Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13273
Revised: February 10, 2014
Accepted: April 30, 2014
Published online: October 7, 2014
Processing time: 343 Days and 23.8 Hours
Endoscopic resection has been an optimal treatment for selected patients with early gastric cancer (EGC) based on advances in endoscopic instruments and techniques. As endoscopic submucosal dissection (ESD) has been widely used for treatment of EGC along with expanding ESD indication, concerns have been asked to achieve curative resection for EGC while guaranteeing precise prediction of lymph node metastasis (LNM). Recently, new techniques including ESD or endoscopic full-thickness resection combined with sentinel node navigation enable minimal tumor resection and a laparoscopic lymphadenectomy in cases of EGC with high risk of LNM. This review covers the development and challenges of endoscopic treatment for EGC. Moreover, a new microscopic imaging and endoscopic techniques for precise endoscopic diagnosis and minimally invasive treatment of EGC are introduced.
Core tip: Endoscopic treatment of early gastric cancer (EGC) has been evolved along with the expansion of ESD indication and toward the question of how to achieve accurate risk assessment of lymph node metastasis (LNM). To achieve curative endoscopic treatment, not only accurate endoscopic diagnosis but precise selection of the patient of EGC without LNM should be preceded. Recently, endomicroscopy has been introduced to provide precise microscopic visualization of histology. Moreover, sentinel node navigation surgery combined ESD and hybrid natural orifice transluminal endoscopic surgery have been reported as a new minimally invasive treatment option for the EGC patients with high risk of LNM.
- Citation: Kim MY, Cho JH, Cho JY. Ever-changing endoscopic treatment for early gastric cancer: Yesterday-today-tomorrow. World J Gastroenterol 2014; 20(37): 13273-13283
- URL: https://www.wjgnet.com/1007-9327/full/v20/i37/13273.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i37.13273
Endoscopic resection for early gastric cancer (EGC) is widely accepted as one of the standard treatments together with surgical treatment[1]. In the 2000s, advances in endoscopic instruments were achieved and various new endoscopic techniques were developed. Still, the major obstacle to endoscopic resection for EGC has been its limitations of predicting lymph node metastasis (LNM). Therefore, approaches to the next level of endoscopic treatment have been evolved in three ways. First, how we can achieve curative endoscopic resection for EGC while guaranteeing precise prediction of LNM. Second, how we can expand a definitive indication for complete resection to secure depth of invasion and lateral margin. Last, how we can manage the patients with high risk of LNM for EGC using new endoscopic techniques. In this review, we will keep track of the progress of endoscopic resection in two time periods and suggest the prospect of the therapeutic strategy for EGC in the future.
In Japan, endoscopic mucosal resection (EMR) technique called the “strip biopsy” for EGC was first described in 1984, which is to lift the lesion with a grasper and to remove the lesion using a double-channel endoscope after submucosal injection of saline under the lesion. In 1988, new EMR technique, which cuts the lateral margin using needle knife (EMR-P), was introduced[2]. It helped the precise en bloc resection by resecting with a snare after peripheral cutting of the lesion. EMR was known to be a minimally invasive and safe technique and became an axis of treatment for EGC[3]. However, this technique requires skillful endoscopists and has a higher perforation risk. Then, EMR with a cap-fitted endoscope (EMR-C) for early esophageal cancer was developed in 1992 and used to treat relatively small EGC[4]. Another technique is EMR with ligation (EMR-L), which was started as a standard endoscopic variceal ligation[5]. These techniques helped to resect the lesion more safely and quickly. It also reduced the risks of perforation and bleeding during the procedure. Absolute indication of EMR for EGC was published by Japanese Gastric Cancer Association in 1998[6,7]: (1) elevated cancers less than 2 cm in diameter, and (2) small (< 1 cm) depressed cancers without ulceration. These lesions must also be differentiated cancers confined to the mucosa, and have no lymphovascular invasion. As long as the lesion was completely removed, EMR showed good long term outcome compared to surgery. The 5-year overall survival rates and recurrence rates did not differ significantly between the EMR and surgery groups (93.6% vs 94.2% and 1.2% vs 1.1%)[8]. Although the risk of metachronous gastric cancer was significantly higher in the EMR group than in the surgery group (5.8% vs 1.1%), most metachronous gastric cancers were successfully retreated by EMR or surgery. A major limitation of EMR was incomplete resection for lesions larger than 2 cm in diameter due to the size limitations of accessories such as snares, caps and ligating devices. Piecemeal resection also caused a high risk of local recurrence (2.3%-36.5%)[9-12]. So, the size limitations for en bloc resection of EGC kept demanding improvement in techniques.
In the late 1990s, ESD was developed for complete removal of EGC regardless of its size and location by dissecting the submucosal layer via the use of through-the scope endoscopic knives[13]. ESD is superior to EMR because it enables en-bloc resection and precise pathologic staging for large EGCs. Now, it has become one of the standard treatments and is being used to achieve en bloc resection for EGCs that would otherwise require piecemeal or surgical resection[10,14,15]. Owing to the development of ESD technique, indications of endoscopic resection for EGC were expanded, based on the analysis of 5265 surgical specimens of EGC patients who underwent gastrectomy with D2 level lymph node dissection[16]. The recent expanded criteria are differentiated type cancers without evidence of lymphovascular invasion, including: (1) mucosal cancer without ulceration, irrespective of tumor size; (2) mucosal cancer with ulceration, less than 3 cm in diameter; and (3) minimal (500 μm from the muscularis mucosa) submucosal invasive cancer less than 3 cm in size. After ESD, the patients with EGC of expanded indication may be followed closely without surgery because they have very small risks for LNM[16-18]. However, it should be mentioned that the data provided from these observations were surgical specimens sliced at 5 mm and not 2 mm as required for ESD specimens.
Asian studies have reported that ESD has achieved a high rate of en bloc resection (89.7%-96.7%) and complete resection (84%-94.7%) (Table 1)[8,10,11,17,19-25]. Recent meta-analyses to compare the efficacy and safety of ESD and EMR for EGC showed that ESD had advantages in en bloc resection rate, histologically complete resection rate and local recurrence rate even for small lesions[26-28]. When the lesions were classified by size, the 5-year recurrence-free rate was significantly lower in the EMR group compared with the ESD group especially for the lesions larger than 10 mm. The 5-year overall survival rates and recurrence-free rates of ESD have been reported to 93.6%-100% and 98.7%-100%. For complications, delayed bleeding occurs more during ESD, with an incidence rate of up to 7%-15.6%. Perforation is higher during ESD (3.6%-4.5% vs 1.0%-1.2%), which was endoscopically managed in most cases. To demonstrate the efficacy and safety of ESD especially for EGCs in expanded indication, well-controlled, prospective randomized trials with a large population and long-term follow-up periods are needed.
| Ref. | Published yearcountry | No.(lesion/patients) | Method | En bloc resection (%) | Complete resection (%) | Follow-up(mo/range) | Complications | Recurrence rate(%) | 5-yr overall survival rate (%) | 5-yr recurrence-free rate (%) | |||||||||
| Bleeding | Perforation | ||||||||||||||||||
| EMR | ESD | EMR | ESD | EMR | ESD | EMR | ESD | EMR | ESD | Local | Metachronous | ||||||||
| Oda et al[15] | 2006 Japan | 714/655 | 411 | 303 | 56.0 | 66.3 | 61.1 | 73.6 | 38 (6-60) | 0.1 | 0 | 1.2 | 3.6 | 7.5 | NA | 99.21 | 94.42 | ||
| Oka et al[11] | 2006 Japan | 1020/896 | 825 | 195 | 42.1 | 83.1 | 23.6 | 83.1 | EMR 83.2 ± 34.6 ESD 19.4 ± 9.2 | 7.6 | 22.6 | 4.8 | 8.7 | 3.1 | NA | NA | NA | ||
| Imagawa et al[21] | 2006 Japan | 196/185 | 196 | 93.0 | 84.0 | 22.8 (12-46) | 6.1 | 0.0 | NA | NA | NA | ||||||||
| Chung et al[19] | 2009 Korea | 534/1000 (dysplasia 466) | 534 | 95.3 | 87.7 | NA | 15.6 | 1.2 | NA | NA | NA | NA | |||||||
| Jang et al[22] | 2009 Korea | 198/198 | 198 | 89.7 | 87.9 | 30 (9-49) | 7.4 | 2.9 | 5.1 | NA | NA | 94.92 | |||||||
| Min et al[24] | 2009 Korea | 346/243 | 103 | 243 | 77.7 | 95.9 | 75.7 | 88.9 | 29 (4-44) | 3.9 | 5.3 | 1.9 | 4.5 | 0.0 | 6 | NA | NA | ||
| Isomoto et al[25] | 2009 Japan | 589/551 | 589 | 94.9 | 94.7 | 30 (6-89) | 1.8 | 4.5 | 0.0 | NA | 97.1 | 1003 | |||||||
| Goto et al[20] | 2009 Japan | 276/231 | 276 | 96.7 | 91.7 | 36 (2-39) | 5.1 | 4.0 | 0.9 | NA | 96.2 | 1003 | |||||||
| Nakamoto et al[10] | 2009 Japan | 202/177 | 80 | 122 | 53.8 | 94.3 | 37.5 | 92.6 | 54 (12-89) | 0 | 1.6 | 2.5 | NA | 100.0 | 100 | ||||
| Choi et al[8] | 2011 Korea | 215/215 | 215 | 71.2 | 81 (56-94) | NA | NA | 93.6 | 98.7 | ||||||||||
| Ahn et al[17] | 2011 Korea | 1370/1244 | Absolute Ix | 32 (22-48) | 98.8-99.02 | ||||||||||||||
| 355 | 182 | 72.4 | 65.9 | 94.4 | 83.0 | 1.4 | 0.8 | 0.3 | 1.2 | 1.5 | 0.5 | 6.1 | 2.2 | 95.8-95.3 | |||||
| Expanded Ix | |||||||||||||||||||
| 497 | 336 | 96.8 | 95.5 | 97.8 | 91.1 | 1.6 | 2.1 | 1.6 | 2.4 | 1.6 | 0.8 | 13.7 | 4.0 | 96.8 | 98.52 | ||||
| Catalano et al[29] | 2009 Italy | 48/45 | 36 | 12 | 72.0 | 92.0 | 56.0 | 92.0 | 31 (12-71) | 8.0 | 8 | 0.0 | 8.0 | 0.0 | NA | NA | NA | ||
| Dinis-Ribeiro et al[30] | 2009 Portugal | 19/19 | 19 | 79.0 | 89.0 | 10 | 5 | 0.0 | 0.0 | NA | NA | NA | |||||||
| Probst et al[34] | 2010 Germany | 91/83 (EGC 66) | 1 | 85 | 100.0 | 88.2 | 90.0 | 68.6 | 27 (1-71) | 3.5 | 1.2 | 6.6 | NA | NA | 90.12 | ||||
| Schumacher et al[36] | 2012 Germany | 30/30 (EGC 21) | 30 | 90.0 | 64.3 | 22 | 4 | 6.0 | 10.7 | NA | NA | NA | |||||||
| Repici et al[35] | 2013 Italy | 42/42 (EGC 10) | 42 | 100.0 | 92.8 | 19 (9-53) | 7.1 | 0.0 | 5.0 | NA | NA | NA | |||||||
| Chaves et al[32] | 2013 Brazil | 62/61 (EGC 55) | 62 | 82.2 | 77.4 | 11.3 (1-30) | 0 | 4.8 | 0.0 | 3.5 | NA | NA | |||||||
As ESD procedure has become widespread in western countries, several small reports have shown a high success rate of en bloc resection (79%-100%) and complete resection (64.3%-100%) (Table 1)[29-36]. For complications, the rate of perforation and bleeding has been reported ranging from 0% to 8% and 0% to 8%. The long-term follow-up data are needed to evaluate the therapeutic outcome.
To guarantee the curative endoscopic resection or stratify the risk of LNM for EGC, several key points should be checked for the pathologic diagnosis of ESD specimen[37]. Complete resection should be confirmed by the precise lateral and vertical margin status. The distance from the lateral margin of the tumor to the margin of the specimen should be described. In case of positive lateral margin, the number of sections and the extent showing positive tumor cells should be documented. If ESD specimen shows a positive vertical margin, the positive tumor site and the distance from the lower edge of muscularis mucosae to the positive margin should be demonstrated. In addition, depth of invasion, histologic type, lymphovascular invasion of the tumor should be evaluated. If the undifferentiated type is mixed within the differentiated type cancer, the proportion of undifferentiated type should be evaluated to predict the risk of vascular invasion and LNM. In case of submucosal invasive cancer, the extent of submucosal invasion and histologic type should be described to determine additional surgery. It is important to identify the muscularis mucosae by using the immunohistochemistry of desmin, because the risk of LNM is higher when the tumor depth is 500 μm or more from the lower edge of muscularis mucosae (≥ sm2) than sm1. For careful microscopic examination of vascular invasion, Victoria blue staining is helpful, and immunohistochemistry of D2-40 is useful for evaluation of lymphatic invasion.
Noncurative resection is defined as the presence of positive lateral or vertical resection margins, submucosal and lymphovascular invasion, or undifferentiated histology, which means high-risks of recurrence or LNM. Conceptually, the patients with incomplete resection after ESD can be managed with laparoscopic gastrectomy with lymph node dissection. However, when only a small portion of positive lateral margins or unclear lateral margins are found on the post-ESD specimen, this may suggest a lower risk of LNM in the cases having no other factor of noncurative resection[38,39]. The rate of residual cancer in the positive lateral margin group (25.0%) was reported to be significantly lower than that in the positive vertical margin group (33.3%) or in the positive lateral and vertical margin group (66.7%) among the patients who underwent curative gastrectomy due to non-curative endoscopic resection for EGC[40]. The patients having mucosal cancer with lateral cut-end-positive status with no LNM can be recommended to have close follow-up or endoscopic treatment[41]. Another report demonstrated that neither residual cancer nor LNM was found in the patients with less than 500 μm submucosal invasion without margin involvement in endoscopically resected specimens among 43 patients who were operated on due to residual mucosal cancer, a mucosal cancer larger than 3 cm, or a submucosal cancer regardless of size or margin involvement[42]. Lymphatic involvement and tumor size have been reported to be independent risk factors for LNM in EGC with submucosal invasion[43,44]. Based on the results of the studies, endoscopic resection may be feasible for highly selective submucosal cancers with no lymphovascular invasion. Gastrectomy with lymph node dissection is recommended to patients with positive vertical margins, submucosal involvement having high risk features or lymphovascular invasion.
Recent issues are on whether laparoscopic lymph node dissection without gastrectomy can be performed if the resection margins are negative in the patients with high-risks of recurrence and non-curative resections based on the presence of other criteria including submucosal invasion, lymphovascular invasion, or undifferentiated adenocarcinoma. One report evaluated the efficacy of diagnostic and therapeutic laparoscopic lymph node dissection after ESD in EGC patients with high-risks of LNM including undifferentiated adenocarcinoma, submucosal cancer, immunohistochemically-positive cytoplasmic staining for vascular endothelial growth factor, lymphovascular invasion, a high lymphatic microvessel density, or high microvessel density[45]. All of the dissected lymph nodes were free of cancer cells in all 9 patients. During 16 mo of follow-up, no patients had an evidence of tumor recurrence. In a retrospective study with a small number of patients, the area for lymph node according to the location of the tumor and/or the lymphatic drainage of the stomach was visualized with standard laparoscopy or infrared-ray electronic laparoscopy after submucosal injection of indocyanine green (ICG) around post-ESD scars[46]. The study showed that 2 out of 20 (10%) patients had lymph node metastases confirmed after lymph node dissection without gastrectomy, and none had local or distant recurrence at a median follow-up of 61 mo. However, this approach cannot be generalized in clinical practice yet.
Traditionally, poorly differentiated adenocarcinomas were candidates for surgery. However, in a retrospective study, 1362 patients with EGC of signet ring cell histology who underwent gastrectomy showed the similar rate of LNM compared with the patients with differentiated EGC[47]. A recent report showed that LNM was significantly associated with female sex, tumor size, depth of tumor invasion and lymphatic involvement in poorly differentiated EGC[48]. Although endoscopic management for the patients with undifferentiated adenocarcinoma is still controversial, small studies have reported successful ESD for lesions smaller than 20 mm without lymphovascular invasion[49-51]. Another study showed that poorly differentiated EGC confined to the mucosa or with minimal submucosal infiltration (≤ 500 μm) could be considered for curative EMR due to the low risk of LNM[52]. Moreover, a study showed that EGC with signet ring cell histology can be treated by EMR, if it is smaller than 25 mm, limited to the sm2 layer, and does not involve the lymphatic-vascular structure[53]. However, larger lesions showing submucosal invasion and ulceration lower the possibility of curative resection with ESD. A recent report showed that ESD for undifferentiated EGC can achieve curative resection with an excellent 5-year mortality rate[54]. En bloc and R0 resection were achieved in 99.0% and 90.7%. Curative resection was achieved in 63.9%. Among the patients who had additional surgery, the rate of local residual tumor and LNM was 4.8% and 9.5%. None had local recurrence or lymph node or distant metastasis in the patients with curative resection during a median follow-up of 76.4 mo. Until now, it is not clear that ESD is just as effective in cases of undifferentiated type EGC because the rate of curative resection is lower for undifferentiated cancer than differentiated cancer, ranging 45%-89%, in spite of high en bloc resection rate.
To expand ESD criteria, instrumental and technical advances in diagnostic and therapeutic endoscopy have been challenged. Early detection of gastric cancer or precancerous lesion as well as precise staging is integral to curative endoscopic resection. Over the past decades, several advances in diagnostic endoscopy including magnifying endoscopy, narrow-band imaging, and virtual chromoendoscopy have allowed improvement in tissue characterization by detailed imaging of the mucosal pit pattern and microvascular structures. However, these techniques could not provide microscopic visualization of histology. Microscopic imaging is aimed not only to predict histology, but to visualize actual microscopic mucosal architectures in real time, high resolution and high magnification. Moreover, it is useful in microscopically guided target biopsy for EGC because it can avoid sampling errors caused by conventional biopsies in ill-defined, large mucosal cancers. Lastly, it helps to determine the margin of EGC before ESD.
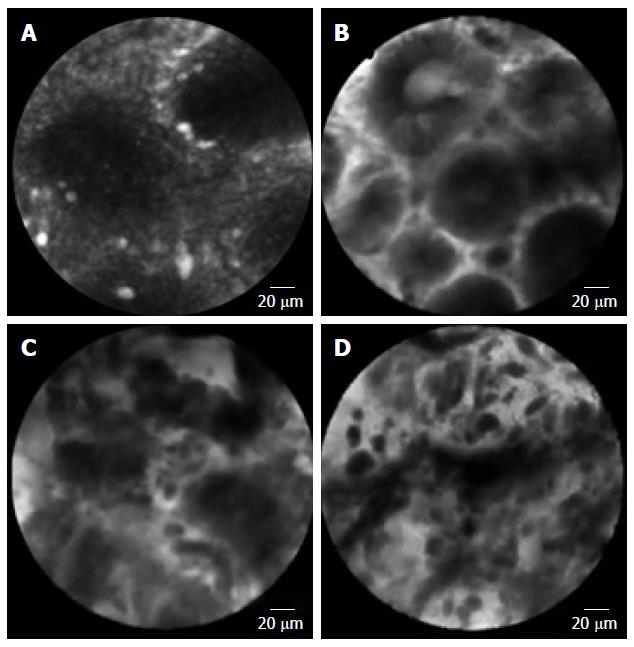
Confocal laser endomicroscopy (CLE) is a system using laser light (currently blue laser light of 488 nm) for excitation and capture of laser-induced fluorescence from the defined lesion. Usually, exogenous fluorophores (intravenous fluorescein, 2.5 mL, 10%) are used to enhance the optical contrast[55-57]. There are 2 types of CLE, endoscopy-based CLE (eCLE)[58], which is integrated into an endoscope, and through-the-scope probe-based CLE (pCLE)[59] that can be inserted through the working channel of endoscopes. Compared with eCLE, pCLE shows somewhat lower resolution, but faster image acquisition. It also provides microscopic video sequences and can be used into the bile duct or through ultrasonography-guided needles. For accurate interpretation of microscopic images, adequate training in the endoscopic technique and knowledge about histopathology of EGC is required. In 2004, the first study on CLE was reported in patients who performed screening colonoscopy[55]. In the stomach, several studies have been reported CLE imaging for Helicobacter pylori infection and gastritis[60,61], intestinal metaplasia[62] and hyperplastic and adenomatous polyps[63]. From the Miami classification[64], the key features used to distinguish non-neoplastic tissue, dysplasia, and adenocarcinoma are as follows: (1) normal or non-neoplastic mucosa, round regular crypts, cobblestone appearance of normal glands; (2) dysplasia, irregular crypt lumen, dark irregular thickened epithelium; and (3) gastric adenocarcinoma, completely disorganized epithelium, fluorescein leakage, dark irregular epithelium. Differentiated and undifferentiated adenocarcinoma can be distinguished based on the presence of discriminable glandular structures[58] (Figure 1). In the studies to evaluate efficacy in pre-ESD pathologic diagnosis or post-ESD surveillance for high-grade neoplasia and superficial gastric cancer, CLE showed high accuracy (91.7%-99%) and decreased biopsies. Moreover, CLE would have directed 10% of the patients to surgery instead of ESD by correctly showing undifferentiated carcinoma[58]. CLE is a promising technology for identifying EGC and has potential to decrease the rate of discrepancy pre- and post-ESD histopathology. The limitation of CLE is that the endoscopists who perform the in vivo CLE diagnosis are unavoidably biased by the endoscopic appearance of the lesion, which may have affected the in vivo CLE diagnosis. The efficacy of CLE should be confirmed in a larger population including more non-neoplastic and dysplastic lesions.
As mentioned above, a major limitation of ESD for curative treatment of EGC is inaccuracy in lymph node status. Ultimately, ESD is a curative treatment modality only if EGCs do not have regional LNM. N staging for EGC is mostly performed by CT or EUS, but diagnostic yields were not so satisfactory. EUS has a limitation not only to evaluate of regional LNM but to predict depth of invasion. It takes a lot out of the patients and endoscopists to decide and follow up after ESD. Finally, it is most important to decide what could be a minimally invasive treatment for EGC patients with a potential to escape the expanded ESD indication. Some patients who underwent surgical operation are diagnosed as mucosal cancer without LNM on the final pathology. In contrast, it is not unusual that some patients are required to have additional surgery or to give careful consideration of additional surgery after ESD. Because of these important problems, a paradigm shift has been emerged.
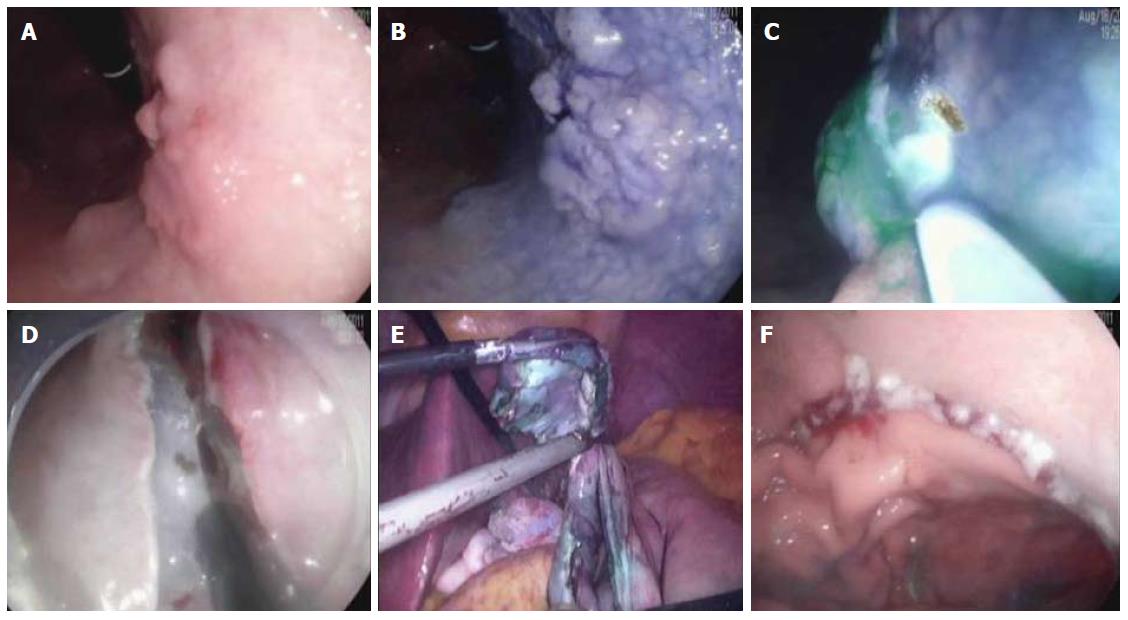
Sentinel lymph node is the hypothetical first lymph node or group of nodes draining a cancer and is considered the first site of micrometastasis along the route of lymphatic drainage. Sentinel node navigation is defined as a novel, minimally invasive surgery based on sentinel node mapping and the sentinel node-targeted diagnosis of nodal metastasis. The concept of sentinel node has evolved from the surgical staging of both breast cancer and melanoma. It avoided unnecessary prophylactic radical lymphadenectomy such as axillary lymph node dissection in breast cancer patients with negative sentinel node for cancer metastasis. Although the clinical application of sentinel node mapping for EGC has been controversial for years, sentinel node mapping, using a dual-tracer method that utilizes radioactive colloids and blue dyes, is currently considered the most reliable method for the stable detection of sentinel nodes in patients with EGC[65,66]. An accumulation of radioactive colloids facilitates the identification of sentinel nodes even in resected specimens, and the blue dye is effective for intraoperative visualization of lymphatic flow, even during laparoscopic surgery. Usually, technetium-99m tin colloid, technetium-99 m sulfur colloid, and technetium-99m antimony sulfur colloid are used as radioactive tracers. Isosulfan blue, patent blue, and indocyanine green (ICG) are currently the preferred dye tracers. The patients with clinical T1N0 (< 4 cm) gastric cancer can undergo sentinel node mapping and biopsy without limitation of tumor location. Radioactive colloids and blue dyes are injected the day before surgery and just before the procedure into four quadrants of the submucosal layer around the primary tumor using an endoscopic puncture needle. Studies are investigating sentinel lymph node navigation using endoscopic injection of radiocolloid dye or ICG[65,67], or CT lymphography[68,69] using nanoscale iodized oil emulsion to increase the accuracy of detecting LNM[70]. A recent meta-analysis showed that the sentinel node detection rate, sensitivity, negative predictive value, and accuracy were 93.7%, 76.9%, 90.3%, and 90.2%, respectively[71]. When considering laparoscopic procedure, sentinel node identification rate, sensitivity, false negative rate, and accuracy were 89.3%, 68.6%, 31.4%, and 92.6%, respectively. Combined ESD and sentinel node navigation surgery might be a feasible, minimally invasive procedure that allows en bloc tumor resection to be achieved while assessing the pathological status of the regional lymph nodes (Figure 2). A case series reported that combined ESD and sentinel node navigation was conducted for 13 patients with clinical T1 N0 (≤ 3 cm) EGC, and was completed in 12 patients[72]. One patient was converted to gastrectomy after sentinel node navigation surgery. En bloc resection was achieved in all other cases.
The risk of LNM in EGC exceeding the indication has known to 5.7%-20%[9]. In other words, at least 80% of patients might potentially save their stomach with curative endoscopic treatment if depth of invasion of the tumor is within the submucosa and microscopic vertical margin is secured after ESD.
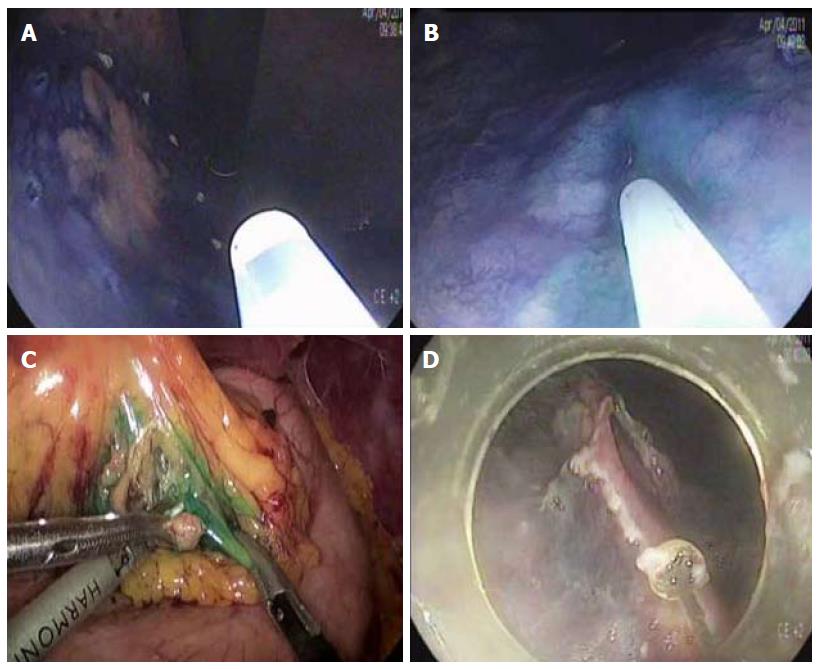
Natural orifice transluminal endoscopic surgery (NOTES) may be applied as a modified treatment for EGC. NOTES means that abdominal operations are performed with an endoscope passed through a natural orifice (e.g., mouth, urethra, anus) and then through an internal incision in the stomach, vagina or colon[73]. This procedure allows flexible endoscope to reach organs outside the lumen of the bowel. NOTES is minimally invasive compared to open surgery is exposed to fewer risks. Hybrid NOTES enables minimal tumor resection using the ESD technique, and laparoscopic lymphadenectomy can be performed simultaneously in cases of EGC with high risk for LNM. Hybrid NOTES for EGC means endoscopic full-thickness gastric resection (EFTGR) with laparoscopic regional lymph node dissection. It consists of endoscopic full-thickness gastric resection and laparoscopic lymphadenectomy after sentinel node navigation. EFTGR consists of five major procedures: (1) marking around the lesion safety margin confirmed by margin biopsies; (2) a circumferential incision as deep as the submucosal layer around the lesion; (3) circumferential endoscopic full-thickness resection around the lesion through the submucosal incision line under the laparoscopic guidance; (4) laparoscopic full-thickness resection around the remaining lesion through the EFTGR incision line inside the peritoneal cavity; and (5) laparoscopic closure of the resection margin[74] (Figure 3). The lymph node dissection is performed before the full-thickness resection. Depending on the location of the lesion, the regional lymph nodes are dissected after sentinel lymph node navigation. The first prospective, pilot study for 14 patients with EGC was published in Korea[75]. The case series concluded that hybrid NOTES could be a bridge between endoscopic resection and laparoscopic surgery and may prevent extensive gastrectomy with lymphadenectomy in patients with EGC. EFTGR has a limited indication because of the potential for tumor dissemination into the abdominal space during the procedure and vagus nerve injury. Until now, several studies have been published, and techniques are being developed to accomplish non-exposed endoscopic wall-inversion surgery[76-78]. This new method may be an alternative to surgery in patients with submucosal cancer with or without ulceration, or mucosal cancer technically difficult to resect with ESD[74,76,78].
The key to improving therapeutic outcomes for EGC is early detection and accurate diagnosis. In spite of many advantages, endomicroscopy including CLE is still limited to some tertiary centers throughout the world. The biggest restraint in using CLE is that most health-care systems do not offer a billing code for this kind of advanced and optimized endoscopy. Clinical use of CLE before ESD will provide more accurate diagnosis of EGC compared with biopsies. Moreover, advance in endoscopic instruments, techniques and training is essential to improve outcomes of patients with EGC. Recently, novel laser system for ESD was introduced. ESD was completed using only the thulium laser, instead of endoscopy knives, without significant complications in all 10 patients[79]. In the near future, the concept of endoscopic surgery including ESD with sentinel node navigation and hybrid NOTES is expected to become one of the treatment options for the selective EGC patients with high risk of LNM.
| 1. | Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, Noguchi Y. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 135] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [PubMed] |
| 3. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62. [PubMed] |
| 5. | Akiyama M, Ota M, Nakajima H, Yamagata K, Munakata A. Endoscopic mucosal resection of gastric neoplasms using a ligating device. Gastrointest Endosc. 1997;45:182-186. [PubMed] |
| 6. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 964] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim do H, Lee JH, Kim MY, Kim BS, Oh ST. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 9. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 500] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 10. | Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 535] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 12. | Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Fukuda K, Fujita Y, Ninomiya K, Tano S, Katurahara M. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc. 2012;26:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] |
| 14. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 15. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 17. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Kang HJ, Kim DH, Jeon TY, Lee SH, Shin N, Chae SH, Kim GH, Song GA, Kim DH, Srivastava A. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 483] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 20. | Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy. 2009;41:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Jang JS, Choi SR, Qureshi W, Kim MC, Kim SJ, Jeung JS, Han SY, Noh MH, Lee JH, Lee SW. Long-term outcomes of endoscopic submucosal dissection in gastric neoplastic lesions at a single institution in South Korea. Scand J Gastroenterol. 2009;44:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 23. | Jung HY. Endoscopic resection for early gastric cancer: current status in Korea. Dig Endosc. 2012;24 Suppl 1:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis. 2009;41:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 528] [Article Influence: 31.1] [Reference Citation Analysis (1)] |
| 26. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 27. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 28. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 295] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 29. | Catalano F, Trecca A, Rodella L, Lombardo F, Tomezzoli A, Battista S, Silano M, Gaj F, de Manzoni G. The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg Endosc. 2009;23:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Chaves DM, Maluf Filho F, de Moura EG, Santos ME, Arrais LR, Kawaguti F, Sakai P. Endoscopic submucosal dissection for the treatment of early esophageal and gastric cancer--initial experience of a western center. Clinics (Sao Paulo). 2010;65:377-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Chaves DM, Moura EG, Milhomem D, Arantes VN, Yamazaki K, Maluf F, Albuquerque W, Conrado AC, Araújo JC, Uejo PH. Initial experience of endoscopic submucosal dissection in Brazil to treat early gastric and esophagheal cancer: a multi-institutional analysis. Arq Gastroenterol. 2013;50:148-152. [PubMed] |
| 33. | Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, Koch S, Houcke P, Cellier C, Heresbach D. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Probst A, Pommer B, Golger D, Anthuber M, Arnholdt H, Messmann H. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy. 2010;42:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Repici A, Zullo A, Hassan C, Spaggiari P, Strangio G, Vitetta E, Ferrara E, Malesci A. Endoscopic submucosal dissection of early gastric neoplastic lesions: a western series. Eur J Gastroenterol Hepatol. 2013;25:1261-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc. 2012;75:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Nagata K, Shimizu M. Pathological evaluation of gastrointestinal endoscopic submucosal dissection materials based on Japanese guidelines. World J Gastrointest Endosc. 2012;4:489-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Oda I, Gotoda T, Sasako M, Sano T, Katai H, Fukagawa T, Shimoda T, Emura F, Saito D. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Tokioka S, Umegaki E, Murano M, Takeuchi N, Takeuchi T, Kawakami K, Yoda Y, Kojima Y, Higuchi K. Utility and problems of endoscopic submucosal dissection for early gastric cancer in elderly patients. J Gastroenterol Hepatol. 2012;27 Suppl 3:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Lee JH, Kim JH, Kim DH, Jeon TY, Kim DH, Kim GH, Park do Y. Is Surgical Treatment Necessary after Non-curative Endoscopic Resection for Early Gastric Cancer? J Gastric Cancer. 2010;10:182-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Nagano H, Ohyama S, Fukunaga T, Seto Y, Fujisaki J, Yamaguchi T, Yamamoto N, Kato Y, Yamaguchi A. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 42. | Ryu KW, Choi IJ, Doh YW, Kook MC, Kim CG, Park HJ, Lee JH, Lee JS, Lee JY, Kim YW. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol. 2007;14:3428-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Abe N, Sugiyama M, Masaki T, Ueki H, Yanagida O, Mori T, Watanabe T, Atomi Y. Predictive factors for lymph node metastasis of differentiated submucosally invasive gastric cancer. Gastrointest Endosc. 2004;60:242-245. [PubMed] |
| 44. | An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Kim YH, Cho JY, Cho WY, Cho YK, Lee TH, Kim HG, Kim JO, Lee JS, Kim YJ, Jin SY. The Efficacy of Diagnostic and Therapeutic Laparoscopic Lymph Node Dissection after Endoscopic Submucosal Dissection in Early Gastric Cancer Laparoscopic Lymph Node Dissection after ESD in EGC. Korean J Gastrointest Endosc. 2010;40:90-96. |
| 46. | Abe N, Takeuchi H, Ohki A, Yanagida O, Masaki T, Mori T, Sugiyama M. Long-term outcomes of combination of endoscopic submucosal dissection and laparoscopic lymph node dissection without gastrectomy for early gastric cancer patients who have a potential risk of lymph node metastasis. Gastrointest Endosc. 2011;74:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Lee JH, Choi IJ, Kook MC, Nam BH, Kim YW, Ryu KW. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg. 2010;97:732-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Lee JH, Choi MG, Min BH, Noh JH, Sohn TS, Bae JM, Kim S. Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg. 2012;99:1688-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Abe N, Mori T, Izumisato Y, Sasaki H, Ueki H, Masaki T, Nakashima M, Sugiyama M, Atomi Y. Successful treatment of an undifferentiated early stage gastric cancer by combined en bloc EMR and laparoscopic regional lymphadenectomy. Gastrointest Endosc. 2003;57:972-975. [PubMed] [DOI] [Full Text] |
| 50. | Kamada K, Tomatsuri N, Yoshida N. Endoscopic submucosal dissection for undifferentiated early gastric cancer as the expanded indication lesion. Digestion. 2012;85:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, Kosaka T, Ono HA, Akiyama H, Moriwaki Y. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, Cho CM, Tak WY, Kweon YO. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Park JM, Kim SW, Nam KW, Cho YK, Lee IS, Choi MG, Chung IS, Song KY, Park CH, Jung CK. Is it reasonable to treat early gastric cancer with signet ring cell histology by endoscopic resection? Analysis of factors related to lymph-node metastasis. Eur J Gastroenterol Hepatol. 2009;21:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 55. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [PubMed] |
| 56. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 271] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 57. | Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, Löhr M. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 58. | Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, Cho JY. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc. 2011;74:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Bok GH, Jeon SR, Cho JY, Cho JH, Lee WC, Jin SY, Choi IH, Kim HG, Lee TH, Park EJ. The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointest Endosc. 2013;77:899-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Kiesslich R, Goetz M, Burg J, Stolte M, Siegel E, Maeurer MJ, Thomas S, Strand D, Galle PR, Neurath MF. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology. 2005;128:2119-2123. [PubMed] |
| 61. | Wang P, Ji R, Yu T, Zuo XL, Zhou CJ, Li CQ, Li Z, Li YQ. Classification of histological severity of Helicobacter pylori-associated gastritis by confocal laser endomicroscopy. World J Gastroenterol. 2010;16:5203-5210. [PubMed] |
| 62. | Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, Liu FG, Xie XJ, Zhu Q, Zhao YA. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy. 2008;40:547-553. [PubMed] |
| 63. | Li WB, Zuo XL, Zuo F, Gu XM, Yu T, Zhao YA, Zhang TG, Zhang JP, Li YQ. Characterization and identification of gastric hyperplastic polyps and adenomas by confocal laser endomicroscopy. Surg Endosc. 2010;24:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 65. | Kelder W, Nimura H, Takahashi N, Mitsumori N, van Dam GM, Yanaga K. Sentinel node mapping with indocyanine green (ICG) and infrared ray detection in early gastric cancer: an accurate method that enables a limited lymphadenectomy. Eur J Surg Oncol. 2010;36:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Kitagawa Y, Fujii H, Kumai K, Kubota T, Otani Y, Saikawa Y, Yoshida M, Kubo A, Kitajima M. Recent advances in sentinel node navigation for gastric cancer: a paradigm shift of surgical management. J Surg Oncol. 2005;90:147-151; discussion 151-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Lips DJ, Schutte HW, van der Linden RL, Dassen AE, Voogd AC, Bosscha K. Sentinel lymph node biopsy to direct treatment in gastric cancer. A systematic review of the literature. Eur J Surg Oncol. 2011;37:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Kim YH, Lee YJ, Park JH, Lee KH, Lee HS, Park YS, Park do J, Kim HH. Early gastric cancer: feasibility of CT lymphography with ethiodized oil for sentinel node mapping. Radiology. 2013;267:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Lim JS, Choi J, Song J, Chung YE, Lim SJ, Lee SK, Hyung WJ. Nanoscale iodized oil emulsion: a useful tracer for pretreatment sentinel node detection using CT lymphography in a normal canine gastric model. Surg Endosc. 2012;26:2267-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol. 2013;20:522-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Wang Z, Dong ZY, Chen JQ, Liu JL. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2012;19:1541-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 72. | Bok GH, Kim YJ, Jin SY, Chun CG, Lee TH, Kim HG, Jeon SR, Cho JY. Endoscopic submucosal dissection with sentinel node navigation surgery for early gastric cancer. Endoscopy. 2012;44:953-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Chun HJ, Keum B, Park S. The Current Status of Natural Orifice Transluminal Endoscopic Surgery (NOTES). Korean J Gastrointest Endosc. 2009;38:121-127. |
| 74. | Hoya Y, Yamashita M, Sasaki T, Yanaga K. Laparoscopic intragastric full-thickness excision (LIFE) of early gastric cancer under flexible endoscopic control--introduction of new technique using animal. Surg Laparosc Endosc Percutan Tech. 2007;17:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Cho WY, Kim YJ, Cho JY, Bok GH, Jin SY, Lee TH, Kim HG, Kim JO, Lee JS. Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection--14 human cases. Endoscopy. 2011;43:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Goto O, Mitsui T, Fujishiro M, Wada I, Shimizu N, Seto Y, Koike K. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 77. | Mitsui T, Niimi K, Yamashita H, Goto O, Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 78. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 79. | Cho JH, Cho JY, Kim MY, Jeon SR, Lee TH, Kim HG, Jin SY, Hong SJ. Endoscopic submucosal dissection using a thulium laser: preliminary results of a new method for treatment of gastric epithelial neoplasia. Endoscopy. 2013;45:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
P- Reviewer: Barbosa AJA, Thong-Ngam D, von Delius S S- Editor: Qi Y L- Editor: A E- Editor: Wang CH