Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13521
Revised: March 10, 2014
Accepted: May 28, 2014
Published online: October 7, 2014
Processing time: 343 Days and 6.3 Hours
AIM: To investigate the role of epidermal growth factor (EGF) in visceral hypersensitivity and its effect on the serotonin transporter (SERT).
METHODS: A rat model for visceral hypersensitivity was established by intra-colonic infusion of 0.5% acetic acid in 10-d-old Sprague-Dawley rats. The visceral sensitivity was assessed by observing the abdominal withdrawal reflex and recording electromyographic activity of the external oblique muscle in response to colorectal distension. An enzyme-linked immunosorbent assay was used to measure the EGF levels in plasma and colonic tissues. SERT mRNA expression was detected by real-time PCR while protein level was determined by Western blot. The correlation between EGF and SERT levels in colon tissues was analyzed by Pearson’s correlation analysis. SERT function was examined by tritiated serotonin (5-HT) uptake experiments. Rat intestinal epithelial cells (IEC-6) were used to examine the EGF regulatory effect on SERT expression and function via the EGF receptor (EGFR).
RESULTS: EGF levels were significantly lower in the rats with visceral hypersensitivity as measured in plasma (2.639 ± 0.107 ng/mL vs 4.066 ± 0.573 ng/mL, P < 0.01) and in colonic tissue (3.244 ± 0.135 ng/100 mg vs 3.582 ± 0.197 ng/100 mg colon tissue, P < 0.01) compared with controls. Moreover, the EGF levels were positively correlated with SERT levels (r = 0.820, P < 0.01). EGF displayed dose- and time-dependent increased SERT gene expressions in IEC-6 cells. An EGFR kinase inhibitor inhibited the effect of EGF on SERT gene upregulation. SERT activity was enhanced following treatment with EGF (592.908 ± 31.515 fmol/min per milligram vs 316.789 ± 85.652 fmol/min per milligram protein, P < 0.05) and blocked by the EGFR kinase inhibitor in IEC-6 cells (590.274 ± 25.954 fmol/min per milligram vs 367.834 ± 120.307 fmol/min per milligram protein, P < 0.05).
CONCLUSION: A decrease in EGF levels may contribute to the formation of visceral hypersensitivity through downregulation of SERT-mediated 5-HT uptake into enterocytes.
Core tip: Results of this study show that visceral hypersensitivity results in a decrease in the plasma and colon tissue levels of epidermal growth factor (EGF). Moreover, the EGF levels were positively correlated with serotonin transporter (SERT) levels. SERT gene expression and protein activity were upregulated in a dose- and time-dependent manner by EGF, and an inhibitor of the EGF receptor kinase blocked SERT gene expression and activity in an intestinal epithelial cell line. The data suggest that decreased EGF levels may contribute to the formation of visceral hypersensitivity through downregulation of SERT activity.
- Citation: Cui XF, Zhou WM, Yang Y, Zhou J, Li XL, Lin L, Zhang HJ. Epidermal growth factor upregulates serotonin transporter and its association with visceral hypersensitivity in irritable bowel syndrome. World J Gastroenterol 2014; 20(37): 13521-13529
- URL: https://www.wjgnet.com/1007-9327/full/v20/i37/13521.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i37.13521
Irritable bowel syndrome (IBS), a common chronic functional gastrointestinal disease, is characterized by abdominal pain and discomfort, and bowel disturbance. The pathogenesis of IBS remains unclear; however, visceral hypersensitivity is the most likely cause for the motor and sensory abnormalities in IBS patients[1]. Recent reports indicate abnormalities in serotonergic signaling systems being involved in the development of IBS, particularly those affecting serotonin (5-HT) levels in the gastrointestinal tract[2]. Therefore, it is of interest to investigate the role of this pathway in the pathogenesis of IBS.
High levels of 5-HT have been found in the intestinal mucosal tissue of IBS patients, especially those with constipation[3]. 5-HT is known to facilitate communication between the enteric nervous system and its effector systems (muscles, secretory endothelium, endocrine cells, and vasculature of the gastrointestinal tract). An increase in 5-HT can lead to gastrointestinal motility disorder and visceral hypersensitivity[4]. Accumulating evidence suggests that alterations in serotonergic signaling exist in the gut of IBS patients, including alterations in 5-HT biosynthesis, release, and/or reuptake[5,6].
The serotonin transporter (SERT) is mainly localized to the apical membrane of intestinal epithelial cells. Due to its role in reuptake of 5-HT, SERT plays an important part in terminating transmitter action and maintaining transmitter homeostasis[7,8]. SERT gene expression is downregulated in the colon[9] and rectal tissues[10] of patients with IBS and inflammatory bowel disease. The downregulation may contribute to the pathophysiology of these gastrointestinal disorders; however, the underlying mechanisms are still not fully understood.
Previous studies have demonstrated that epidermal growth factor (EGF) upregulates the reuptake of 5-HT by increasing SERT transcription in human intestinal epithelial cells[11,12]. EGF is a 53-amino acids peptide with a variety of biologic functions. In the gut, EGF plays an important role in intestinal proliferation, differentiation, and maturation[13]. EGF affects various processes by binding to the EGF receptor (EGFR), which is expressed on the basolateral surface of both human and rat intestinal epithelial cells[14] and is associated with certain bowel diseases, such as inflammatory bowel disease[15,16]. Our preliminary findings demonstrated that plasma EGF levels were decreased in IBS patients. To date, the role of EGF in IBS patients remains unknown. Some studies report that SERT-mediated alterations of 5-HT levels in the intestinal space are related to IBS-like syndrome[17,18].
We hypothesized that EGF regulates SERT expression via EGFR and SERT, consequently mediating the 5-HT reuptake, which may be involved in visceral hypersensitivity. In this study, a rodent model of visceral hypersensitivity was established by a two-week colonic infusion of 0.5% acetic acid to produce persistent chronic hypersensitivity[18-20]. SERT expression was evaluated in this model, and the association of EGF levels with SERT expression was assessed. Lastly, the effect of EGF treatment on SERT expression and its function via EGFR in intestinal epithelial cells were investigated.
Sprague-Dawley male rats were purchased from Beijing Vital River Laboratories Animal Technology Co., Ltd. (Beijing, China). Rats were housed with ad libitum food in standard rodent cages at 22 °C in a 12 h light-dark controlled room. All procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Nanjing, China), and were in accordance with the guidelines of the International Association for the Study of Pain (IASP; Washington, DC, United States).
Ten-day-old male rats (n = 10) received an intracolonic infusion of 0.2 mL of 0.5% (v/v) acetic acid in saline, 2 cm from the anus. Control 10-d-old pups (n = 10) were infused with an equal volume of saline alone[19,20]. All of the experiments were conducted on 8-wk-old rats. Two parallel electrodes were implanted in the external oblique muscles of control and colonic-sensitized rats under anesthesia with pentobarbital sodium (50 mg/kg, intraperitoneal). The end of electrode was extended to the back of the necks in both groups for electromyography (EMG) recording. All postoperative rats were housed quietly for seven days in individual cages to recuperate.
The visceral sensitivity of the rats was assessed by observing the abdominal withdrawal reflex (AWR) and EMG activity of the external oblique muscle in response to colorectal distension (CRD), as previously described[19-21]. Briefly, the rats were anesthetized with ether, followed by insertion of a flexible balloon, attached to a graded pressure system, into the colon 8 cm from the anus and fixed on the tail. The EMG activity of the external oblique muscle in response to CRD was measured at different pressures (20, 40, 60, and 80 mmHg). After the rats awoke, CRD was performed with 20 s of distention, followed by a complete balloon deflation for 2 min to rest between different distentions. Using a PowerLab data acquisition system (8SP; AD Instruments, Sydney, Australia) to record EMG, the area under the curve was analyzed for each EMG signal using an in-house written computer program. AWR in response to CRD was assessed by an observer without prior knowledge of the test/control groups. AWR was scored as follows: 0 = normal behavior with no response, 1 = contraction of abdominal muscles, 2 = lifting of the abdominal wall, and 3 = body arching and lifting of pelvis[20].
After behavior testing, colon tissue specimens (4 cm proximal to the anus) were removed from rats of both groups and fixed in 10% (w/v) buffered formalin. Tissue specimens were embedded in paraffin, cut into 4 μm sections, and stained with hematoxylin and eosin. A pathologist blindly assessed and assigned an inflammatory grade to each section.
EGF levels in plasma and colon tissues were quantified using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R and D Systems Inc., Minneapolis, MN, United States). Following rat anesthetization, 1 mL blood samples were removed from the orbital canthus vein plexus and collected into heparin tubes followed by centrifugation at 1000 ×g for 5 min at room temperature. The plasma (supernatant) was then collected and stored at -70 °C until analysis. At the same time, colon tissue samples (0.1 g) were homogenized in phosphate-buffered saline with a glass homogenizer on ice. The homogenates were then sonicated with an ultrasonic cell disrupter (New Cheese Biotech Company, NingBo, China) and centrifuged for 5 min at 5000 ×g at 4 °C. The supernatants were collected and stored at -70 °C until EGF analysis.
Rat intestinal epithelial cells (IEC-6) at the 10th culture passage were obtained from the American Type Culture Collection (Manassas, VA, United States) and were cultured as previously described[22]. Briefly, IEC-6 cells were grown in Dulbecco’s Modified Eagle Medium (Gibco of Thermo Fisher Scientific Inc., Waltham, MA, United States), supplemented with 10% FBS (Gibco), 2 mmol/L L-glutamine, 10 mL/L antibiotic solution containing penicillin G (10000 U/mL) and streptomycin (10000 μg/mL; Gibco), and 0.01 mg/mL insulin (Sigma-Aldrich, St. Louis, MO, United States). IEC-6 cells were seeded in polystyrene plastic culture dishes (Corning Inc., Corning, NY, United States), grown for four days (37 °C and 5% CO2), and used up to the 25th passage. The medium was changed every 2-3 d. All cell treatments were conducted in serum-free medium following a 1 h period of serum starvation. IEC-6 cell monolayers were treated with 0-160 ng/mL EGF (0203B16; PeproTech, Rocky Hill, NJ, United States) for 0-48 h or with 10 μmol/L EGFR specific kinase inhibitor PD153035 (Sigma-Aldrich) to block EGFR action.
Intestinal tissue samples were homogenized in potency lysate buffer [25 mmol/L Tris-HCl (pH 7.5); 5 mmol/L EDTA, 5 mmol/L EGTA, 0.5 mmol/L PMSF, 25 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mmol/L sodium vanadate] with a glass homogenizer on ice. Cells were lysed in ice-cold cell lysis buffer [20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1% (w/v) Triton X-100, 0.1% (w/v) sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L EDTA, 1 mmol/L Na3VO4, 2 μg/mL leupeptin). Protein concentrations were determined by a BCA protein assay kit (#23250; Thermo Fisher Scientific Inc.). The remaining supernatant (36 μL) was combined (1:1) with 1 × sodium dodecyl sulfate polyacrylamide gel electrophoresis loading buffer, and boiled for 5 min. Approximately 30 μg of protein from each sample was run on a 10% polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane for immunoblotting as previously reported[23,24]. The membranes were blocked with 5% (w/v) non-fat milk, and SERT protein was detected using rabbit anti-SERT polyclonal antibody (AB9726, 1:500; EMD Millipore, Billerica, MA, United States). Mouse anti-GAPDH (Macclesfield, Cheshire, United Kingdom) polyclonal antibody (1:3000) was used as a reference. The membranes were incubated with either horseradish peroxidase-conjugated goat anti-rabbit (BS13278, 1:3000; Bioworld, Louis Park, MN, United States) or goat anti-mouse secondary antibodies (BS12478, 1:3000; Bioworld). All the antibodies were diluted with 5% (w/v) non-fat milk. The SERT/GAPDH ratio was calculated from the films with the Quantity One Analysis Software (Bio-Rad, Hercules, CA, United States), and the results were expressed in densitometric units.
The total RNA from control and EGF-treated cells was extracted using TRIzol reagent® (Invitrogen of Thermo Fisher Scientific Inc.). After cDNA was synthesized with a two-step reverse transcription kit (Takara, Dalian, China), real-time PCR was performed on an Applied Biosystems 7500 Real-time PCR System using the SYBR Premix Ex Taq Kit (Applied Biosystems of Thermo Fisher Scientific Inc.) in a 96-well plate. PCR cycle parameters were as follows: initial denaturation at 95 °C at 30 s, 40 cycles of 95 °C for 5 s and 60 °C for 34 s, followed by a dissociation stage for recording the melting curve. The cycle threshold (Ct) values of all genes were obtained, and the relative level of SERT gene was normalized to a housekeeping β-actin gene. Data were analyzed according to the relative expression using the 2-ΔΔCt method. Each sample was run in triplicate, and the mean values are presented. Primers used for the PCR were: forward, 5’-GACTCCTCCCCTCTAAGCCA-3’ and reverse, 5’-CACGGAAAGAAGTGGTCGGA- 3’ for SERT; forward, 5’-CTAAGGCCAACCGTGAAAAG-3’ and reverse, 5’-TCTCAGCTGTGGTGGTGAAG-3’ for β-actin.
[3H]-5-HT uptake was examined as previously described[8,25]. Briefly, cells were plated in poly-D-lysine-coated (0.1 mg/mL) 12-well plates (Corning, Inc.) for 48 h before the uptake experiments. Cells (at 80%-90% confluence) were treated either with the optimal dose of EGF (40 ng/mL) in a serum-free medium or with an equal volume of the albumin-containing medium for 24 h. For the [3H]-5-HT uptake assay, the growth medium was completely removed, and 1 mL of Krebs-Ringer’s (KRH) buffer (130 mmol/L NaCl, 1.3 mmol/L KCl, 2.2 mmol/L CaCl2, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 1.8 g/L glucose, 10 mmol/L HEPES, pH 7.4) was used to wash the cells twice. Next, cells were incubated in KRH buffer containing 100 μmol/L pargyline and 100 μmol/L ascorbic acid in a 5% CO2 homothermal chamber (37 °C) for 10 min. The uptake experiment was initiated by adding 0.1 μM [3H]-5-HT (27.9 Ci/mmol, NET498; PerkinElmer, Waltham, MA, United States) and incubating the cells at 37 °C for another 10 min. The uptake assays were terminated by three rapid washes with cold KRH buffer. The cells were then lysed with 600 μL of 0.5 N NaOH, and 100 μL of the cell lysate was diluted in OptiPhase SuperMix scintillation mixture (Wallac, Gaithersburg, MD, United States) for direct quantification of radioactivity in a Wallac liquid scintillation counter (PerkinElmer). The total protein in the cell lysates was determined using a Bradford assay kit (Beyotime Biotechnology, Haimen, China). Specific SERT-mediated uptake was calculated by subtracting the uptake of [3H]-5-HT in the presence of 100 μM paroxetine, a SERT inhibitor (Enzo, Farmingdale, NY, United States), from the total uptake.
The data were analyzed using SPSS 18.0 software (SPSS Inc., Chicago, IL, United States). Results are expressed as mean ± SD. Two-tailed Student’s t tests were used to analyze differences between two mean values. Two-way repeated measures analysis of variance (ANOVA) was used to evaluate whether the AWR scores and/or EMG area under the curves were altered in the test and/or control groups at different pressures. The remaining data were analyzed using one-way ANOVAs. Values were considered statistically significant if P was < 0.05.
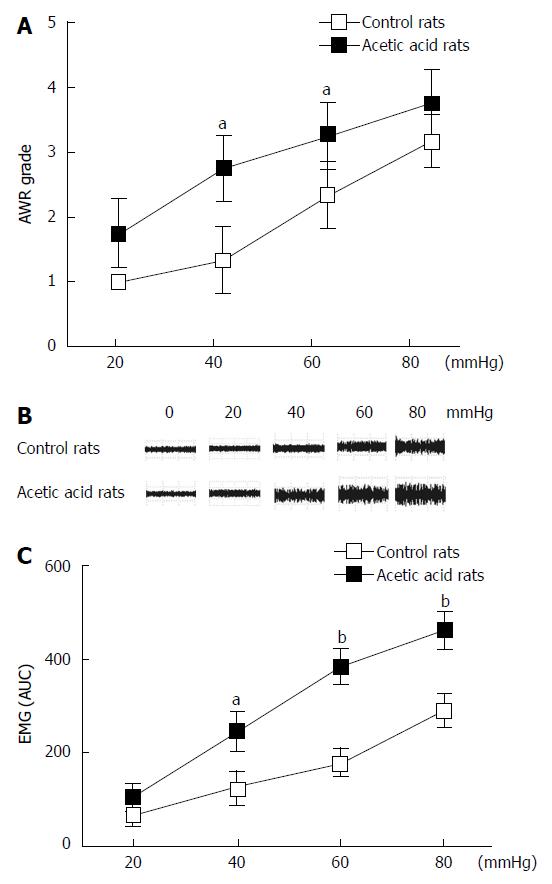
Neonatal rats (n = 10) treated with 0.5% acetic acid developed visceral hypersensitivity. Compared with the control rats (n = 10), the AWR scores in the acetic acid-treated rats were higher during CRD at all tested distension pressures (Figure 1A). These differences were significant at distension pressures of 40 mmHg (P < 0.01) and 60 mmHg (P < 0.05). Similar to the AWR results, acetic acid-treated rats showed increased EMG responses to CRD at three distension pressures tested (Figure 1B). The EMG area under the curve of the acetic acid-treated rats was significantly higher compared to the controls at 40 mmHg (P < 0.05), 60 mmHg (P < 0.01), and 80 mmHg (P < 0.01) (Figure 1C). No evidence of inflammation or structural abnormalities was found in the control or acetic acid-treated rats, indicating successful establishment of visceral hypersensitivity.
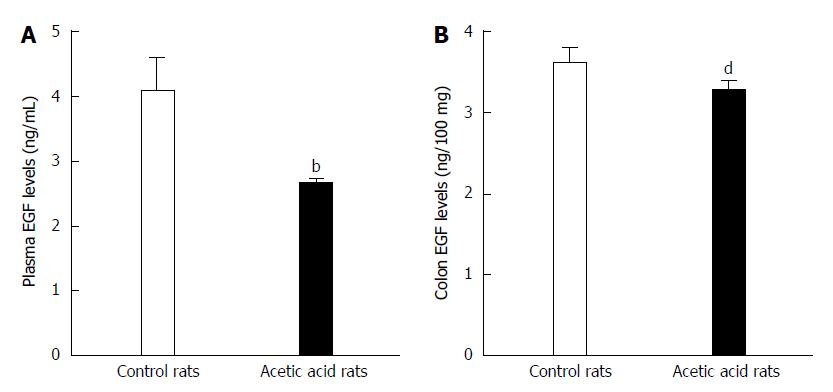
ELISA was used to detect any potential differences in EGF levels between the visceral hypersensitive and control group rats. As shown in Figure 2A, the visceral-sensitized rats had significantly lower plasma EGF levels compared with control rats (2.639 ± 0.107 ng/mL vs 4.066 ± 0.573 ng/mL, P < 0.01). The EGF levels in colon tissue from visceral-sensitized rats were also significantly decreased compared with control rats (3.244 ± 0.135 ng/100 mg vs 3.582 ± 0.197 ng/100 mg colon tissue, P < 0.01) (Figure 2B).
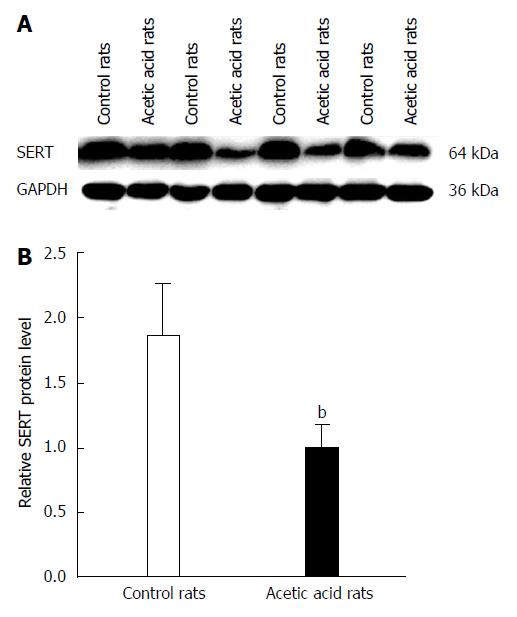
Western blot analysis showed a decrease in SERT protein levels in colon tissue of the visceral-sensitized rats (Figure 3). Analysis of the relationship between EGF and SERT levels in colon tissue showed a positive correlation (r = 0.820, P < 0.01).
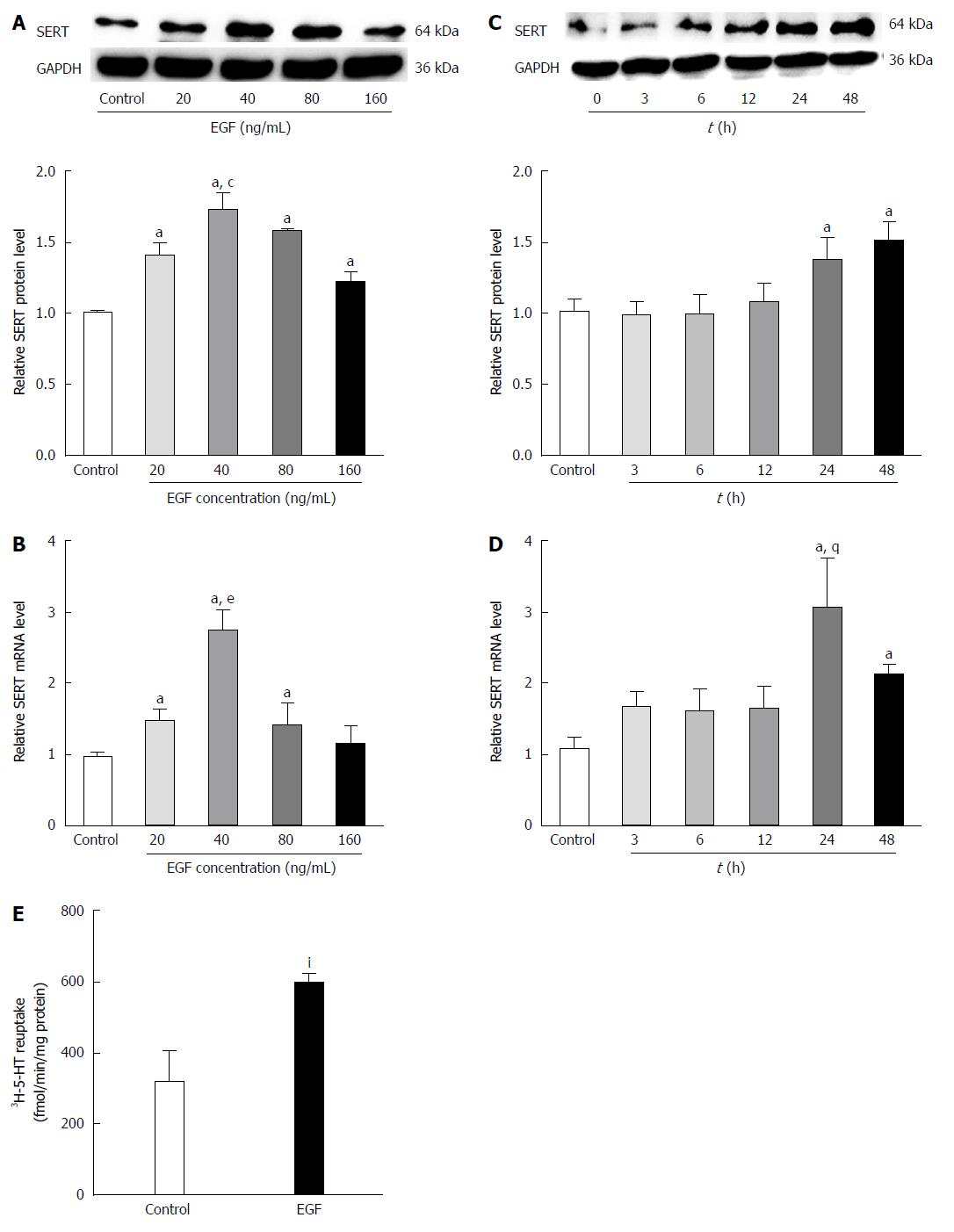
To observe the effect of EGF on SERT levels, IEC-6 cells were treated with various concentrations of EGF for 24 h followed by western blot analysis of SERT protein levels. Our results indicate that EGF upregulates SERT in a dose-dependent manner, with a peak at a dose of 40 ng/mL (Figure 4A). IEC-6 cells were also treated with the optimal dose of EGF (40 ng/mL) for 0, 3, 6, 12, 24, or 48 h. SERT expression was upregulated after a 12 h EGF treatment, with maximal effects after 24-48 h (Figure 4C). The real-time PCR analysis of the EGF effects on SERT gene expression showed that EGF increased SERT mRNA expression in a dose- and time-dependent manner in IEC-6 cells (Figure 4B and D).
A [3H]-5-HT uptake assay was used to determine whether the high SERT expression, induced by EGF, influenced the reuptake activity of SERT in IEC-6 cells. Uptake of [3H]-5-HT was significantly higher in cells pre-treated with EGF (40 ng/mL) for 24 h than in the solvent controls (592.908 ± 31.515 fmol/min per milligram vs 316.789 ± 85.652 fmol/min per milligram protein, P < 0.05) (Figure 4E).
To determine whether the effects of EGF on SERT expression and function were mediated via EGFR, IEC-6 cells were pre-treated with the EGFR kinase-specific inhibitor PD153035 (10 μmol/L) for 30 min, followed by a 24 h treatment with EGF (40 ng/mL). The results show that PD153035 blocks the EGF upregulation of SERT expression (Figure 5A), suggesting that EGF upregulates SERT expression via EGFR.
To investigate whether EGFR is also necessary for EGF to affect SERT reuptake activity, the [3H]-5-HT uptake assay was performed in the IEC-6 cells. PD153035 (10 μM) pre-treatment abolished the EGF-induced increase in [3H]-5-HT uptake in IEC-6 cells (Figure 5B), suggesting that EGF influences SERT function in 5-HT uptake via EGFR.
The gastrointestinal tract is a major source of endogenous 5-HT, which regulates the gastrointestinal tract’s sensory, motor, and secretory functions through the interaction with different receptor subtypes. 5-HT is synthesized by tryptophan hydroxylases in enterochromaffin cells and in brainstem and myenteric plexus neurons. It is inactivated by SERT-mediated uptake into enterocytes or neurons. Recent studies have reported that patients with IBS have higher 5-HT levels and lower SERT gene expression in rectal mucosa compared with healthy controls[3,5]. These findings suggest that SERT may play an important role in the pathophysiology of IBS. In this study, SERT expression was decreased in colon tissues from visceral-sensitized rats, suggesting a potential association of SERT with sensitivity of the gastrointestinal tract.
High expression of EGF in the gastrointestinal tract[26] plays a vital role in the regulation of gastrointestinal function and protects epithelial cells from damage by various factors[27-29]. EGF has also been shown to upregulate SERT gene expression in human intestinal epithelial[11] and glial cells[30]. In our preliminary study, the plasma EGF levels in IBS patients were significantly decreased compared with healthy controls (see supplemental Figure 1). Additionally, the levels of EGF in plasma and colon tissues of visceral-sensitized rats were significantly lower than in controls. Furthermore, the Pearson’s correlation analysis showed a positive correlation between EGF and SERT protein levels. These results suggest that EGF may be involved in the development of visceral hypersensitivity via SERT-mediated 5-HT uptake into enterocytes. Consequently, one has to ask how EGF affects SERT gene expression and function. The results of the present study show that EGF upregulates SERT mRNA expression in a dose- and time-dependent manner, suggesting regulation is at the transcriptional level. EGF-stimulated SERT gene expression is known to be dependent on tyrosine kinase activation of EGFR in human choriocarcinoma cells[31]. In this study, inhibition of EGFR blocked the effect of EGF on SERT expression and function in IEC-6 cells, thus suggesting that EGF promotes SERT-mediated 5-HT uptake into enterocytes through EGFR binding. These results are in agreement with the report by Gill et al[11]. EGF plays important roles in restoring tissue and enhancing SERT expression in intestinal epithelial cells; hence, promoting reuptake of 5-HT into enterocytes. Clinical studies support the beneficial effects of EGF in decreasing inflammation and diarrhea[32,33]. The beneficial effects of EGF, which may contribute to a change in visceral sensitivity, are worthy of further investigation.
In summary, plasma EGF levels were decreased both in IBS patients and in visceral-sensitized rats, and were correlated with SERT protein expression. Furthermore, treatment of cells with EGF upregulated SERT expression and function via EGFR. These results suggest that EGF downregulates SERT-mediated 5-HT uptake into enterocytes, potentially contributing to the development of visceral hypersensitivity.
There are some limitations to this study. First, we did not confirm the findings concerning EGFR signaling on SERT expression and change of visceral hypersensitivity in vivo. Future studies are needed to use mice expressing dominant-negative EGFR point mutations to test whether the EGFR signaling pathway is involved in SERT expression and visceral hypersensitivity. Although accumulating evidence indicates that alterations in 5-HT signaling occurs in IBS, the underlying mechanism remains not well understood. Further studies are needed to better understand the pathogenesis of visceral hypersensitivity.
The pathogenesis of irritable bowel syndrome (IBS) remains poorly understood. Visceral hypersensitivity is the most likely culprit responsible for sensory abnormalities in IBS patients. Serotonergic signaling abnormalities are involved in the development of IBS. Serotonin transporter (SERT) gene expression is downregulated in the colon and rectal tissues of IBS patients. Epidermal growth factor (EGF) can upregulate 5-HT reuptake levels by increasing SERT expression.
Visceral hypersensitivity plays an important role in the pathogenesis of IBS. However, the mechanism of visceral hypersensitivity formation remains unclear. Downregulation of SERT-mediated 5-HT uptake into enterocytes by EGF can potentially contribute to the development of visceral hypersensitivity.
There have been no studies evaluating the role that plasma EGF plays in the pathogenesis of IBS. This is the first study showing a significant decrease in the plasma level of EGF in IBS patients and rats with visceral hypersensitivity. Furthermore, SERT gene expression and protein activity were enhanced following treatment with EGF. Hence, a decrease in EGF levels may contribute to the formation of visceral hypersensitivity through downregulation of SERT-mediated 5-HT uptake into enterocytes.
The results of the study add to our understanding of EGF upregulation of SERT expression and function and its association with formation of visceral hypersensitivity. This may provide a future strategy for therapeutic intervention in the treatment of patients with IBS.
Altered 5-HT signaling in the gut contributes to visceral hypersensitivity of IBS. Downregulated expression and function of SERT is considered a pathophysiology of various functional gastrointestinal disorders, especially IBS. EGF can increase SERT expression and function in intestinal epithelial cells, consequently decreasing 5-HT levels, thus presenting a potential therapeutic effect for visceral hypersensitivity in IBS.
In this manuscript, the authors show that EGF levels are decreased in colon tissues and plasma of rats with visceral hypersensitivity. Furthermore, SERT expression is decreased at both protein and mRNA levels in these animals with respect to controls. The authors also show that EGF treatment induces SERT expression via EGFR in rat intestinal crypt cells. This research is important because it sheds light on the role that decreased EGF levels play in SERT regulation and subsequent development of visceral hypersensitivity.
| 1. | Talley NJ, Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet. 2002;360:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L, Yi-Xuan L. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz J Med Biol Res. 2012;45:948-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech Coloproctol. 2014;18:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 577] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 6. | Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Latorre E, Mendoza C, Matheus N, Castro M, Grasa L, Mesonero JE, Alcalde AI. IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine. 2013;61:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). J Pharmacol Exp Ther. 2003;306:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Gill RK, Anbazhagan AN, Esmaili A, Kumar A, Nazir S, Malakooti J, Alrefai WA, Saksena S. Epidermal growth factor upregulates serotonin transporter in human intestinal epithelial cells via transcriptional mechanisms. Am J Physiol Gastrointest Liver Physiol. 2011;300:G627-G636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Gil C, Najib A, Aguilera J. Serotonin transport is modulated differently by tetanus toxin and growth factors. Neurochem Int. 2003;42:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Dvorak B. Milk epidermal growth factor and gut protection. J Pediatr. 2010;156:S31-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Niederlechner S, Baird C, Petrie B, Wischmeyer E, Wischmeyer PE. Epidermal growth factor receptor expression and signaling are essential in glutamine’s cytoprotective mechanism in heat-stressed intestinal epithelial-6 cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G543-G552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Alexander RJ, Panja A, Kaplan-Liss E, Mayer L, Raicht RF. Expression of growth factor receptor-encoded mRNA by colonic epithelial cells is altered in inflammatory bowel disease. Dig Dis Sci. 1995;40:485-494. [PubMed] |
| 16. | Dubé PE, Yan F, Punit S, Girish N, McElroy SJ, Washington MK, Polk DB. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J Clin Invest. 2012;122:2780-2792. [PubMed] |
| 17. | Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053-G1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Kerckhoffs AP, Ter Linde JJ, Akkermans LM, Samsom M. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 21. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 565] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 22. | Gulec S, Collins JF. Silencing the Menkes copper-transporting ATPase (Atp7a) gene in rat intestinal epithelial (IEC-6) cells increases iron flux via transcriptional induction of ferroportin 1 (Fpn1). J Nutr. 2014;144:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Gendron FP, Mongrain S, Laprise P, McMahon S, Dubois CM, Blais M, Asselin C, Rivard N. The CDX2 transcription factor regulates furin expression during intestinal epithelial cell differentiation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G310-G318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Gordillo-Bastidas D, Oceguera-Contreras E, Salazar-Montes A, González-Cuevas J, Hernández-Ortega LD, Armendáriz-Borunda J. Nrf2 and Snail-1 in the prevention of experimental liver fibrosis by caffeine. World J Gastroenterol. 2013;19:9020-9033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Koldzic-Zivanovic N, Seitz PK, Cunningham KA, Thomas ML, Hughes TK. Serotonin regulation of serotonin uptake in RN46A cells. Cell Mol Neurobiol. 2006;26:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Rao RK. Biologically active peptides in the gastrointestinal lumen. Life Sci. 1991;48:1685-1704. [PubMed] |
| 27. | Rao R, Porreca F. Epidermal growth factor protects mouse ileal mucosa from Triton X-100-induced injury. Eur J Pharmacol. 1996;303:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Rao RK, Thomas DW, Pepperl S, Porreca F. Salivary epidermal growth factor plays a role in protection of ileal mucosal integrity. Dig Dis Sci. 1997;42:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Tepperman BL, Vozzolo BL, Soper BD. Effect of maternal sialoadenectomy on ontogenic response of rat gastric mucosa to luminal H+. Am J Physiol. 1993;265:G354-G360. [PubMed] |
| 30. | Kubota N, Kiuchi Y, Nemoto M, Oyamada H, Ohno M, Funahashi H, Shioda S, Oguchi K. Regulation of serotonin transporter gene expression in human glial cells by growth factors. Eur J Pharmacol. 2001;417:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Kekuda R, Torres-Zamorano V, Leibach FH, Ganapathy V. Human serotonin transporter: regulation by the neuroprotective agent aurintricarboxylic acid and by epidermal growth factor. J Neurochem. 1997;68:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | McCole DF, Rogler G, Varki N, Barrett KE. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology. 2005;129:591-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Riegler M, Sedivy R, Sogukoglu T, Cosentini E, Bischof G, Teleky B, Feil W, Schiessel R, Hamilton G, Wenzl E. Effect of growth factors on epithelial restitution of human colonic mucosa in vitro. Scand J Gastroenterol. 1997;32:925-932. [PubMed] |
P- Reviewer: Troncoso MF, Yang P S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Wang CH