical studies need to be conducted before drawing any significant conclusions on the prevalence and risk factors of NAFLD in geriatric patients.
| Kagansky et al[50], 2004 | In this prospective study, 91 octogenarians who were admitted to the rehabilitation departments of a geriatric hospital were compared to 46 NAFLD young patients | NAFLD is a common and benign finding in the elderly population, in whom it is not associated with the metabolic syndrome |
| Frith et al[3], 2009 | In this retrospective, cohort study set in a tertiary liver clinic in United Kingdom. Three hundred and fifty one consecutive biopsy-proven NAFLD patients were divided into an older (≥ 60), a middle-aged (≥ 50 to < 60) and a younger (< 50) group | NAFLD affects mainly the middle-aged and the elderly. Older patients show more risk factors and more severe laboratori alterations and histological changes, with cirrhotics having a significantly more advanced age than those with milder disease |
| Noureddin et al[51], 2013 | A cross-sectional analysis of adult participants who were prospectively enrolled in the NASH Clinical Research Network studies. Participants were included based on availability of the centrally reviewed liver histology data within 1 year of enrollment, resulting in 61 elderly (age ≥ 65 yr) and 735 nonelderly (18-64 yr) participants | The main outcomes were the presence of NASH and advanced fibrosis. Compared to nonelderly patients with NAFLD, elderly patients had a higher prevalence of NASH (56% vs 72%, P = 0.02), and advanced fibrosis (25% vs 44%, P = 0.002). Compared to nonelderly patients with NASH, elderly patients with NASH had higher rates of advanced fibrosis (35% vs 52%, P = 0.03), as well as other features of severe liver disease including the presence of ballooning degeneration, acidophil bodies, megamitochondria, and Mallory-Denk bodies (P ≤ 0.05 for each) |
Our understanding of the natural history of NAFLD is based on relatively few studies conducted in the United States and northern Europe (reviewed in[12,53,54]). Based on such studies, it may be concluded that NAFLD carries a risk of both hepatic and extrahepatic disease.
Data suggest that in middle-aged individuals (especially in the 45-54 year-old age group), NAFLD is a strong independent risk factor for cardiovascular (CV) mortality[55]. NASH-cirrhosis is expected to occur in older ladies with multiple metabolic derangements and moderately raised liver enzymes because of the following four reasons: (1) the male sex is typically prone to premature coronary deaths; (2) NAFLD occurs in women later than in men (Table 2); (3) female gender and older age are significantly associated with severe fibrosis[30]; and (4) younger ages are associated with more elevated liver enzymes[35]. Such a prediction is confirmed by Ludwig et al[56] and by more recent evidence. Caldwell et al[57,58], by comparing different series of cirrhosis due to varying etiologies, concluded by inference that “cryptogenic cirrhosis” featuring a prevalence of female gender, associated metabolic conditions and slightly raised liver enzymes is nothing but NASH-cirrhosis.
Bugianesi et al[59] group went further in identifying the risk of hepatocellular carcinoma (HCC) as a part of the NAFLD disease spectrum, confirming early hypotheses published as early as in 1990[60].
Risk factors for HCC in NAFLD include advanced age, type 2 diabetes (T2D) and cirrhosis[61-63]. Of concern, however, is that HCC may also develop against the background of non-cirrhotic NAFLD[64,65] accounting for the finding that these patients are missed by surveillance programs and are diagnosed later than those associated with viral etiology[66,67]. This is of importance given that, by expanding the percentage of cancers amenable to effective treatments, surveillance for HCC is deemed to improve the survival rates of elderly patients[68].
In the elderly, decreased cytochrome P450 activity can affect drug metabolism, increasing the susceptibility to drug-induced liver injury. Immune responses against pathogens or neoplastic cells are reduced in the elderly, although these individuals may be predisposed to autoimmunity through impairment of dendritic cell maturation and reduction of regulatory T cells. These changes in immune functions could alter the pathogenesis of viral hepatitis and autoimmune liver diseases, as well as the development of HCC[69].
As is now clear from consistent evidence in the literature, NAFLD stands not only as a primary disease of the liver but rather as a systemic condition with an inherent increased risk for CV disease (CVD). Evidence for a link between NAFLD and CVD, notably including atherosclerosis, which occurs independent of components of MS, has extensively been reviewed by our group and others[12,54,70-75]. Such a view is specifically confirmed in elderly people.
By evaluating 810 males and 1273 elderly women in Japan, Akahoshi et al[76] demonstrated that NAFLD was associated with coronary risk factors independent of obesity in either gender.
Evidence for those NAFLD patients who are T2D-free at baseline to develop the disease during the follow-up period have been extensively reviewed elsewhere[12,54]. Clearly, in the elderly, the combination of T2D with NAFLD is likely to manifest a high-risk interaction[62].
Of particular concern is that NAFLD was associated with osteoporotic fractures in middle-aged and elderly men in a recent Chinese study[77].
Finally, a line of research has highlighted NASH (but not simple steatosis) as an independent risk factor for (particularly right-sided) colonic adenomas and advanced neoplasms[78]. Given the strong age-dependency of the disease, findings from this study suggest that colorectal cancer screening is strongly indicated in elderly individuals with NASH.
In conclusion, NAFLD is neither an uncommon nor benign disease in the aged individual.
Elderly people have a high risk for strongly age-dependent hepatic and extra-hepatic complications.
Awareness of NAFLD epidemiology and natural history allows prompt recognition of the disease and the management of complications.
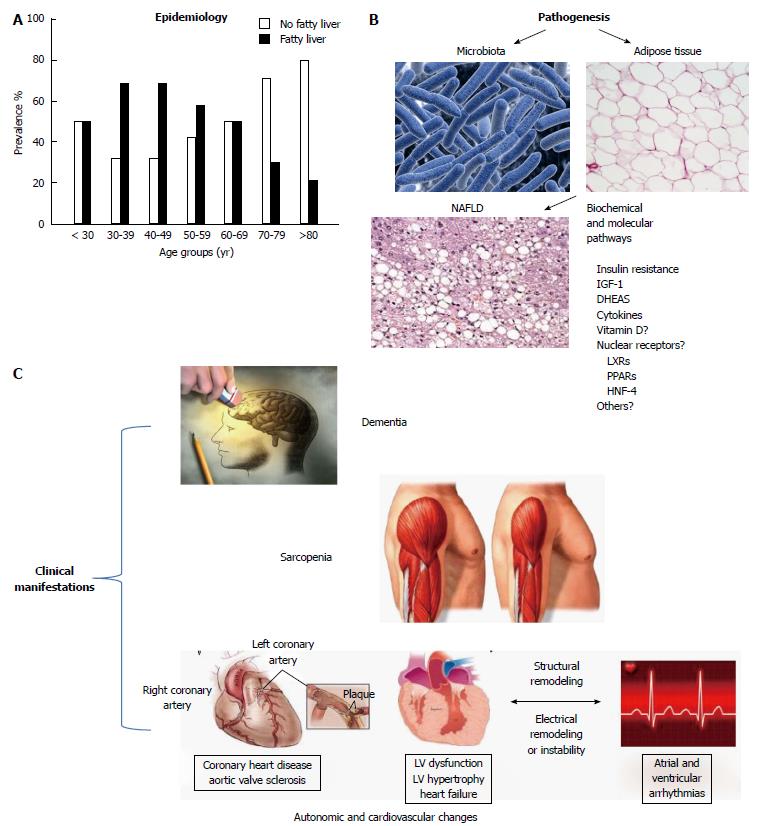
Figure 1A summarizes the epidemiology of NAFLD with respect to age (adapted from References[39,75]).
An age-specific pattern of liver disease cannot be defined[79,80]; nonetheless, a number of pathophysiological changes have been described with aging, which can ultimately affect hepatic lipid metabolism.
The reduced liver function, which occurs with aging, has been causally associated with a decline in hepatic blood flow and liver volume[80]. Specific alterations in the metabolism of drugs with a high hepatic extraction rate have been described[81]. However, the metabolic impact of such changes remains unclear. The possible role of the alterations in drug disposition on the progression of liver disease has been commented above[69].
Age-related alterations in hepatic cholesterol homeostasis are poorly defined. Epidemiological evidence supports increased serum cholesterol values that tend to plateau in very old individuals[82] and an increased prevalence of gallstone disease[83] with aging. Again, the underlying mechanisms are incompletely defined; a limited number of reports on in vivo studies in humans have described a reduction in low density lipoprotein turnover[84] and an increase in biliary cholesterol output, possibly related to a decrease in cholesterol degradation to bile acid[85,86]. Data from humans also suggest that a reduction of whole body, and presumably hepatic, cholesterol synthesis occurs with aging[87].
The pathogenesis of NAFLD undoubtedly involves IR[88-91]. However, the role of IR as a determinant of age-related liver disease has been recently questioned on the grounds that it may represent a compensatory rather than a causal mechanism[92]. Age-related decreases in hormones such as insulin-like growth factor-1 and dehydroepiandrosterone contribute to NAFLD development via increased IR[93-97].
The role of glucocorticoids in the balance between liver injury and protection is controversial[98]. Hypercortisolism may associate with NAFLD, even if its prevalence in this condition is lower than expected[99]; the anti-inflammatory effects of endogenous corticosteroids have been evoked in this regard.
The molecular mechanisms leading to hepatic steatogenesis may theoretically involve increased fat intake (dietary uptake and lipogenesis) or decreased fat output (beta-oxidation, lipoprotein secretion)[88,100,101]. The reduced beta-oxidation was related to reduced hepatic expression of the nuclear receptors of the peroxysome proliferator-activated receptors family (PPARs), whereas the expression of liver X receptors (LXRs) was enhanced[102,103]; the role of other nuclear receptors of the hepatocyte nuclear factor (HNF) family cannot be ruled out as well[96,102].
Interestingly, an inverse correlation between NAFLD and serum levels of adipocyte-fatty acid binding protein, an intracellular lipid transporter, has been reported in the elderly[104].
Human NAFLD in the elderly is frequently more severe and therefore carries a worse prognosis than in younger age groups. This finding has also been substantiated in a murine model of steatohepatitis[105]. Aging-related alterations of the pro-inflammatory vs anti-inflammatory balance (described as the “inflamm-aging” theory) may represent the biological background of these findings[106].
The pivotal role of inflammation in the pathogenesis of liver steatosis has been emphasized in the literature; the link between insulin resistance and inflammation and the possible role of JNKs in the progression of this condition has been clearly defined, along with the involvement of endoplasmic reticulum stress and of the unfolded protein response[71].
In addition, hormonal changes involving the GH/IGF axis have been related to inflammation in NAFLD. In this context, the adipokine pattern plays an important role; in particular, circulating levels and tissue expression of adiponectin seem to be inversely correlated with NAFLD and its progression[107]; a condition of low-grade chronic inflammation, where interleukins, in particular IL-6, play a relevant role, is also present[107]. These findings together underscore the complex relationship between insulin resistance and inflammation. Increased spleen volume has also been correlated with this condition and with the alterations in the hormonal and inflammatory pattern[108].
Finally, the role of the apoptosis-antiapoptosis balance has to be considered; serum concentrations of Bcl-2, a well-known anti-apoptotic marker, were found to be decreased in subjects with NAFLD and NASH[109-111]. Serum levels of Bcl-2 also tended to decrease with increasing age[111], even if the data in very elderly individuals are missing.
Altered distribution of inflammatory cytokines in the different body compartments may further contribute to worsening NAFLD course in the elderly[112].
Aging is typically associated with profound alterations in the amount and distribution of body fat depots with a shift occurring from subcutaneous to visceral localization. Such a shift, in turn, is frequently associated with chronic conditions linked to increased CVR[113,114] such as IR (see above) and other stigmata of MS[113].
The relationship between IR and epicardial fat content, a marker of coronary risk, has been highlighted[115]; evidence in humans, although limited, seems to suggest that in the elderly, epicardial fat accumulation and liver steatosis may be associated[116]. Animal models have confirmed that the amount of adipocytes in fat depots increases with aging[117].
Together with the well-known deleterious effects of visceral fat accumulation and IR, subcutaneous fat loss, and the age-related reduced capacity of subcutaneous fat cells to store lipids may also lead to metabolic complications via lipotoxic effects[118].
Furthermore, the role of preadipocytes, fat cell progenitors, in fat distribution and function has been highlighted in animals[114] and humans[119]. The decline in preadipocyte proliferation and differentiation described with aging may account for an imbalance between lipogenesis and lipolysis, ultimately leading to an increased exposure to free fatty acids and potential lipotoxicity[120,121]. Among the different compounds, saturated fatty acids appear to be particularly hepato-toxic[114,122]. Such toxic potential translates into stress responses, oxidative stress and increased expression of proinflammatory cytokines[123].
The constellation of the alterations in fat cell biochemistry, proliferative capacity and response to inflammation and immune response accompany the process of fat cell senescence[118].
Indeed, fine tuning of the cross-talk between adipogenic and anti-adipogenic pathways[120] and together with previously mentioned age-related hormonal derangements (GH and IGF-1) and activation of proinflammatory cytokines cascade may play a specific role in NAFLD development in the elderly.
The expression of nuclear receptors and transcriptional modulators in adipose tissue is fundamental in such regulation; the role of PPARγ and other coactivators such as CCAAT/enhancer-binding protein α and β has been clarified[124-127]. A role for liver X receptors cannot be ruled out as well, considering its role in adipogenesis[128], even if no definite evidence with respect to aging is presently available.
Closely related the above mentioned findings, it is important to recall the possible therapeutic implications of reducing abdominal fat, and not only BMI, in improving survival in the elderly, as recently reported[129].
The human gut microbiome is a complex “superorganism” that is mostly confined to the colon, which contains at least 1014 microorganisms, equal to 1.5 kg of microbes of 5000 species with a predominance of anaerobic bacteria)[130-132]. The collective genome of gut microbiota, termed the metagenome, is composed of almost 5 million genes[133]. Microbiota is considered an “organ within an organ”, with its own functions: it modulates the expression of genes involved in mucosal barrier development, maturation and maintenance of the GI sensory and motor functions and the mucosal immune system[134,135]. It also has a critical role in supporting normal digestion, affects energy extraction from the diet and produces essential nutrients such as vitamins (e.g., vitamin K, vitamin B12 and folic acid) and amino acids, which humans are unable to produce themselves[136,137]. In addition, the intestinal microbiota participates in the defense against pathogens. Compositional changes have been linked with inflammatory bowel disease, irritable bowel syndrome and metabolic disorders, such as T2D and obesity[138-140].
During adulthood, the composition of the intestinal microbiota at the species level is relatively stable. This relative stability, however, decreases in old age[141,142].
Aging produces modifications in the homeostasis of GI microbiota with changes in the prevalence of different bacteria[143]. There is a general agreement regarding large inter-individual variability in older subjects, with a certain country specificity, possibly related to differences in lifestyle and dietary habits[144].
A decrease in Firmicutes has been reported in the Italian population over 60 years and centenarians, which is in contrast with other European populations. The decrease of both Clostridium cluster XIV and Faecalibacterium prausnitzii group members was also correlated with frailty, hospitalization, and antibiotic and non-steroidal anti-inflammatory treatment. An age-related increase in Bacteroidetes was found in elderly subjects from Northern Europe but not in Italian elderly and centenarians[142,145].
Recent studies observed higher levels of health-promoting bifidobacteria in the elderly in contrast with the common assumption of their decreased levels with age[145-147]. Certainly, there is an age-related increase in facultative anaerobes (like streptococci, staphylococci, enterococci, and enterobacteria), and pro-inflammatory pathobionts are present in the healthy gut microbiota at low concentrations[142,148,149].
A decrease in immunomodulatory species belonging to the Clostridium clusters IV and XIV associated with increased pro-inflammatory pathobionts seems to promote inflammatory disorders, and elderly subjects generally have reduced immune function, particularly a deterioration in cell-mediated responses, that affect gut microbiota composition[150].
During aging, the impairment of the gut-associated lymphoid tissue and the reduced efficiency of the innate immune defenses are responsible for uncontrolled microbial growth on the enterocyte surface. Indeed, aging is also accompanied by a chronic, low grade overall inflammatory status responsible for the development of pathobionts that can support inflammation with compromised host longevity[151]. Finally, decreased cellular immune functions can lead to an increased emphasis on antibody-associated responses in the elderly, the subsequent excess of antibodies reflects an increased autoimmunity to self-antigens, predisposing the person to the development of autoimmune diseases[152-154].
In addition to immunosenescence and inflamm-aging, lifestyle and dietary habits are other major factors that are responsible for age-related changes in microbiota. Diet impacts on the selection of microbial species are able to optimize energy extraction from dietary substrates[148,155,156].
Age-related changes in taste and smell sensations, tooth loss and chewing difficulties strongly influence diet with a reduced intake of fiber, non-starch carbohydrates and proteins, producing modifications in the composition of the bowel ecosystem through a reduction in microbial fermentation. In contrast, reduced acid secretion in the stomach can lead to bacterial overgrowth and subsequent nutrient and vitamin malabsorption[157-160].
An unbalanced diet with inadequate intake of vitamin D and B12, calcium and protein can affect both the composition and activity of intestinal microbiota[161-164]. The common decreased sensation of thirst adversely affects bowel motility and bodily water balance[165].
Lifestyle differences, notably including dietary habits, may further affect the geographic-dependent modulation of age on microbiota[148].
The poor quality of the diet associated with diminished physical activity in the elderly is also responsible for reduced intestinal motility, which will result in bacterial overgrowth and enhanced metabolic activity, which are difficult to control in an immunosenescent host[148,155,166]. Age-related neurodegeneration in the enteric nervous system may further contribute to impaired bowel peristalsis[166,167].
Increased incidence of disease will be conducive to increased drug consumption by elderly individuals, and this will eventually alter the composition of intestinal microbiota. Most drugs cause hyposalivation responsible for changes in oral microbiota, candidiasis and dysphagia[168]. Proton pump inhibitors, by lowering gastric pH, may lead to malabsorption of nutrients and microbial overgrowth with associated risk of Clostridium difficile (C. difficile) diarrhea[169]. Similarly, broad-spectrum antibiotics may lead to overgrowth of pathogenic bacteria such as C. difficile; however, non-absorbable antibiotics (e.g., rifaximin) play a therapeutic role in restoring intestinal microbiota imbalance in several intestinal disorders. The frequent use of nonsteroidal anti-inflammatory drugs is associated with mucosal gastric and duodenal damage with alteration in the local ecosystem[170]. Finally, the use of opioids may lead to reduced intestinal motility and subsequent bacterial overgrowth[171].
Figure 1B illustrates in a schematic way some aspects of the pathophysiology and pathogenesis of the relationship between aging and NAFLD.
The relationship between NAFLD and the typical clinical syndromes associated with aging is ill-defined. Some of the clinical manifestations associated with NAFLD are schematically summarized in Figure 1C.
Sarcopenia is characterized by reduced muscle mass and strength[172] and replacement of muscle with adipose and fibrous tissue; sarcopenia represents one of the hallmarks of frailty, according to its commonly adopted definition[173].
The documentation of an association between fat accumulation in the two tissues (liver and muscle) is relatively recent: morphological imaging has shown that fat content in the paravertebral muscles is correlated with aging and steatosis based on CT analysis and suggested that a reduction in muscle fat can be associated with an improvement of steatosis[174]. A very recent paper by Hong et al[175], on behalf of the Korean Sarcopenic Obesity Study, has provided substantial evidence of a correlation between fat liver content, expressed as the liver attenuation index at CT, and the skeletal muscle mass index determined by DXA.
Sarcopenia and NAFLD may share a number of pathophysiological mechanisms. First, IR has been consistently associated with both conditions. The relationship between IR (and the inherent presence of the MS) and NAFLD has long been recognized[89-91] and critically reviewed[92], as discussed above. The relationship between IR and sarcopenia has been outlined more recently: mediated by mitochondrial dysfunction, IR seems to be a key mediator of the loss of muscle mass[176]. Closing the circle, intramuscular steatosis, which occurs with aging, is associated with IR[177].
A strong relationship links body mass and insulin sensitivity in younger subjects[178] and presumably in the elderly as well.
Interestingly, Hong et al[175] reported an inverse correlation between IR and muscle mass, and a direct correlation between IR and liver fat accumulation, supporting a common pathophysiological substrate of the two conditions mediated by IR itself. Consistent with this view, the MS that is associated with IR has also been associated with NAFLD and sarcopenia[179].
Inflammation represents another factor that potentially links sarcopenia to NAFLD. The association between NASH and inflammation[13], or “inflamm-aging[106], is well known, as discussed above. A number of epidemiological studies have also shown an association between low grade inflammation and sarcopenia[180]. Several circulating markers of inflammation, such as TNF-α and interleukins (IL-6 in particular) as well as CRP, have clearly been associated with sarcopenia and hepatic steatosis[175,180]. Inflammation is obviously related to IR, as discussed above, and the possible pathophysiological role of increased cytokine release from adipose tissue in the elderly[89] needs to be emphasized.
Finally, a possible role for vitamin D needs to be emphasized. Several lines of evidence have shown that low vitamin D levels are associated with sarcopenia, on the one hand[181], and with NAFLD on the other[182]. A possible common pathophysiological substrate involving shared molecular pathways[183] is highly plausible. However, no such association was observed in Asian populations[175,184].
Cognitive decline is another typical feature of pathological aging, which might be associated with liver disease in general. Apart from the well-known association between end-stage liver failure and encephalopathy, the presence of more subtle mechanisms linking “milder” liver disease and some forms of cognitive decline deserves full consideration. For instance, primary biliary cirrhosis and hepatitis C have been correlated with some forms of neuropsychological impairment even in earlier stages of the disease[185,186]. NAFLD has recently been postulated to be an additional risk factor for dementia[187] and is associated with carotid atherosclerosis[39,188] which, in turn, can heavily impair cognitive function.
Apart from the obvious effects of cerebrovascular disease, hormonal alterations associated with NAFLD and possibly linked to IR may play a role; among these, the reduction of IGF-1 levels, as previously discussed[93]. Interestingly, IR was found to be associated with visual and cognitive performance deficits that are strictly linked with frailty and dementia[189].
A possible link with vitamin B12, folate and homocysteine levels also needs to be considered. An association between low levels of vitamin B12 and NAFLD is described in the literature[190]. A similar relationship between dementia and vitamin B12 deficiency is well known[191]; an increased plasma concentration of homocysteine, often caused by cobalamine and folate deficiency, is also a strong, independent risk factor for the development of dementia and Alzheimer’s disease[192]. Although there are no studies that compare the three conditions, the presence of a common pathway linking dementia with NAFLD (vitamin B12 and folate deficiency, higher levels of homocysteine) seems plausible.
Autonomic changes can be measured to assess heart rate variability (HRV), that defines non-invasively and with a high repeatability cardiac autonomic function[193,194]. Frequent symptoms of cardiac autonomic dysregulation are fatigue, orthostatic hypotension and vasovagal syncope.
A strong association links NAFLD, autonomic changes, and MS. Liu et al[195] showed an association between NAFLD and the natural logarithm of standard deviation of NN (lnSDNN) after adjustment for the main CVR factors. Moreover, these authors showed a relationship between MS and an index of sympathetic activity at the symbolic dynamics analysis of HRV[196], indicating activation of sympathetic tone in NAFLD patients.
Studies have reported a high prevalence of autonomic dysfunction in patients with chronic liver disease[197]. Most NAFLD patients do not present with symptoms directly attributable to their liver disease. It is increasingly recognized, however, that those with NAFLD report a wide ranging spectrum of non-specific symptoms, such as fatigue and daytime sleepiness, which may be the presenting complaint and may dramatically impact quality of life[198]. Fatigue is a significant issue in NAFLD, the severity of which does not mirror the severity of hepatic histological changes and dysfunction but is associated with daytime sleepiness and autonomic dysfunction[198]. Dysfunction of the autonomic nervous system leads to symptoms such as postural dizziness and syncope and is also associated with a number of clinical consequences in hepatic diseases such as cognitive dysfunction, falls and fall-related injuries. Falls are also considered a direct consequence of autonomic nervous system dysfunction[198]. Newton demonstrated that a history of falls is common in NAFLD (43%). The proportion of recurrent fallers was significantly higher in a NAFLD cohort compared to controls, with injuries, emergency medical attention, fracture rates and hospital admission all being significantly more common in the NAFLD group, independent of the presence of T2D or the severity of liver disease[198].
Fatigue is a significant symptom in NAFLD that impacts quality of life and is unrelated to liver disease severity. Newton et al[199] demonstrated a relationship between fatigue and altered autonomic response, higher orthostatic symptoms, and a higher prevalence of positive response to head-up tilt test.
Recognition of systemic symptoms in NAFLD has important implications for patients because many are potentially modifiable with targeted interventions. Several authors demonstrated that an 8-wk resistance exercise program improves sympathovagal balance, expressed as LF/HF ratio measured at rest, and HR and blood pressure response to submaximal exercise[200].
The spectrum of CVD associated with NAFLD extends beyond the commonly recognized premature atherosclerosis. In particular, arrhythmia and non-rheumatic aortic valve sclerosis (a common finding in geriatric practice) have been recently included among CV complications of NAFLD[75].
The prevalence of atrial fibrillation (AF) increases from approximately 1% in individuals less than 55 years of age to approximately 10%-12% in those older than 80 years of age[201]. Along with older age, many pathologic conditions such as obesity, hypertension, coronary heart disease, heart failure and heart valve disease are the strongest risk factors for new-onset AF, which is a disease associated with high rates of hospitalization and death. Not unexpectedly, given that NAFLD is associated with multiple abnormalities in cardiac structure and function and shares multiple cardiometabolic risk factors with AF, an association between NAFLD and an increased risk of incident AF in patients older than 60 years and T2D, independent of other CVR factors[202] has recently been reported. It may be postulated that NAFLD is a marker of ectopic fat accumulation in other tissues, including both the myocardium and pericardium. Rijzewijk et al[203] and Ng et al[204] showed that the intramyocardial fat content, as detected by proton magnetic resonance spectroscopy, was greater in patients with T2D than in nondiabetic controls and was associated with LV diastolic dysfunction. Moreover, it is possible that NAFLD is not only associated with the risk of AF as the consequence of the shared risk factors but that NAFLD per se might partly contribute to the development and persistence of AF, through the systemic release of pathogenic mediators from the steatotic and inflamed liver, including C-reactive protein, interleukin-6, tumor necrosis factor-alpha, plasminogen activator inhibitor-1 and other inflammatory cytokines; these pathogenic mediators are remarkably higher in patients with NAFLD than in those without, as commented above, and may play a role in the development and persistence of AF, possibly by inducing structural and/or electrical remodeling of the atria[205,206].
Finally, plasma homocysteine levels, a well-known risk factor for CV and cerebrovascular disease, are higher in patients with NAFLD[207]. No direct evidence for a correlation that links aging, NAFLD, homocysteine levels and CV disease is available; however, an association of these factors, as mentioned above for dementia, seems likely.
Given that geriatric NAFLD is not a benign disease, elderly patients have a high necessity of treatment. Of concern, lifestyle changes including diet and exercise, which are universally recommended as a first-line approach[19] and which may particularly improve autonomic dysfunction[200], are unlikely to be accepted by elderly individuals. Therefore, the results appear to be largely unpredictable[208] in this specific age-group, particularly regarding increased physical activity[209].
The findings that decreased choline intake is associated with increased fibrosis in postmenopausal NAFLD women[210] and that fish consumption decreases HCC risk[211,212] fully support the recommendation for a balanced diet in the elderly NAFLD patient[213].
Drug treatment is an alternative/adjunctive approach to lifestyle changes. Such a choice might, in principle, appear to be more effective than lifestyle changes. However, to date, no drug has been specifically approved for use in NAFLD[208]. Moreover, current guidelines provide no specific therapeutic recommendations for those older than 65 years[19]. Finally, aging and NAFLD per se may expose these patients to a higher risk of polypharmacy and adverse drug reactions including drug-induced liver injury[214-216].
In the elderly, special attention should be paid to the early identification of cirrhosis and to the diagnosis and treatment of its complications. No significant differences in diagnostic investigations or treatment options are observed between the elderly and the young[217] except that liver transplantation is more rarely performed in the former age group. Fibrosis progression is best detected and monitored through non-invasive techniques[218]. However, after careful consideration of the cost/benefit balance of this procedure, liver biopsy might be considered in selected elderly patients[51].
Next we discuss the principles of treatment of the main metabolic risk factors associated with NAFLD in the elderly.
Dyslipidemia in NAFLD individuals generally requires aggressive management[219]. Statins, the main cholesterol-lowering class of drugs, are the most effective and widely used for the primary and secondary prevention of CVD[220]. Statins are remarkably safe in NAFLD patients[70,221,222]. The available small sample of mostly non-randomized studies have shown limited evidence of hepatic histological benefit in NAFLD[19,223]. Recently, two studies have shown that statins reduce CV events in patients with increased transaminases presumably due to NAFLD. Moreover, one of them reported that the CVD benefit was greater than in patients with normal liver tests[224]. Given that CVD is the main cause of death in NAFLD patients[75], statins play a key role in NAFLD management[225]. Finally, statins may also exert a potentially beneficial role both on portal hypertension and HCC chemoprevention[226-230].
Therapeutic options in the elderly must carefully balance the potential CV benefits vs the possible side effects and risk of drug interactions. No universal guidelines may be proposed, and treatment decisions need to be tailored considering the specific features of the individual patient, such as biological age, comorbidities and functional organ impairment[231].
Metformin is the first-line drug for the treatment of T2D. Despite beneficial effects on IR, it exerts only a small effect on serum aminotransferases and fails to improve liver histology in NAFLD[232]. Accordingly, metformin should not be used in non-diabetic NAFLD patients[19] although it has important beneficial effects on NAFLD complications/comorbidities: metformin moderately reduces the risk of HCC[233-235] and significantly decreases arterial stiffness, a predictor of cardiovascular mortality[236]. A particular concern in the elderly is represented by the frequent impairment of liver function, requiring the need for individualized dosage and warning that metformin is contra-indicated in the presence of overt kidney failure.
Glitazones (i.e., pioglitazone) are recommended by current guidelines for the treatment of subjects with NASH. Large randomized controlled trials reported a beneficial effect of pioglitazone on systemic IR and liver histology, although the advantage was limited for fibrosis[237-239]. However, the beneficial effects on liver histology disappeared after treatment discontinuation[240]. Moreover, pioglitazone - due to increased subcutaneous fat depots - is associated with weight gain; the increased risk of congestive heart failure and bone fractures and a slightly increased risk of bladder cancer limit its clinical appeal[19], especially in the elderly.
Finally, preliminary evidence for potentially favorable action has been described in an experimental model with pharmacological agents inducing a small increase in mitochondrial uncoupling, leading to an improvement of T2D and IR associated with decreased hepatic steatosis[241].
Angiotensin receptor blockers are widely used antihypertensive agents with a well-characterized safety profile. Chronic liver damage up-regulates the local tissue renin-angiotensin system, which contributes to the recruitment of inflammatory cells and the development of fibrosis[70]. Some animal and small sample human studies have suggested that angiotensin receptor blockers improve serum liver enzyme levels and histological features of NAFLD; however, further larger clinical trials are needed to corroborate these findings[75].
Recently, telmisartan has prevented hepatocarcinogenesis via inhibited hepatic angiogenesis in a rat NASH model[242].
When considering treatment with blood pressure-lowering agents, attention needs to be paid to the risk of orthostatic hypotension (see above).
The association between NAFLD and obesity in the elderly may be less relevant due to a physiologic weight loss occurring with aging[39]. Lifestyle modification through hypocaloric diet and increased physical activity is the mainstay of the treatment of obesity. A 5% to 10% weight loss is recommended to improve steatosis and NASH[19]. Consolidated habits and common physical limitations hamper lifestyle changes in the elderly. No specific drugs are approved for weight-loss, and indications to perform bariatric surgery are restricted by reduced life expectancy and comorbidities of elderly patients.
Vitamin E (800 IU/d) has been shown able to improve NASH histological features (except for fibrosis)[19]. However, no long-term follow-up data are available, and this vitamin is not recommended in those with diabetes[243]. Of major concern, high-dose Vitamin E supplementation has been linked to increased all-cause mortality[244].
Vitamin D deficiency is common in the elderly[245]. An increasing body of evidence suggests that low vitamin D status is associated with NAFLD and its histological severity as well as to T2D, MS and CV events[75]. Randomized clinical trials are needed to determine whether vitamin D supplementation improves NAFLD and reduces the incidence of cardiovascular outcomes.
Dietary prebiotic consumption, which modulates gut microbiota[246], although associated with subjective satiety, reduced postprandial glucose and insulin concentrations, exhibits inconsistent results regarding total energy intake, body weight, gut peptides, insulin sensitivity, serum lipids, inflammatory markers and immune function[247]. Moreover, despite encouraging results in animals[248-250], the effect of probiotics on metabolic parameters have provided conflicting results in humans. Transplantation of intestinal microbiota from lean donors improves insulin sensitivity in subjects with MS[251]. The wide use of this interesting approach, however, is likely to be limited by the potential risk of transmitting the recipient (unknown) pathogens. Further studies are needed to identify strategies to target gut microbiota composition as an innovative NAFLD treatment in the elderly.
Aging is a major social and economic challenge that will steadily increase in the coming decades. NAFLD is expected to play an important role in age-related liver disease in the setting of the secular worldwide trend towards an increment of metabolic diseases, notably including those associated with reduced insulin sensitivity such as obesity and T2D.
Of concern, NAFLD in the elderly is expected to carry a substantial burden of NASH, cirrhosis and HCC, which along with the worrying extra-hepatic manifestations and correlates of disease, makes proper identification and management of this condition a major task for clinical geriatricians and geriatric hepatologists. Careful consideration of the aspects that are peculiar to elderly subjects, including the typical geriatric syndromes (frailty, multimorbidity, polypharmacy, and dementia), is also required.
Proper treatment strategies will have to consider the peculiarities of the geriatric population and will require a multidisciplinary approach. Non-pharmacological treatment (diet and physical exercise) has to be individually tailored considering the physical limitations of most elderly people and the need for an adequate caloric supply. Similarly, the choice for drug treatment must carefully balance the benefits and costs in terms of adverse events and pharmacological interactions, in the common context of both multiple comorbidities and polypharmacy.
Further epidemiological and pathophysiological insight is warranted. More accurate understanding of the molecular mechanisms of geriatric NAFLD will help in identifying the most appropriate diagnostic and therapeutic approach in individual elderly patients.
This review article is dedicated to the memory of late Professor Paola Loria, M.D.
| 1. | World Health Organization. 2008-2013 Action Plan for the Global Strategy for the Prevention and Control of Noncommunicable Disease. Geneva, Switzerland: World Health Organization 2009; . |
| 2. | Jones K, Timchenko L, Timchenko NA. The role of CUGBP1 in age-dependent changes of liver functions. Ageing Res Rev. 2012;11:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Frith J, Day CP, Henderson E, Burt AD, Newton JL. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Mak KM, Chu E, Lau KH, Kwong AJ. Liver fibrosis in elderly cadavers: localization of collagen types I, III, and IV, α-smooth muscle actin, and elastic fibers. Anat Rec (Hoboken). 2012;295:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Anantharaju A, Feller A, Chedid A. Aging Liver. A review. Gerontology. 2002;48:343-353. [PubMed] |
| 6. | Jin J, Wang GL, Timchenko L, Timchenko NA. GSK3beta and aging liver. Aging (Albany NY). 2009;1:582-585. [PubMed] |
| 7. | Singh P, Goode T, Dean A, Awad SS, Darlington GJ. Elevated interferon gamma signaling contributes to impaired regeneration in the aged liver. J Gerontol A Biol Sci Med Sci. 2011;66:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Cuervo AM. Cell biology. Autophagy’s top chef. Science. 2011;332:1392-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Mallikarjuna K, Shanmugam KR, Nishanth K, Wu MC, Hou CW, Kuo CH, Reddy KS. Alcohol-induced deterioration in primary antioxidant and glutathione family enzymes reversed by exercise training in the liver of old rats. Alcohol. 2010;44:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Gan L, Chitturi S, Farrell GC. Mechanisms and implications of age-related changes in the liver: nonalcoholic Fatty liver disease in the elderly. Curr Gerontol Geriatr Res. 2011;2011:831536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (34)] |
| 12. | Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G. Cardiovascular and systemic risk in nonalcoholic fatty liver disease - atherosclerosis as a major player in the natural course of NAFLD. Curr Pharm Des. 2013;19:5177-5192. [PubMed] |
| 13. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [PubMed] |
| 14. | Ovchinsky N, Lavine JE. A critical appraisal of advances in pediatric nonalcoholic Fatty liver disease. Semin Liver Dis. 2012;32:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Della Corte C, Alisi A, Saccari A, De Vito R, Vania A, Nobili V. Nonalcoholic fatty liver in children and adolescents: an overview. J Adolesc Health. 2012;51:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Lonardo A, Carani C, Carulli N, Loria P. ‘Endocrine NAFLD’ a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. J Hepatol. 2006;44:1196-1207. [PubMed] |
| 17. | Koehler EM, Schouten JN, Hansen BE, van Rooij FJ, Hofman A, Stricker BH, Janssen HL. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J Hepatol. 2012;57:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 18. | Wang Z, Xu M, Peng J, Jiang L, Hu Z, Wang H, Zhou S, Zhou R, Hultström M, Lai EY. Prevalence and associated metabolic factors of fatty liver disease in the elderly. Exp Gerontol. 2013;48:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (4)] |
| 20. | Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 21. | Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Tani S, Goto M. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142-149. [PubMed] |
| 22. | Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954-961. [PubMed] |
| 23. | Shen L, Fan JG, Shao Y, Zeng MD, Wang JR, Luo GH, Li JQ, Chen SY. Prevalence of nonalcoholic fatty liver among administrative officers in Shanghai: an epidemiological survey. World J Gastroenterol. 2003;9:1106-1110. [PubMed] |
| 24. | Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508-514. [PubMed] |
| 25. | Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol. 2006;40:745-752. [PubMed] |
| 26. | Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138-143. [PubMed] |
| 27. | Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26:856-863. [PubMed] |
| 28. | Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, Hu PJ. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419-6424. [PubMed] |
| 29. | Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239-244. [PubMed] |
| 30. | Miyaaki H, Ichikawa T, Nakao K, Yatsuhashi H, Furukawa R, Ohba K, Omagari K, Kusumoto Y, Yanagi K, Inoue O. Clinicopathological study of nonalcoholic fatty liver disease in Japan: the risk factors for fibrosis. Liver Int. 2008;28:519-524. [PubMed] |
| 31. | Hu X, Huang Y, Bao Z, Wang Y, Shi D, Liu F, Gao Z, Yu X. Prevalence and factors associated with nonalcoholic fatty liver disease in Shanghai work-units. BMC Gastroenterol. 2012;12:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 33. | Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J Gastroenterol. 2012;18:237-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 408] [Article Influence: 29.1] [Reference Citation Analysis (2)] |
| 35. | Al-hamoudi W, El-Sabbah M, Ali S, Altuwaijri M, Bedewi M, Adam M, Alhammad A, Sanai F, Alswat K, Abdo A. Epidemiological, clinical, and biochemical characteristics of Saudi patients with nonalcoholic fatty liver disease: a hospital-based study. Ann Saudi Med. 2012;32:288-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Foster T, Anania FA, Li D, Katz R, Budoff M. The prevalence and clinical correlates of nonalcoholic fatty liver disease (NAFLD) in African Americans: the multiethnic study of atherosclerosis (MESA). Dig Dis Sci. 2013;58:2392-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. Am J Gastroenterol. 2013;108:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 38. | Yan J, Xie W, Ou WN, Zhao H, Wang SY, Wang JH, Wang Q, Yang YY, Feng X, Cheng J. Epidemiological survey and risk factor analysis of fatty liver disease of adult residents, Beijing, China. J Gastroenterol Hepatol. 2013;28:1654-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Lonardo A, Lombardini S, Scaglioni F, Ballestri S, Verrone AM, Bertolotti M, Carulli L, Ganazzi D, Carulli N, Loria P. Fatty liver, carotid disease and gallstones: a study of age-related associations. World J Gastroenterol. 2006;12:5826-5833. [PubMed] |
| 40. | Bedogni G, Miglioli L, Masutti F, Castiglione A, Crocè LS, Tiribelli C, Bellentani S. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. 2007;46:1387-1391. [PubMed] |
| 41. | Caballería L, Pera G, Auladell MA, Torán P, Muñoz L, Miranda D, Alumà A, Casas JD, Sánchez C, Gil D. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol. 2010;22:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Soresi M, Noto D, Cefalù AB, Martini S, Vigna GB, Fonda M, Manzato E, Cattin L, Fellin R, Averna MR. Nonalcoholic fatty liver and metabolic syndrome in Italy: results from a multicentric study of the Italian Arteriosclerosis society. Acta Diabetol. 2013;50:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] |
| 44. | Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore). 2012;91:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 45. | Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, Unalp-Arida A, Bass N. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 46. | North KE, Graff M, Franceschini N, Reiner AP, Feitosa MF, Carr JJ, Gordon-Larsen P, Wojczynski MK, Borecki IB. Sex and race differences in the prevalence of fatty liver disease as measured by computed tomography liver attenuation in European American and African American participants of the NHLBI family heart study. Eur J Gastroenterol Hepatol. 2012;24:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Nakajima T, Nakashima T, Yamaoka J, Shibuya A, Itoh Y, Yoshikawa T. Age is a negative, and visceral fat accumulation is a positive, contributor to hepatic steatosis, regardless of the fibrosis progression in Non-alcoholic Fatty Liver Disease. J Gastroenterol Hepatol Res. 2012;1:315-319. |
| 48. | Carulli L, Lonardo A, Lombardini S, Marchesini G, Loria P. Gender, fatty liver and GGT. Hepatology. 2006;44:278-279. [PubMed] |
| 49. | van der Poorten D, Samer CF, Ramezani-Moghadam M, Coulter S, Kacevska M, Schrijnders D, Wu LE, McLeod D, Bugianesi E, Komuta M. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology. 2013;57:2180-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 50. | Kagansky N, Levy S, Keter D, Rimon E, Taiba Z, Fridman Z, Berger D, Knobler H, Malnick S. Non-alcoholic fatty liver disease--a common and benign finding in octogenarian patients. Liver Int. 2004;24:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, Merriman R, Hameed B, Doo E, Kleiner DE. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Suzuki A, Diehl AM. Should nonalcoholic fatty liver disease be treated differently in elderly patients? Nat Clin Pract Gastroenterol Hepatol. 2005;2:208-209. [PubMed] |
| 53. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 934] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 54. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1346] [Article Influence: 103.5] [Reference Citation Analysis (1)] |
| 55. | Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, Schwimmer JB. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 56. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 57. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [PubMed] |
| 58. | Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578-584. [PubMed] |
| 59. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [PubMed] |
| 60. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. [PubMed] |
| 61. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1030] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 62. | Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 63. | Kodama K, Tokushige K, Hashimoto E, Taniai M, Shiratori K. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2013;37 Suppl 1:E247-E252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 65. | Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (2)] |
| 66. | Giannini EG, Marabotto E, Savarino V, Trevisani F, di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M, Borzio F. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 2009;7:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Stroffolini T, Trevisani F, Pinzello G, Brunello F, Tommasini MA, Iavarone M, Di Marco V, Farinati F, Del Poggio P, Borzio F. Changing aetiological factors of hepatocellular carcinoma and their potential impact on the effectiveness of surveillance. Dig Liver Dis. 2011;43:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Trevisani F, Cantarini MC, Labate AM, De Notariis S, Rapaccini G, Farinati F, Del Poggio P, Di Nolfo MA, Benvegnù L, Zoli M. Surveillance for hepatocellular carcinoma in elderly Italian patients with cirrhosis: effects on cancer staging and patient survival. Am J Gastroenterol. 2004;99:1470-1476. [PubMed] |
| 69. | Tajiri K, Shimizu Y. Liver physiology and liver diseases in the elderly. World J Gastroenterol. 2013;19:8459-8467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 150] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 70. | Maurantonio M, Ballestri S, Odoardi MR, Lonardo A, Loria P. Treatment of atherogenic liver based on the pathogenesis of nonalcoholic fatty liver disease: a novel approach to reduce cardiovascular risk? Arch Med Res. 2011;42:337-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Tarantino G, Caputi A. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J Gastroenterol. 2011;17:3785-3794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 72. | Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 73. | Loria P, Lonardo A, Targher G. Is liver fat detrimental to vessels?: intersections in the pathogenesis of NAFLD and atherosclerosis. Clin Sci (Lond). 2008;115:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Loria P, Lonardo A, Bellentani S, Day CP, Marchesini G, Carulli N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: an open question. Nutr Metab Cardiovasc Dis. 2007;17:684-698. [PubMed] |
| 75. | Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 76. | Akahoshi M, Amasaki Y, Soda M, Tominaga T, Ichimaru S, Nakashima E, Seto S, Yano K. Correlation between fatty liver and coronary risk factors: a population study of elderly men and women in Nagasaki, Japan. Hypertens Res. 2001;24:337-343. [PubMed] |
| 77. | Li M, Xu Y, Xu M, Ma L, Wang T, Liu Y, Dai M, Chen Y, Lu J, Liu J. Association between nonalcoholic fatty liver disease (NAFLD) and osteoporotic fracture in middle-aged and elderly Chinese. J Clin Endocrinol Metab. 2012;97:2033-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 78. | Wong VW, Wong GL, Tsang SW, Fan T, Chu WC, Woo J, Chan AW, Choi PC, Chim AM, Lau JY. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 80. | Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing. 2009;38:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Marchesini G, Bua V, Brunori A, Bianchi G, Pisi P, Fabbri A, Zoli M, Pisi E. Galactose elimination capacity and liver volume in aging man. Hepatology. 1988;8:1079-1083. [PubMed] |
| 82. | Wilson PW, Anderson KM, Harris T, Kannel WB, Castelli WP. Determinants of change in total cholesterol and HDL-C with age: the Framingham Study. J Gerontol. 1994;49:M252-M257. [PubMed] |
| 83. | Attili AF, Capocaccia R, Carulli N, Festi D, Roda E, Barbara L, Capocaccia L, Menotti A, Okolicsanyi L, Ricci G. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology. 1997;26:809-818. [PubMed] |
| 84. | Ericsson S, Eriksson M, Vitols S, Einarsson K, Berglund L, Angelin B. Influence of age on the metabolism of plasma low density lipoproteins in healthy males. J Clin Invest. 1991;87:591-596. [PubMed] |
| 85. | Einarsson K, Nilsell K, Leijd B, Angelin B. Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N Engl J Med. 1985;313:277-282. [PubMed] |
| 86. | Bertolotti M, Abate N, Bertolotti S, Loria P, Concari M, Messora R, Carubbi F, Pinetti A, Carulli N. Effect of aging on cholesterol 7 alpha-hydroxylation in humans. J Lipid Res. 1993;34:1001-1007. [PubMed] |
| 87. | Bertolotti M, Mussi C, Pellegrini E, Magni A, Del Puppo M, Ognibene S, Carulli L, Anzivino C, Baldelli E, Loria P. Age-associated alterations in cholesterol homeostasis: evidence from a cross-sectional study in a Northern Italy population. Clin Interv Aging. 2014;9:425-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12:950-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 89. | Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 373] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 90. | Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753-4761. [PubMed] |
| 91. | Lonardo A, Caldwell , CH , Loria P. Clinical physiology of NAFLD: a critical overview of pathogenesis and treatment. Expert Rev Endocrinol Metab. 2010;5:403-423. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Barzilai N, Ferrucci L. Insulin resistance and aging: a cause or a protective response? J Gerontol A Biol Sci Med Sci. 2012;67:1329-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Ceda GP, Dall’Aglio E, Magnacavallo A, Vargas N, Fontana V, Maggio M, Valenti G, Lee PD, Hintz RL, Hoffman AR. The insulin-like growth factor axis and plasma lipid levels in the elderly. J Clin Endocrinol Metab. 1998;83:499-502. [PubMed] |
| 94. | Paolisso G, Tagliamonte MR, Rizzo MR, Giugliano D. Advancing age and insulin resistance: new facts about an ancient history. Eur J Clin Invest. 1999;29:758-769. [PubMed] |
| 95. | Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Ble A, Egan J, Paolisso G, Najjar S, Jeffrey Metter E. Association between hormones and metabolic syndrome in older Italian men. J Am Geriatr Soc. 2006;54:1832-1838. [PubMed] |
| 96. | Bertolotti M, Gabbi C, Anzivino C, Crestani M, Mitro N, Del Puppo M, Godio C, De Fabiani E, Macchioni D, Carulli L. Age-related changes in bile acid synthesis and hepatic nuclear receptor expression. Eur J Clin Invest. 2007;37:501-508. [PubMed] |
| 97. | Arturi F, Succurro E, Procopio C, Pedace E, Mannino GC, Lugarà M, Procopio T, Andreozzi F, Sciacqua A, Hribal ML. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab. 2011;96:E1640-E1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 98. | Loria P, Carulli L, Bertolotti M, Lonardo A. Endocrine and liver interaction: the role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol. 2009;6:236-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 99. | Tarantino G, Finelli C. Pathogenesis of hepatic steatosis: the link between hypercortisolism and non-alcoholic fatty liver disease. World J Gastroenterol. 2013;19:6735-6743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 100. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [PubMed] |
| 101. | Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Sanguino E, Roglans N, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC. Atorvastatin reverses age-related reduction in rat hepatic PPARalpha and HNF-4. Br J Pharmacol. 2005;145:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Kuhla A, Blei T, Jaster R, Vollmar B. Aging is associated with a shift of fatty metabolism toward lipogenesis. J Gerontol A Biol Sci Med Sci. 2011;66:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 104. | Masetti M, Bianchi G, Gianotti G, Giovagnoli M, Vizioli L, Zorzi V, Rossi V, Forti P, Zoli M. Adipocyte-fatty acid binding protein and non-alcoholic fatty liver disease in the elderly. Aging Clin Exp Res. 2014;26:241-247. [PubMed] |
| 105. | Fontana L, Zhao E, Amir M, Dong H, Tanaka K, Czaja MJ. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology. 2013;57:995-1004. [PubMed] |
| 106. | Salvioli S, Monti D, Lanzarini C, Conte M, Pirazzini C, Bacalini MG, Garagnani P, Giuliani C, Fontanesi E, Ostan R. Immune system, cell senescence, aging and longevity--inflamm-aging reappraised. Curr Pharm Des. 2013;19:1675-1679. [PubMed] |
| 107. | Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16:4773-4783. [PubMed] |
| 108. | Savastano S, Di Somma C, Pizza G, De Rosa A, Nedi V, Rossi A, Orio F, Lombardi G, Colao A, Tarantino G. Liver-spleen axis, insulin-like growth factor-(IGF)-I axis and fat mass in overweight/obese females. J Transl Med. 2011;9:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:6198-6202. [PubMed] |
| 110. | García-Monzón C, Lo Iacono O, Mayoral R, González-Rodríguez A, Miquilena-Colina ME, Lozano-Rodríguez T, García-Pozo L, Vargas-Castrillón J, Casado M, Boscá L. Hepatic insulin resistance is associated with increased apoptosis and fibrogenesis in nonalcoholic steatohepatitis and chronic hepatitis C. J Hepatol. 2011;54:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 111. | Tarantino G, Scopacasa F, Colao A, Capone D, Tarantino M, Grimaldi E, Savastano S. Serum Bcl-2 concentrations in overweight-obese subjects with nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:5280-5288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Nakamura Y, Sekikawa A, Kadowaki T, Kadota A, Kadowaki S, Maegawa H, Kita Y, Evans RW, Edmundowicz D, Curb JD. Visceral and subcutaneous adiposity and adiponectin in middle-aged Japanese men: the ERA JUMP study. Obesity (Silver Spring). 2009;17:1269-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 114. | Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65:242-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 115. | Bambace C, Sepe A, Zoico E, Telesca M, Olioso D, Venturi S, Rossi A, Corzato F, Faccioli S, Cominacini L. Inflammatory profile in subcutaneous and epicardial adipose tissue in men with and without diabetes. Heart Vessels. 2014;29:42-48. [PubMed] |
| 116. | Stramaglia G, Greco A, Guglielmi G, De Matthaeis A, Vendemiale GL. Echocardiography and dual-energy x-ray absorptiometry in the elderly patients with metabolic syndrome: a comparison of two different tecniques to evaluate visceral fat distribution. J Nutr Health Aging. 2010;14:6-10. [PubMed] |
| 117. | Bertrand HA, Lynd FT, Masoro EJ, Yu BP. Changes in adipose mass and cellularity through the adult life of rats fed ad libitum or a life-prolonging restricted diet. J Gerontol. 1980;35:827-835. [PubMed] |
| 118. | Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 867] [Cited by in RCA: 836] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 119. | Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T. Dynamics of fat cell turnover in humans. Nature. 2008;453:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1625] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 120. | Bays HE, González-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 121. | Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J, Wang T, Wong S, Cartwright A, Hegardt FG. Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. Am J Physiol Endocrinol Metab. 2007;292:E1041-E1051. [PubMed] |
| 122. | Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 501] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 123. | Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84-91. [PubMed] |
| 124. | Tang QQ, Zhang JW, Daniel Lane M. Sequential gene promoter interactions of C/EBPbeta, C/EBPalpha, and PPARgamma during adipogenesis. Biochem Biophys Res Commun. 2004;319:235-239. [PubMed] |
| 125. | Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757-767. [PubMed] |
| 126. | Siersbaek R, Nielsen R, Mandrup S. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 2010;584:3242-3249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 127. | Floyd ZE, Stephens JM. Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARγ. Biochim Biophys Acta. 2012;1822:1090-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 128. | Gerin I, Dolinsky VW, Shackman JG, Kennedy RT, Chiang SH, Burant CF, Steffensen KR, Gustafsson JA, MacDougald OA. LXRbeta is required for adipocyte growth, glucose homeostasis, and beta cell function. J Biol Chem. 2005;280:23024-23031. [PubMed] |
| 129. | Finelli C, Sommella L, Gioia S, La Sala N, Tarantino G. Should visceral fat be reduced to increase longevity? Ageing Res Rev. 2013;12:996-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 130. | Hattori M, Taylor TD. The human intestinal microbiome: a new frontier of human biology. DNA Res. 2009;16:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 131. | Moore WE, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961-979. [PubMed] |
| 132. | Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810. [PubMed] |
| 133. | de Vos WM, Nieuwdorp M. Genomics: A gut prediction. Nature. 2013;498:48-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 134. | Bauer E, Williams BA, Smidt H, Verstegen MW, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol. 2006;7:35-51. [PubMed] |
| 135. | Rolfe RD. Colonization resistance. Gastrointestinal Microbiolog. London: Chapman and Hall 1997; 501-536. |
| 136. | Cummings JH, Macfarlane GT. Role of intestinal bacteria in nutrient metabolism. JPEN J Parenter Enteral Nutr. 1997;21:357-365. [PubMed] |
| 137. | Deguchi Y, Morishita T, Mutai M. Comparative studies on synthesis of water-soluble vitamins among human species of bifidobacteria. Agric Biol Chem. 1985;49:13-19. |
| 138. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [PubMed] |
| 139. | Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. [PubMed] |
| 140. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [PubMed] |
| 141. | Biagi E, Candela M, Fairweather-Tait S, Franceschi C, Brigidi P. Aging of the human metaorganism: the microbial counterpart. Age (Dordr). 2012;34:247-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 142. | Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 979] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 143. | O’Toole PW, Claesson MJ. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int Dairy J. 2010;20:281-291. |
| 144. | Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2387] [Article Influence: 170.5] [Reference Citation Analysis (0)] |
| 145. | Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027-1033. [PubMed] |
| 146. | van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71:6438-6442. [PubMed] |
| 147. | Lahtinen SJ, Tammela L, Korpela J, Parhiala R, Ahokoski H, Mykkänen H, Salminen SJ. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age (Dordr). 2009;31:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 148. | Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4586-4591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1277] [Article Influence: 85.1] [Reference Citation Analysis (2)] |
| 149. | Cheng J, Palva AM, de Vos WM, Satokari R. Contribution of the intestinal microbiota to human health: from birth to 100 years of age. Curr Top Microbiol Immunol. 2013;358:323-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 150. | Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res. 2013;69:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 151. | Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 152. | Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920-1925. [PubMed] |
| 153. | Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda). 2008;23:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 154. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3922] [Cited by in RCA: 3595] [Article Influence: 211.5] [Reference Citation Analysis (4)] |
| 155. | Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4098] [Cited by in RCA: 4687] [Article Influence: 312.5] [Reference Citation Analysis (1)] |
| 156. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3175] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 157. | Boyce JM, Shone GR. Effects of ageing on smell and taste. Postgrad Med J. 2006;82:239-241. [PubMed] |
| 158. | Gupta A, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841-846. [PubMed] |
| 159. | Laurin D, Brodeur JM, Bourdages J, Vallée R, Lachapelle D. Fibre intake in elderly individuals with poor masticatory performance. J Can Dent Assoc. 1994;60:443-446, 449. [PubMed] |
| 160. | Elphick HL, Elphick DA, Sanders DS. Small bowel bacterial overgrowth. An underrecognized cause of malnutrition in older adults. Geriatrics. 2006;61:21-26. [PubMed] |
| 161. | Oudshoorn C, van der Cammen TJ, McMurdo ME, van Leeuwen JP, Colin EM. Ageing and vitamin D deficiency: effects on calcium homeostasis and considerations for vitamin D supplementation. Br J Nutr. 2009;101:1597-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 162. | Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP, Vieth R, Pettifor JM, Dawson-Hughes B, Lamberg-Allardt CJ. 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2010;121:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 163. | Dali-Youcef N, Andrès E. An update on cobalamin deficiency in adults. QJM. 2009;102:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 164. | de Groot LC, Verheijden MW, de Henauw S, Schroll M, van Staveren WA. Lifestyle, nutritional status, health, and mortality in elderly people across Europe: a review of the longitudinal results of the SENECA study. J Gerontol A Biol Sci Med Sci. 2004;59:1277-1284. [PubMed] |
| 165. | Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc. 2001;33:1524-1532. [PubMed] |
| 166. | Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol. 2012;302:G1-G9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 167. | Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 168. | Genser D. Food and drug interaction: consequences for the nutrition/health status. Ann Nutr Metab. 2008;52 Suppl 1:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 169. | Karlström O, Fryklund B, Tullus K, Burman LG. A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. The Swedish C. difficile Study Group. Clin Infect Dis. 1998;26:141-145. [PubMed] |
| 170. | Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 171. | Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:11S-18S. [PubMed] |
| 172. | Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889-896. [PubMed] |
| 173. | Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [PubMed] |
| 174. | Kitajima Y, Eguchi Y, Ishibashi E, Nakashita S, Aoki S, Toda S, Mizuta T, Ozaki I, Ono N, Eguchi T. Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol. 2010;45:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 175. | Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 321] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 176. | Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, Lattanzio F. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15:890-895. [PubMed] |
| 177. | Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 178. | Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022-1027. [PubMed] |
| 179. | Sanada K, Iemitsu M, Murakami H, Gando Y, Kawano H, Kawakami R, Tabata I, Miyachi M. Adverse effects of coexistence of sarcopenia and metabolic syndrome in Japanese women. Eur J Clin Nutr. 2012;66:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 180. | Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 181. | Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766-5772. [PubMed] |
| 182. | Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 183. | Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE, Kowdley KV. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 184. | Li L, Zhang L, Pan S, Wu X, Yin X. No significant association between vitamin D and nonalcoholic fatty liver disease in a Chinese population. Dig Dis Sci. 2013;58:2376-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 185. | Newton JL, Hollingsworth KG, Taylor R, El-Sharkawy AM, Khan ZU, Pearce R, Sutcliffe K, Okonkwo O, Davidson A, Burt J. Cognitive impairment in primary biliary cirrhosis: symptom impact and potential etiology. Hepatology. 2008;48:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 186. | Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, Wesnes KA, Taylor-Robinson SD. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433-439. [PubMed] |
| 187. | Yilmaz Y, Ozdogan O. Liver disease as a risk factor for cognitive decline and dementia: an under-recognized issue. Hepatology. 2009;49:698. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 188. | Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045-1050. [PubMed] |
| 189. | Abbatecola AM, Ferrucci L, Marfella R, Paolisso G. Insulin resistance and cognitive decline may be common soil for frailty syndrome. Arch Intern Med. 2007;167:2145-2146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 190. | Koplay M, Gulcan E, Ozkan F. Association between serum vitamin B12 levels and the degree of steatosis in patients with nonalcoholic fatty liver disease. J Investig Med. 2011;59:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 191. | O’Leary F, Allman-Farinelli M, Samman S. Vitamin B12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr. 2012;108:1948-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 192. | Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476-483. [PubMed] |
| 193. | Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178-193. [PubMed] |
| 194. | Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043-1065. [PubMed] |
| 195. | Liu YC, Hung CS, Wu YW, Lee YC, Lin YH, Lin C, Lo MT, Chan CC, Ma HP, Ho YL. Influence of non-alcoholic fatty liver disease on autonomic changes evaluated by the time domain, frequency domain, and symbolic dynamics of heart rate variability. PLoS One. 2013;8:e61803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 196. | Guzzetti S, Borroni E, Garbelli PE, Ceriani E, Della Bella P, Montano N, Cogliati C, Somers VK, Malliani A, Porta A. Symbolic dynamics of heart rate variability: a probe to investigate cardiac autonomic modulation. Circulation. 2005;112:465-470. [PubMed] |
| 197. | Frith J, Newton JL. Autonomic dysfunction in chronic liver disease. Liver Int. 2009;29:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 198. | Newton JL. Systemic symptoms in non-alcoholic fatty liver disease. Dig Dis. 2010;28:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 199. | Newton JL, Pairman J, Wilton K, Jones DE, Day C. Fatigue and autonomic dysfunction in non-alcoholic fatty liver disease. Clin Auton Res. 2009;19:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 200. | Jakovljevic DG, Hallsworth K, Zalewski P, Thoma C, Klawe JJ, Day CP, Newton J, Trenell MI. Resistance exercise improves autonomic regulation at rest and haemodynamic response to exercise in non-alcoholic fatty liver disease. Clin Sci (Lond). 2013;125:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 201. | Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 202. | Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Rodella S, Zoppini G, Mantovani W, Barbieri E, Byrne CD. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8:e57183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 203. | Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 204. | Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, Siebelink HM, Smit JW, Diamant M, Romijn JA. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation. 2010;122:2538-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 205. | Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 206. | Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886-2891. [PubMed] |
| 207. | de Carvalho SC, Muniz MT, Siqueira MD, Siqueira ER, Gomes AV, Silva KA, Bezerra LC, D’Almeida V, de Oliveira CP, Pereira LM. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD). Nutr J. 2013;12:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 208. | Mazzella N, Ricciardi LM, Mazzotti A, Marchesini G. The role of medications for the management of patients with NAFLD. Clin Liver Dis. 2014;18:73-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 209. | Scaglioni F, Marino M, Ciccia S, Procaccini A, Busacchi M, Loria P, Lonardo A, Malavolti M, Battistini NC, Pellegrini M. Short-term multidisciplinary non-pharmacological intervention is effective in reducing liver fat content assessed non-invasively in patients with nonalcoholic fatty liver disease (NAFLD). Clin Res Hepatol Gastroenterol. 2013;37:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 210. | Guerrerio AL, Colvin RM, Schwartz AK, Molleston JP, Murray KF, Diehl A, Mohan P, Schwimmer JB, Lavine JE, Torbenson MS. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr. 2012;95:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 211. | Fedirko V, Trichopolou A, Bamia C, Duarte-Salles T, Trepo E, Aleksandrova K, Nöthlings U, Lukanova A, Lagiou P, Boffetta P. Consumption of fish and meats and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Oncol. 2013;24:2166-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 212. | Sawada N, Inoue M, Iwasaki M, Sasazuki S, Shimazu T, Yamaji T, Takachi R, Tanaka Y, Mizokami M, Tsugane S. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology. 2012;142:1468-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 213. | Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 214. | Stine JG, Sateesh P, Lewis JH. Drug-induced liver injury in the elderly. Curr Gastroenterol Rep. 2013;15:299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 215. | Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 216. | Tarantino G, Conca P, Basile V, Gentile A, Capone D, Polichetti G, Leo E. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415. [PubMed] |
| 217. | Premoli A, Paschetta E, Hvalryg M, Spandre M, Bo S, Durazzo M. Characteristics of liver diseases in the elderly: a review. Minerva Gastroenterol Dietol. 2009;55:71-78. [PubMed] |
| 218. | Estep JM, Birerdinc A, Younossi Z. Non-invasive diagnostic tests for non-alcoholic fatty liver disease. Curr Mol Med. 2010;10:166-172. [PubMed] |
| 219. | Corey KE, Chalasani N. Management of dyslipidemia as a cardiovascular risk factor in individuals with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:1077-1084; quiz e59-e60. [PubMed] |
| 220. | Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1871] [Cited by in RCA: 2016] [Article Influence: 134.4] [Reference Citation Analysis (0)] |
| 221. | Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690-695. [PubMed] |
| 222. | Argo CK, Loria P, Caldwell SH, Lonardo A. Statins in liver disease: a molehill, an iceberg, or neither? Hepatology. 2008;48:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 223. | Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, Gasbarrini A, Loguercio C, Lonardo A, Marchesini G. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 224. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 225. | Lonardo A, Loria P. If steatosis is the atherosclerosis of the liver, are statins the “aspirin” for steatosis? Dig Liver Dis. 2012;44:451-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 226. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 227. | Ramírez G, Briceño J, Rojas A. Statins and portal hypertension: a new pharmacological challenge. Curr Vasc Pharmacol. 2012;10:767-772. [PubMed] |
| 228. | Lonardo A, Loria P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1654-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 229. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 362] [Article Influence: 27.8] [Reference Citation Analysis (1)] |
| 230. | Pradelli D, Soranna D, Scotti L, Zambon A, Catapano A, Mancia G, La Vecchia C, Corrao G. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev. 2013;22:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 231. | Vigna GB, Zuliani G, Fellin R. Dyslipidemias in the older subject: features, significance and treatment dilemmas. Clin Lipidol. 2011;6:339-350. |
| 232. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 233. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881-891; quiz 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 234. | Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 235. | Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 313] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 236. | Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1036] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 237. | Ratziu V, Caldwell S, Neuschwander-Tetri BA. Therapeutic trials in nonalcoholic steatohepatitis: insulin sensitizers and related methodological issues. Hepatology. 2010;52:2206-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 238. | Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 239. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 506] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 240. | Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, Loomba R, Liang TJ, Premkumar A. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 241. | Perry RJ, Shulman GI. Treating fatty liver and insulin resistance. Aging (Albany NY). 2013;5:791-792. [PubMed] |
| 242. | Tamaki Y, Nakade Y, Yamauchi T, Makino Y, Yokohama S, Okada M, Aso K, Kanamori H, Ohashi T, Sato K. Angiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitis. J Gastroenterol. 2013;48:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 243. | Younossi ZM, Reyes MJ, Mishra A, Mehta R, Henry L. Systematic review with meta-analysis: non-alcoholic steatohepatitis - a case for personalised treatment based on pathogenic targets. Aliment Pharmacol Ther. 2014;39:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 244. | Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [PubMed] |
| 245. | Pérez-López FR, Chedraui P, Fernández-Alonso AM. Vitamin D and aging: beyond calcium and bone metabolism. Maturitas. 2011;69:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 246. | D’Aversa F, Tortora A, Ianiro G, Ponziani FR, Annicchiarico BE, Gasbarrini A. Gut microbiota and metabolic syndrome. Intern Emerg Med. 2013;8 Suppl 1:S11-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 247. | Kellow NJ, Coughlan MT, Reid CM. Metabolic benefits of dietary prebiotics in human subjects: a systematic review of randomised controlled trials. Br J Nutr. 2014;111:1147-1161. [PubMed] |
| 248. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3224] [Cited by in RCA: 3621] [Article Influence: 201.2] [Reference Citation Analysis (0)] |
| 249. | Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, Matsuzaki T, Miyazaki K, Ishikawa F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 250. | Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 251. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-916.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2075] [Article Influence: 148.2] [Reference Citation Analysis (0)] |
P- Reviewer: de Oliveira C, Kayadibi H, Penkova-Radicheva MP, Rouabhia S, Tarantino G S- Editor: Gou SX L- Editor: A E- Editor: Wang CH