Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12588
Revised: May 10, 2014
Accepted: June 12, 2014
Published online: September 21, 2014
Processing time: 169 Days and 20.1 Hours
AIM: To investigate hepatic function after combined transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) with a short-term interval (0-2 d).
METHODS: A total of 115 patients with compensated liver cirrhosis underwent RFA combined with TACE at a time-interval of 0-2 d for the treatment of hepatocellular carcinoma (HCC) < 5.0 cm. There were 21 patients who received further hepatic directed treatment altering liver function within 12 mo after the combined therapy for HCC-recurrence, and were excluded. The remaining 94 patients who survived without HCC-recurrence were included in this retrospective study.
RESULTS: At 1 mo after treatment, Child-Pugh scores (CPs) remained unchanged in 89 of 94 patients (94.7%), and transiently increased by one-point in 5 patients (5.3%). However, the score returned to baseline score at 3 mo and was maintained until 6 mo in all patients. The baseline CPs of 8 or more was identified as a factor for transient rise of CPs after the treatment (CPs 8/9 vs 5/6/7; 21.4% vs 2.5%; P = 0.022). At 12 mo follow-up, CPs was unchanged in 90 patients (95.7%), and increased by one-point in 4 patients (4.3%). The rise of CPs at 12 mo was not statistically associated with the initial transient rise of CPs. There were procedure-related complications in 3 patients (3.2%), but the complications were resolved by medical and interventional treatments without hepatic functional sequelae.
CONCLUSION: The combined TACE and RFA with an interval of 0-2 d are safe for the management of HCC < 5 cm in cirrhotic patients.
Core tip: This study investigated whether an interval of 0-2 d for combined transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) is acceptable for recovery of liver functional reserve in cirrhotic patients with hepatocellular carcinoma (HCC) < 5 cm. Of 94 enrolled patients, 89 (94.7%) did not show changes in their Child-Pugh scores (CPs) after treatment. Only 5 patients experienced a transient rise of CPs by one-point and their CPs was restored to the baseline within 3 mo after treatment. Therefore, we suggest that the combined TACE and RFA using a short-term interval are safe for treating HCC < 5 cm in cirrhotic patients.
- Citation: Choe WH, Kim YJ, Park HS, Park SW, Kim JH, Kwon SY. Short-term interval combined chemoembolization and radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol 2014; 20(35): 12588-12594
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12588.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12588
Percutaneous radiofrequency ablation (RFA) has become established as a curative treatment for small sized hepatocellular carcinoma (HCC)[1,2]. However, the likelihood of complete ablation using RFA decline rapidly as tumor size increases. The complete remission (CR) after RFA for HCC ≤ 2 cm is approximately 90%[3], but the local tumor progression rate for HCC > 2 cm is substantial, and varies from 20% to 40%[4,5]. Because local tumor progression could have an adverse effect on patient survival or distant HCC recurrence, it is necessary to obtain complete coagulation necrosis of the tumor and a sufficient safety margin. Thus, the combination of RFA with transcatheter arterial chemoembolization (TACE) has several theoretical advantages[6,7]. Occlusion of hepatic arterial flow by TACE could reduce the heat sink effects and contribute to a larger RFA ablation zone. In addition, the hyperthermia induced by RFA may enhance the effect of anticancer agents delivered by TACE. Several recent reports demonstrated promising results and indicated the combination therapy is more effective than either of the mono therapies in preventing incomplete necrosis of HCCs[8-11].
The time-interval between sequential TACE and RFA treatments should be chosen carefully to achieve a balance between successful tumor eradication and adequate preservation of liver function. A longer time interval between the two treatments might preserve liver function because sufficient time is allowed for hepatic functional recovery. However, this extended time prolongs the hospital stay or increases the number of times a patient is admitted, which might undermine the benefits of this treatment option. Conversely, a short interval can lead to better local efficacy because of more synergistic effect for the combination of TACE and RFA. However, a short interval might increase the potential risk of damage of liver function reserves, especially in patients with liver cirrhosis. Theoretically, the optimal time interval is as short as possible when adequate preservation of hepatic functional reserves is guaranteed. However, until now there has been no clear consensus regarding the time interval between TACE and RFA for balancing local therapeutic efficacy and safety[12-15].
The purpose of this study is to investigate the serial changes of liver function parameters and Child-Pugh score (CPs) during a 1-year follow-up period after combination TACE and RFA treatments. The treatments were separated by a time-interval of 0-2 d and were used to treat HCC in patients with liver cirrhosis. The risk factors for aggravation of liver function (rise of CPs) after the treatment were also assessed.
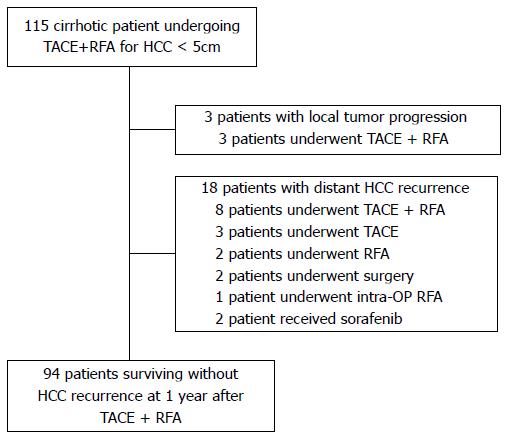
Between July 2005 and September 2011, a total of 115 consecutive patients with HCC underwent sequential application of TACE and RFA at a time interval of 0-2 days with curative intent at Konkuk University Medical Center, Seoul, Korea. The inclusion criteria of the combined treatment were as follows: (1) patients with a solitary HCC less than 5 cm in diameter or those with two or three nodules each less than or equal to 3 cm in diameter; (2) cirrhosis classified as Child-Pugh classification A or B; (3) absence of vascular invasion or extrahepatic metastasis; (4) absence of severe coagulopathy (i.e., prothrombin activity < 40% or platelet count < 40000/mL); and (5) tumor located at least 1 cm away from the central bile duct. There were 21 patients who received subsequent management for recurrence of HCC (3 patients with local tumor progression at the treated site; 18 patients with distant HCC recurrence) during the 1-year follow up after the initial combined treatment. These 21 patients were excluded from the analysis because subsequent treatment causes additional alteration of liver function. Thus, the local tumor progression rate and distant recurrence rate during 1 year follow-up period were 2.6% and 15.7%, respectively. Ninety-four patients who survived without HCC recurrence were included in this retrospective study for evaluation of serial changes of liver function during a 1 year follow-up after combined treatment of TACE and RFA (Figure 1).
The diagnosis of HCC was based on the typical imaging features (arterial enhancement followed by delayed washout) on dynamic contrast enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI)[16]. Local tumor progression was defined as a new enhancing lesion within or adjacent to the ablation site on dynamic liver CT or MRI. Distant recurrence was defined as a new HCC in the liver distant from the index tumor or in extra-hepatic regions. The clinical diagnosis of liver cirrhosis was based on imaging findings such as CT or MRI, together with compatible clinical features of portal hypertension, such as esophageal varices or splenomegaly with thrombocytopenia. This study protocol received Institutional review board approval.
All TACE procedures were performed on an inpatient basis by an interventional radiologist with more than 10 years of TACE experience. After celiac and superior mesenteric arteriography using a 5-French catheter (Cook, Bloomington, IN, United States), the hepatic artery was catheterized. The tumor feeding branch off either the right or left hepatic artery was selectively cannulated using a 3-French microcatheter (Microferret; Cook). Thereafter, selective embolization of the tumor-feeding artery was performed while sparing the majority of hepatic parenchymal arterial supply using an emulsion of iodized oil (Lipiodol; Andre Gurbet, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea). Infusion of this emulsion was performed until arterial flow stasis was achieved and/or iodized oil was visualized in the portal branches. Further embolization with gelatin sponge particles (1-2 mm in diameter; Gelfoam; Upjohn, Kalamazoo, MI) was also performed. After embolization, angiography was performed to assess the extent of vascular occlusion and presence of any residual tumor staining.
Within 2 d after TACE, RFA was performed percutaneously by one of two radiologists with more than 5 years of experience in RFA for hepatic tumors. The patients were treated under local anesthesia with conscious sedation or general anesthesia. After anesthesia, RFA was performed using a 17-gage straight electrode with a 2- or 3-cm exposed tip (Cool-tip; Valleylab, Boulder, CO, United States) connected to an RF generator (Cool-tip RF generator, Radionics). Single or multiple overlapping ablations were performed based on lesion size/geometry and imaging feedback to achieve at least a 0.5-1.0 cm ablative margin around the target tumor. The time and frequency of RF application were varied depending on the number and size of HCC nodules. After the RFA procedure, tract ablation was performed during electrode retraction to prevent bleeding or tract seeding. CT was performed immediately after RFA to assess technical success, which was defined as the presence of a non-enhancing area surrounding the index tumor with a sufficient ablative margin of 0.5 cm or larger on portal phase CT images. In cases of insufficient ablative margin, a second session RFA was performed within 24 h.
A CT was performed at the one month follow-up and was used to evaluate the complete remission (CR) of HCC by the combined treatment. CR was defined as the presence of a non-enhancing area surrounding the index tumor with an ablative margin of 0.5 cm or larger on portal phase CT images. Thereafter, the patients were followed up every 3 mo using liver function tests, such as serum alanine aminotransferase (ALT), albumin (Alb), total bilirubin (T-Bil), prothrombin time (PT), and CPs with dynamic liver CT or MRI. The presence of major complications were also evaluated based on a previous guideline that was defined as any event leading to substantial morbidity and disability, increasing in the level of care, lengthening hospital stay or requiring blood transfusion or interventional drainage procedure.
The statistical calculations were performed with software (SPSS, Chicago, Illinois). The continuous variables are shown as the mean with standard deviation (SD) or median with range. The categorical values are expressed as the frequency with percentage. Interval changes of liver functional parameters were analyzed using a paired t-test, and significant differences were corrected with Bonferroni adjustment for multiple comparisons. Univariate analysis by Fisher’s exact test was performed to identify the variables which were associated with the aggravation of CPs. Potential variables that had an association (P < 0.10) with the rise of CPs in univariate analysis were included in a multivariate logistic regression analysis to determine the independent risk factors increasing CPs after TACE+RFA. Statistical significance was defined by a P-value less than 0.05.
Among the 94 enrolled patients, 69 patients were men and 25 patients were women. The mean patient age was 58.5 ± 8.9 years. Hepatitis B surface antigen and antibodies to hepatitis C were positive in 72 (76.6%) and 13 (13.8%) patients, respectively. Seventy-two patients had chronic hepatitis B, and 60 patients (83.3%) were concomitantly treated with antiviral agents. None of the 14 patients with chronic hepatitis C were treated with anti-HCV agents. Two (2.1%) patients were co-infected with both viruses. Nine (9.6%) patients who had no evidence of viral hepatitis reported high alcohol consumption, and two (2.1%) patients had cirrhosis of unknown origin. There were 67 (71.3%) patients considered class A, and 27 (28.7%) patients were class B based on the Child-Pugh classification system. The mean CPs was 5.9 ± 1.2. There were 21 cases with left lobe HCC, 66 cases with right lobe HCC and 7 cases involving both hepatic lobes. Seventy patients had a single HCC nodule, 21 had two nodules, and 3 patients had three nodules. The mean tumor diameter was 24.1 ± 7.6 mm. The demographics of the enrolled patients in this study are summarized in Table 1.
| Parameters | Total patients (n = 94) |
| Gender (n), Male/Female | 69:25 |
| Age (yr), ≥ 60 : < 60 yr | 46:48 |
| Etiology of HCC (n), HBV: HCV: HBV and HCV co-infection: Alcohol: Cryptogenic | 70:11:2:9:2 |
| Antiviral agents (n), Yes/No | 60/34 |
| Albumin (g/dL), mean ± SD | 3.65 ± 0.52 |
| Bilirubin (mg/dL) , mean ± SD | 1.17 ± 0.69 |
| Prothrombin time (INR), mean ± SD | 1.21 ± 0.16 |
| AST (IU/L), mean ± SD | 55.3 ± 29.7 |
| ALT (IU/L), mean ± SD | 44.4 ± 40.4 |
| PLT (× 103/mm3), mean ± SD | 97.0 ± 41.6 |
| Controllable ascites : no ascites (n) | 26:62 |
| Varices (n), Yes/No | 48/46 |
| Child-Pugh score, 5:6:7:8:9 | 51:16:13:11:3 |
| Chid-Pugh classification, A: B | 67:27 |
| Tumor size (cm), > 3.0 : ≤ 3.0 | 22:72 |
| Tumor number, multiple: single | 24:70 |
| Tumor location, bilobar: unilobar | 7:87 |
| AFP (ng/mL), > 100: ≤ 100 | 19:75 |
| Interval between TACE and RFA (d), 0:1:2 | 27:35:32 |
The mean number of overlapping ablations per tumor was 2.2 ± 1.3 and the mean total time of ablation per tumor was 23.9 min ± 15.0 min. The mean diameter of the long axis of the coagulation necrosis induced by RFA was 39.9 ± 7.5 mm and the short axis was 32.2 ± 6.4 mm based on CT images. On 1-mo follow-up CT images, CR was achieved in all 94 patients.
The serum ALT levels tended to increase slightly from 44.41 ± 40.48 IU/L prior to TACE+RFA to 51.52 ± 46.19 IU/L at 1 mo after TACE+RFA (paired t-test, P = 0.083). However, the levels gradually decreased at 3, 6, and 9 mo and then returned to baseline. Serum Alb levels significantly decreased from 3.65 ± 0.52 g/dL before the treatment to 3.50 ± 0.51 g/dL at 1 mo after the combined treatment (paired t-test with Bonferroni correction, P = 0.005). The Alb levels were recovered by 3 mo and subsequently remained at similar levels for 6, 9, and 12 mo. Serum T-Bil levels at 1, 3, 6, 9 and 12 mo were maintained at the pre-treatment levels. Serum PT levels were also unchanged 12 mo after the treatment (Table 2).
| Pre-TACE+RFA | After TACE+RFA | |||||
| Liver function parameters | Baseline | At 1 mo | At 3 mo | At 6 mo | At 9 mo | At 12 mo |
| ALT (IU/L) | 44.41 ± 40.48 | 51.52 ± 46.19 | 38.71 ± 28.16 | 38.50 ± 23.88 | 38.05 ± 27.72 | 37.69 ± 20.94 |
| Albumin (g/dL) | 3.65 ± 0.52 | 3.50 ± 0.511 | 3.64 ± 0.50 | 3.69 ± 0.48 | 3.67 ± 0.50 | 3.66 ± 0.48 |
| Bilirubin (mg/dL) | 1.17 ± 0.69 | 1.23 ± 0.80 | 1.20 ± 0.69 | 1.22 ± 0.76 | 1.23 ± 0.73 | 1.23 ± 0.79 |
| Prothrombin time (INR) | 1.21 ± 0.16 | 1.22 ± 0.19 | 1.20 ± 0.17 | 1.20 ± 0.17 | 1.20 ± 0.18 | 1.20 ± 0.19 |
At 1 mo after the treatment, the CPs remained unchanged in 89 of the 94 patients (94.7%). There was an increase of CPs by one-point in 5 patients (5.3%), but their CPs returned to pre-treatment scores at 3 mo after the treatment. To evaluate the factors affecting the transient rise of CPs at 1 mo after the combined treatment, the following potential variables were examined: gender, age (≥ 60 vs < 60 years), etiology of HCC (hepatitis B virus vs others), CP class (B vs A), CPs (score 8/9 vs 5/6/7), tumor size (> 3.0 vs≤ 3.0 cm), multiplicity of HCC (≥ 2 vs single) and presence of major complications (Table 3). In the univariate analysis, high baseline CPs (score 8/9 vs 5/6/7, P = 0.022) and large tumor (> 3.0 vs≤ 3.0 cm, P = 0.082) were significantly or marginally associated with transient rise of CPs. However, in the multivariate analysis, the high CPs (score 8/9) was identified as the only independent factor for transient worsening of CPs (score 8/9 vs score 5/6/7; P = 0.026, OR = 9.20, 95%CI: 1.31-64.70).
| Factors | n | Rise of CPs at 1mo events | P | Rise of CPs at 12 mo events | P | |||
| Gender | Male | 69 | 4 | (5.8%) | 1.000 | 2 | (2.9%) | 0.287 |
| Female | 25 | 1 | (4.0%) | 2 | (8.0%) | |||
| Age (yr) | ≥ 60 | 46 | 1 | (2.2%) | 0.362 | 2 | (4.3%) | 1.000 |
| < 60 | 48 | 4 | (8.3%) | 2 | (4.2%) | |||
| Etiology of HCC | HBV | 72 | 4 | (5.6%) | 1.000 | 2 | (2.8%) | 0.232 |
| Others | 22 | 1 | (4.5%) | 2 | (9.1%) | |||
| CP class | A | 67 | 2 | (3.0%) | 0.141 | 2 | (3.0%) | 0.576 |
| B | 27 | 3 | (11.1%) | 2 | (7.4%) | |||
| CP score | score 5-7 | 80 | 2 | (2.5%) | 0.022 | 3 | (3.8%) | 0.481 |
| score 8-9 | 14 | 3 | (21.4%) | 1 | (7.1%) | |||
| Diameter of HCC (cm) | > 3.0 | 22 | 3 | (13.6%) | 0.082 | 1 | (4.5%) | 0.856 |
| ≤ 3.0 | 72 | 2 | (2.8%) | 3 | (4.2%) | |||
| Multiplicity of HCC | ≥ 2.0 | 24 | 3 | (12.5%) | 0.103 | 1 | (4.2%) | 1.000 |
| single | 70 | 2 | (2.9%) | 3 | (4.3%) | |||
| AFP (ng/mL) | > 100 | 19 | 1 | (5.3%) | 1.000 | 0 | (0.0%) | 0.579 |
| ≤ 100 | 75 | 4 | (5.3%) | 4 | (5.3%) | |||
| TACE-RFA interval (d) | same day | 27 | 3 | (11.1%) | 0.141 | 2 | (7.4%) | 0.576 |
| 1-2 | 67 | 2 | (3.0%) | 2 | (3.0%) | |||
| Major complications | Yes | 3 | 0 | (0.0%) | 1.000 | 1 | (33.3%) | 0.124 |
| No | 91 | 5 | (5.5%) | 3 | (3.3%) | |||
| Rise of CPs at 1 mo | Yes | 5 | 1 | (20.0%) | 0.199 | |||
| No | 89 | 3 | (3.4%) | |||||
At 3 and 6 mo after treatment, the CPs maintained pre-treatment scores in all patients. At 9 and 12 mo after the treatment, CPs was not changed in 91 patients (96.8%) and 90 patients (95.7%), respectively. There was an increase of CPs by one-point at 9 and 12 mo observed in 3 (3.2%) and 4 (4.3%) patients, respectively. There was no patient whose CPs increased by two points or more at any time. The rise of CPs at 12 mo was not statistically associated with the initial transient rise of CPs in univariate and multivariate analyses. Other clinical parameters were not associated with long-term aggravation of CPs.
Major complications developed in three of 94 patients (3.2%): hepatic arterial bleeding (n = 1) and biloma with abscess (n = 2). The hepatic arterial bleeding was detected immediately after the procedures, and was successfully treated with transcatheter arterial embolization using microcoils. Two patients experienced infected bilomas, which occurred in the ablated area 4 and 10 mo after combination therapy, respectively. These patients were treated with percutaneous drainage of the biloma and administration of antibiotics. The bilomas were resolved within 1 mo without hepatic functional deterioration. Minor complications developed in 10 patients (10.6%), including moderate pleural effusion (n = 1), subsegmental hepatic infarction (n = 2), non-infected biloma (n = 4), and perihepatic hematoma (n = 2) or partial portal vein thrombosis (n = 1). All the minor complications were asymptomatic and did not alter the CPs. The minor complications did not require any specific invasive treatment. There was no mortality from hepatic failure directly related to the procedure complications.
In our study, 5 (5.4%) of 94 patients who had short-term interval (0-2 d) combined treatment for HCC with mean size of 2.4 ± 0.8 cm showed transient rise of CPs by 1 point at 1 mo follow-up. The only identified risk factor for this transient hepatic functional change was baseline CPs ≥ 8. At the 3 mo follow-up, the scores returned to baseline and were maintained up to 6 mo in all patients. This result suggested that a short term interval combined treatment did not induce long- term hepatic functional deterioration.
There have been two studies comparing RFA treatment alone with TACE combined with RFA that focused on hepatic functional alteration after the treatment. Koda et al[12] reported that there was no significant difference of hepatic functional damage between the RFA alone group (n = 25) and the TACE+RFA group (n = 28). Regardless of treatment option, a transient hepatic functional deterioration at 1 mo followed by recovery at 6 mo was observed in early cirrhotic patients. The deterioration of hepatic function at 6 mo was found in patients with baseline CPs ≥ 8. The mean tumor size was 2.9 ± 1.3 cm and the exact time interval between TACE and RFA was not specified. Kuroda et al[13] reported similar results in patients who received only RFA therapy (n = 25) or combined treatment (n = 20). Baseline CPs ≥ 9 was the only significant risk factor for hepatic functional deterioration at 12 mo. There was no risk factor identified for combination therapy. In their study, the mean tumor size was 2.5 ± 0.8 cm and the average time interval between TACE and RFA was 16.5 d. In the above two studies, the addition of TACE prior to RFA did not impose increased risks of hepatic functional deterioration on the patients compared to RFA monotherapy. The long- term hepatic functional deterioration induced by the treatment was dependent on the baseline hepatic function. In our study, none of the patients experienced long-term hepatic functional deterioration induced by the treatment even though our study population was much larger the prior two studies. One of the reasons for this discrepancy might be a difference in the dominant etiology of cirrhosis: a majority (75%) of our patients had HBV whereas a majority (68%-82%) of patients in the above two studies had HCV as a cirrhosis etiology.
There were two single-arm investigations examining hepatic functional alterations induced by combined treatment in patients with HBV as a dominant etiology. Kang et al[14] showed that CPs had returned to the baseline at 1 mo follow-up in all 50 patients who underwent combined treatment within a single day. This result agrees with our study in which almost all (94.6%) patients had no alteration of CPs at 1 mo follow-up. Conversely, in a study by Li et al[15] 13 (31%) of 42 patients had suffered deterioration of CPs by two or more points at 1 mo follow-up. The median time interval between TACE and RFA was 3 d, and the range was 1-7 d. In their study, CPs ≥ 9 was determined to be a risk factor for hepatic functional deterioration at 1 mo follow-up. A possible explanation for suboptimal results at 1 mo in the study by Li et al[15] is that they included patients with larger tumors that had a mean size of 3.5 cm, whereas in our study and that of Kang et al[14] the mean tumor size was 2.4 cm. In addition, it is not clear whether their observation was transient or not because the follow-up period for hepatic function was limited to 1 mo. In our study, hepatic functional deterioration at 1 mo was transient in all 5 patients.
In our study, baseline CPs ≥ 8 was identified as a risk factor for hepatic functional alteration at 1 mo, although it was mild and transient and was not a clinically significant issue. In previous studies and our study a poor baseline hepatic function (e.g., CPs ≥ 8 or 9) is consistently a significant risk factor for transient or persistent hepatic functional deterioration after the treatment. Therefore, additional attention is needed when applying the combined treatment for patients with limited hepatic functional reserve.
At 12 mo after the treatment, CPs were unchanged compared to baseline score in 90 (95.7%) of 94 patients; the other 4 patients (4.3%) experienced a 1 point increase in CPs in our study. Because all 5 patients who had alteration of CPs at 1 mo recovered baseline hepatic functional status at both 3 and 6 mo follow-up, this mild deterioration at 12 mo follow-up might not be related to the treatment. This change would be associated with natural course of liver cirrhosis. Statistically, the rise of CPs at 12 mo was not significantly associated with any factors including baseline liver functional reserves, TACE-RFA interval, and procedure-related complications. During the entire follow-up period after treatment the CPs was not changed by 2 or more points in any patients.
Major complications developed in three of 94 patients and were successfully managed with interventional procedures without deterioration of hepatic function. The incidence of major complications (3.2%) in our study involving short-term (0-2 d) interval sequential application of TACE and RFA is comparable to the rate for long-term interval sequential application of TACE and RFA and for RFA therapy alone[17,18].
This study has several limitations. First, it did not include a control population such as a RFA alone treatment group or combined treatment with a longer time interval because of its retrospective nature. These control groups would have been necessary to clearly confirm the safety of short-term interval combined treatment. However, we believe that our results regarding liver function change and complications after the short-term interval treatment are acceptable when compared to already published historical series. Second, we excluded 21 patients who received subsequent treatment of HCC recurrence within the 1 year follow-up period after the combined treatment. The patients were excluded because subsequent HCC-directed treatments can cause additional alteration of hepatic function. This exclusion of these patients might have a selection bias. Third, approximately 60% of patients were concomitantly treated with antiviral agents, which might have biased the hepatic function after the treatment compared to those in previous studies that did not administer antiviral agents.
In conclusion, the combination of TACE and RFA is safe for the management of HCC less than 5 cm. A time interval of 0-2 d is sufficient to allow for recovery of liver functional reserve in patients with cirrhosis. However, patients with CPs of 8 or more are at increased risk for transient liver function damage. Therefore, it might be necessary to carefully monitor these patients when using the combined treatment with a short term interval.
Radiofrequency ablation (RFA) has been considered to be one of the most effective loco-regional therapies for small sized hepatocellular carcinoma (HCC). However, local tumor progression at a RFA site is not negligible, and it is tumor-size dependent. To obtain a larger therapeutic region, the combination of transcatheter arterial chemoembolization (TACE) and RFA has been attempted, and several recent reports indicated that the combination therapy is more effective than either of the mono therapies in preventing incomplete necrosis of HCCs.
The time-interval between TACE and RFA should be chosen carefully to achieve a balance between successful tumor eradication and adequate preservation of liver function. The optimal time interval is as short as possible when adequate preservation of hepatic functional reserves is guaranteed. However, until now there has been no clear consensus regarding the time interval between TACE and RFA for balancing local therapeutic efficacy and safety.
Combination with TACE and RFA at a time interval of 0-2 d was applied to treat HCCs less than 5.0 cm in patients with liver cirrhosis. The results demonstrated that this combined treatment was safe for the management of small or intermediate HCC. A time interval of 0 to 2 d was sufficient to allow for recovery of liver functional reserve in patients with cirrhosis.
The combined TACE and RFA with an interval of 0-2 d is safe for the management of HCC less than 5 cm in cirrhotic patients. However, patients with Child Pugh score (CPs) of 8 or more are at increased risk for transient liver function damage. Therefore, it might be necessary to carefully monitor these patients when using the combined treatment with a short term interval.
TACE is one of the most widely performed treatments for unresectable HCCs, which slows tumor progression and improves survival by combining the effect of targeted chemotherapy with ischemic necrosis by arterial embolization. RFA is a localized thermal treatment technique. RFA has emerged as an accepted therapy for early HCC because of its effectiveness and safety. Nowadays, RFA is generally considered as an alternative treatment to liver resection for small sized HCCs, especially for patients with impaired liver function.
In this study, the authors retrospectively investigated the safety of combination of TACE and RFA with a short term interval. The results suggest that the combined TACE and RFA is safe for treating HCC less than 5 cm in cirrhotic patients, even though the interval between two treatments is short term. Because the appropriate time interval between TACE and RFA is still under debate, this paper is of interest to the readers of the Journal.
| 1. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4519] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 2. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 3. | Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 416] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 6. | Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 346] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, Adachi Y, Takeda K. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, Kim PN. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81:e189-e193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 9. | Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452-5460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 251] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 10. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872-3882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Koda M, Ueki M, Maeda Y, Mimura KI, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. The influence on liver parenchymal function and complications of radiofrequency ablation or the combination with transcatheter arterial embolization for hepatocellular carcinoma. Hepatol Res. 2004;29:18-23. [PubMed] |
| 13. | Kuroda H, Kasai K, Kakisaka K, Yasumi Y, Kataoka K, Ushio A, Miyamoto Y, Sawara K, Oikawa K, Kondo K. Changes in liver function parameters after percutaneous radiofrequency ablation therapy in patients with hepatocellular carcinoma. Hepatol Res. 2010;40:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Kang SG, Yoon CJ, Jeong SH, Kim JW, Lee SH, Lee KH, Kim YH. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009;20:1570-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Li JX, Wu H, Huang JW, Zeng Y. The influence on liver function after transcatheter arterial chemoembolization combined with percutaneous radiofrequency ablation in patients with hepatocellular carcinoma. J Formos Med Assoc. 2012;111:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Song do S, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. Clin Mol Hepatol. 2012;18:258-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Livraghi T. Radiofrequency ablation of hepatocellular carcinoma. Surg Oncol Clin N Am. 2011;20:281-299, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
P- Reviewer: Chen JL, Chetty R, Morales-Gonzalez JA S- Editor: Qi Y L- Editor: A E- Editor: Du P