Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12260
Revised: January 14, 2014
Accepted: April 27, 2014
Published online: September 14, 2014
Processing time: 311 Days and 0.8 Hours
AIM: To develop an algorithm to improve the diagnosis and treatment of patients with biliary candidiasis.
METHODS: We performed a prospective study of 127 patients who underwent endoscopic retrograde cholangiopancreatography, for various biliary disorders, at 3 tertiary referral centers in Germany from July 2011 through July 2012 (ClinicalTrials.gov: NCT01109550). Bile, buccal, and stool samples were collected. When indicated, endoscopic transpapillary bile duct biopsies were performed to clarify the etiology of bile duct strictures and to prove invasive fungal infections.
RESULTS: Candida species were detected in 38 of the 127 bile samples (29.9%). By multivariate analysis patients’ age and previous endoscopic sphincterotomy were independent risk factors for biliary candidiasis (P < 0.05). Patients with immunosuppression (P = 0.058) and recent long-term antibiotic therapy (> 7 d) (P = 0.089) tend to be at risk for biliary candidiasis. One patient was negative in mycological culture of bile fluid but invasive biliary candidiasis was diagnosed histologically. Of Candida subspecies detected, 36.7% were azole-resistant, such as C glabrata. Eight patients received anti-mycotic therapy, based on our algorithm. Of these, 3 had cancer with biliary tract involvement, 2 had secondary sclerosing cholangitis, 1 had retroperitoneal fibrosis, and 5 had septicemia. In all patients contamination was ruled out by smears of the endoscope channel.
CONCLUSION: Gastroenterologists should be aware of frequent candida colonization in patients with cholangitis and biliary disorders. Our suggested algorithm facilitates the further clinical management.
Core tip: This prospective multicenter study evaluates the clinical impact of microbial analysis of bile fluid in diagnosing biliary candidiasis. Additionally, a diagnostic algorithm is established to facilitate the clinical management and to improve antimicrobial therapy in patients with cholangitis and involvement of fungal species.
- Citation: Lenz P, Eckelskemper F, Erichsen T, Lankisch T, Dechêne A, Lubritz G, Lenze F, Beyna T, Ullerich H, Schmedt A, Domagk D. Prospective observational multicenter study to define a diagnostic algorithm for biliary candidiasis. World J Gastroenterol 2014; 20(34): 12260-12268
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12260
The clinical presentation of acute cholangitis remains crucial for its diagnosis and the therapeutic approach comprises decompression via endoscopic retrograde cholangiopancreatography (ERCP) and broad-spectrum antibiotic therapy[1].
Up to now, few studies investigated the impact of microbiological analysis of bile fluid in patients with biliary disease[2-7]. Overall, they conclude that bile analysis may be useful to guide the therapeutic procedure in patients with biliary infections. Candidiasis plays an increasing role in nosocomial infections in recent years, especially on intensive care units[8-14]. Accordingly Candida and other fungal species have increasingly been reported to be involved in cholangitis[15,16]. The term “biliary candidiasis” is commonly used when candida species are detected in microbiological bile fluid analysis and the pathogenicity of its presence might be regarded as an unrecognized clinical problem.
Over the years, the number of publications dealing with biliary candidiasis is increasing, but nevertheless the scientific debate is mainly focused on case reports or series. Interestingly, most of the studies were conducted in European countries although fungal infections play an increasing role worldwide[8-14]. Apart from our work, larger clinical trials dealing with biliary candidiasis are still missing.
In a first prospective observational study of 123 consecutive patients undergoing ERCP for various indications, the authors detected Candida species in 44%[17]. The question how positive findings should influence a patient’s therapy has not been answered yet.
This study is aimed at evaluating the incidence of candida in patients suspected of having cholangitis in a multicenter setting. Furthermore, a diagnostic algorithm including additional parameters (candida antigen testing, histology of transpapillary biopsies) should be established to guide therapeutic decisions in biliary candidiasis.
The study was designed as observational multicenter trial. As study centers the University Hospitals of Muenster, Hannover, and Essen participated with their high volume endoscopy units. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was a priori approved by the responsible local Ethics Committees. Furthermore, the study was officially registered at ClinicalTrials.gov (NCT01109550).
Inclusion criteria comprised suspected cholangitis and biliary stricture of unknown origin. Exclusion criteria included contraindications of the performed ERCP-procedure. Baseline characteristics of our study population are given in Table 1. The present study was conducted at three German institution of tertiary care care (Department of Medicine B, University of Muenster; Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover Department of Gastroenterology and Hepatology, University Hospital Essen). The distribution of the primary diagnosis and indications for performing the ERCP-procedure (Table 1) shows that we have a high percentage of complex cases (e.g., carcinoma of the biliary tract) and the simple diagnosis “choledocholithiasis” accounts only for a less proportion of patients. Furthermore, 9 of 127 patients (7.1%) were liver transplant recipients, which also reflect a distinct risk profile of our study population. ERCP was performed in all participating centers using standard methods with a conventional video duodenoscope (TF160R, Olympus Optical Co., Ltd., Tokyo, Japan)[18,19]. The common bile duct (CBD) was selectively intubated with a filling catheter and bile samples aspirated into a sterile syringe and promptly delivered to the respective Institute of Medical Microbiology. To exclude contamination artifacts smears of the endoscope working channel and elevator were taken before and after the examination. Furthermore, buccal smears and stool samples were taken to document the individual transient flora. Endoscopic transpapillary bile duct biopsy for diagnosis of invasive fungal infection was performed when clinically indicated to clarify the etiology of a bile duct stricture. The biopsies were formalin-fixed and analyzed by the responsible Institute of Pathology. Additionally, candida-antigen-serology (Platelia Candida Ag Plus assay, Bio-Rad, France)[20] and blood cultures (BACTEC™, Becton, Dickinson and Company, Sparks, MD, United States) were obtained routinely four hours after the examination. Informed consent was obtained from all patients. All authors had access to the study data and reviewed and approved the final manuscript.
| No biliary candidiasis (n = 89) | Biliary candidiasis (n = 38) | P value | |
| Age (mean ± SD), yr | 58.3 ± 15.6 | 64.7 ± 15.0 | < 0.05 |
| Male/female | 52/37 | 20/18 | NS |
| Primary diagnosis | NS | ||
| Suspected carcinoma of the biliary tract | 16 (18.0) | 10 (26.3) | |
| Stenosing papillitis | 11 (12.4) | 3 (7.9) | |
| Carcinoma | 9 (10.1) | 6 (15.8) | |
| PSC | 9 (10.1) | 5 (13.2) | |
| CBD stenosis of unknown origin | 13 (14.6) | 5 (13.2) | |
| Choledocholithiasis | 9 (10.1) | 3 (7.9) | |
| Cholestatic hepatitis | 4 (4.5) | 1 (2.6) | |
| Pancreatitis | 4 (4.5) | - | |
| Other | 14 (15.7) | 5 (13.2) | |
| Indication for ERCP | NS | ||
| CBD stenosis | 18 (20.2) | 10 (26.3) | |
| Suspected carcinoma | 15 (16.9) | 8 (21.1) | |
| Carcinoma | 9 (10.1) | 6 (15.8) | |
| Cholestasis | 9 (10.1) | 3 (7.9) | |
| Choledocholithiasis | 8 (9.0) | 1 (2.6) | |
| Cholangitis | 7 (7.9) | 2 (5.3) | |
| PSC | 6 (6.7) | 5 (13.2) | |
| Papillitis stenosans | 4 (4.5) | - | |
| Pancreatitis | 2 (2.2) | - | |
| Cholestatic hepatitis | 1 (1.1) | 1 (2.6) | |
| Other | 10 (11.2) | 2 (5.3) |
The specimen were cultivated both on Kimmig agar plates and in Sabouraud bouillons and further differentiation was done by standard micromorphologic and biochemical methods[21]. The detection and identification takes at least 2 in maximum 8 d when received at the Department of Microbiology. According to the literature the term “biliary candidiasis” was used when the fungal culture of the bile specimen was positive[17,22-29] and two patients’ groups were formed-with (group II) and without biliary candidiasis (group I). Candida species identification was conducted by the API ID 32 C test system (bioMérieux, France) according to the instructions of the manufacturer[30]. Antifungal susceptibility testing was performed by the Etest method as described previously but not conducted at all participating centers[31].
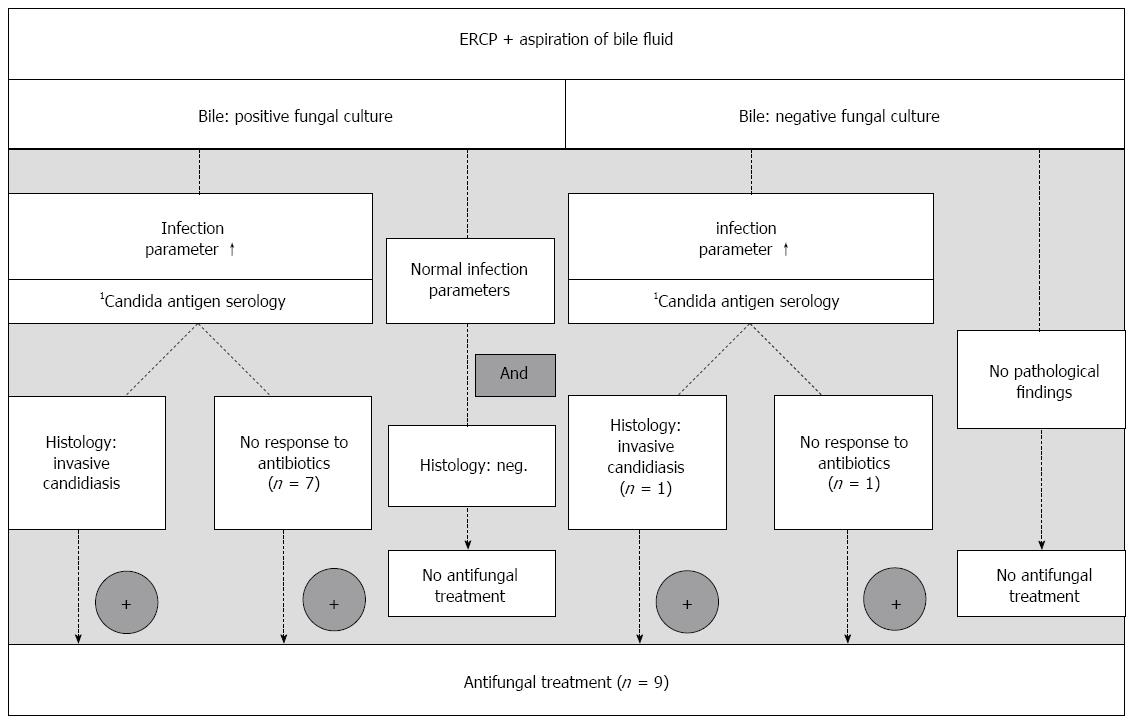
The diagnosis of biliary candidiasis with need for antifungal treatment were made following the guidelines of the Infectious Diseases Society of America[32,33] and the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology[34]. Based on our own experience[17,23,24], we developed the following diagnostic algorithm:
Positive fungal bile culture: (1) Antifungal treatment when elevated infection parameters [body temperature > 38 °C, leukocytosis > 10000/μL, C-reactive protein (CRP) > 0.5 mg/dL] or presence of signs of systemic inflammatory response (SIRS) (blood pressure < 90/50 mmHg) and detection of an invasive candidiasis in the transpapillary biopsy or no response to antibacterial treatment; or (2) no antifungal treatment when patient in good clinical condition and without infection parameters or signs of SIRS or no histologic detection of an invasive candidiasis in the transpapillary biopsy specimen.
No positive fungal culture of the bile: (1) Antifungal treatment when elevated infection parameters or signs of SIRS (see above) and no response to antibacterial treatment (persistent signs of inflammation, see above, despite use of broad-spectrum antibiotics); or (2) no antifungal treatment when no indication within the clinical appearance.
The antifungal treatment was initiated immediately after mediation of the positive finding from the respective institute of microbiology. Patients enrolled in the study were treated with echinocandins (caspofungin, Cancidas™) (loading dose of 70 mg, followed by 50 mg per day for 13.1 ± 5.4 d) as first choice according to the criteria above since our pilot study showed high percentages of azole-resistant fungal species in bile fluid[17]. Additionally, echinocandins are known to reach therapeutic levels in the bile[35] and most of the patients included in the study had elevated liver enzymes speaking in favor of this group of antimycotics as opposed to azoles[36,37]. Azoles were used when oral antimycotic therapy was preferred for prompt release of the patient, for example.
Continuous variables are summarized by the mean and standard deviation. The continuous variables were analyzed using Wilcoxon-Test. Categorical variables are presented in total and as percentage. They were analyzed using χ2-test. Laboratory parameters were analyzed by the Wilcoxon-Mann-Whitney test. Logistic regression was used for multivariate analysis of possible risk factors for biliary candidiasis. The influence of the possible risk factors was investigated using the Wald test. Two-sided P -values of < 0.05 were considered as significant. The test results are regarded in an exploratory and not in an inferential sense, so that it was not necessary to correct P -values for multiple testing. A significant test result provides an indication for clinical relevance, yet clinical relevance needs to be proven in future studies.
Between July 2011 and July 2012, 127 patients were enrolled in this study with various biliary diseases. The gender distribution was similar in both groups (Table 1). Candida species in the bile were detected in 38 of the 127 patients (29.9%). There was a significant difference in age between patients with and without biliary candidiasis (64.7 ± 15.0 vs 58.3 ± 15.6, P < 0.05).
The proportion of patients diagnosed with “biliary candidiasis” based on positive fungal cultures of bile samples was not significantly different between the participating study centers [Muenster: 28/91 (30.8%), Hannover: 7/20 (35.0%), Essen: 3/16 (18.8%), P = 0.541].
Regarding laboratory findings at the time of intervention, no significant difference could be observed (Table 2).
| CRP | Hemoglobin | Leukocytes | Thrombocytes | Bilirubin | Creatinine | GGT | AP | AST | ALT | PCHE | Lipase | |
| (mg/dL) | (g/dL) | (1000/μL) | (1000/μL) | (mg/dL) | (mg/dL) | (U/L) | (U/L) | (U/L) | (U/L) | (U/L) | (U/L) | |
| Group I | ||||||||||||
| mean ± SD | 3.9 ± 6.1 | 12.2 ± 1.8 | 7.9 ± 4.5 | 258.5 ± 151.5 | 4.0 ± 7.2 | 1.1 ± 0.7 | 507.6 ± 766.7 | 374.4 ± 301.4 | 120.0 ± 134.6 | 124.0 ± 182 | 2161.5 ± 3580.3 | 196.2 ± 568.1 |
| Group II | ||||||||||||
| mean ± SD | 3.9 ± 3.3 | 11.6 ± 2.0 | 7.6 ± 3.7 | 270.6 ± 144.0 | 5.1 ± 7.7 | 1.2 ± 1.0 | 430.1 ± 403.4 | 395.8 ± 281.6 | 106.8 ± 90.8 | 101.1 ± 110.8 | 576.4 ± 1144.5 | 226.5 ± 623.6 |
| P | 0.090 | 0.090 | 0.900 | 0.525 | 0.341 | 0.767 | 0.424 | 0.722 | 0.940 | 0.870 | 0.501 | 0.790 |
A list of potential risk factors of biliary candidiasis is given in Table 3 and analyzed by multivariate analysis. Regarding iatrogenic alterations of the biliary tract, previous endoscopic sphincterotomy (EST) is a significant risk factor for biliary candidiasis (65.8% with biliary candidiasis vs 57.3% without biliary candidiasis, P = 0.012). Previous ERCP seems not to be a significant risk factor in multivariate analysis (68.4% vs 59.6%, P = 0.574). Immunosuppression (neutropenia, status under immunosuppressive drugs or chemo-therapy, progressive cancer disease) tends to be predominant in patients with positive fungal cultures of their bile compared to the negative group (68.4% vs 47.2%, P = 0.058). Furthermore, long-term antibiotic treatment exceeding seven d (28.9% vs 10.1%, P = 0.089) tends to be associated with biliary candidiasis. The incidence of Candida species in the bile of patients without prior ERCP was 12/48 (25%).
| Risk factors | No biliary candidiasis (n = 89) | Biliary candidiasis (n = 38) | P value | Exp(B) | 95%CI for exp(B) |
| Age | 58.3 ± 15.6 | 64.7 ± 15.0 | 0.036 | 1.034 | 1.002-1.067 |
| Malignancy | 20 (22.5) | 15 (39.5) | 0.242 | 0.494 | 0.152-1.609 |
| Cholangio-carcinoma | 8 (9.0) | 7 (18.4) | 0.372 | 0.497 | 0.107-2.310 |
| Immunosup-pression | 42 (47.2) | 26 (68.4) | 0.058 | 0.403 | 0.158-1.031 |
| ICU | 2 (2.2) | 2 (5.3) | 1.000 | ||
| Mechanical ventilation | 2 (2.2) | 1 (2.6) | 1.000 | 2.3E+9 | |
| Antibiotic therapy | 42 (47.2) | 26 (68.4) | 0.200 | 0.530 | 0.201-1.399 |
| Long-term antibiotic therapy (> 7 d) | 9 (10.1) | 11 (28.9) | 0.089 | 0.354 | 0.107-1.170 |
| Previous EST | 51 (57.3) | 25 (65.8) | 0.012 | 0.246 | 0.082-0.739 |
| Previous ERCP | 53 (59.6) | 26 (68.4) | 0.574 | 1.318 | 0.504-3.444 |
Distribution of candida subspecies in the bile: Most of the fungal species in the bile were identified as Candida albicans (C. albicans) (60.5%), followed by Candida glabrata (C. glabrata), which accounts for 15.8% of the cases (Table 4). Together with Candida tropicalis (10.5%), Candida krusei (2.6%) and mixed cultures (7.9%) the percentage of potentially azole-resistant candida strains adds up to 36.7%. The mixed cultures include C. albicans et glabrata (n = 2) and C. albicans et tropicalis (n = 1).
| No biliary candidiasis (n = 89) | Biliary candidiasis (n = 38) | P value | |
| Bile samples | |||
| Positive | 89/127 (70.1) | 38/127 (29.9) | |
| Candida albicans (C. albicans) | 23 (60.5) | ||
| Candida tropicalis (C. tropicalis) | 4 (10.5) | ||
| Candida dubliniensis (C. dubliniensis) | 1 (2.6) | ||
| Candida glabrata (C. glabrata) | 6 (15.8) | ||
| Candida krusei | 1 (2.6) | ||
| C. albicans et glabrata | 2 (5.3) | ||
| C. albicans et tropicalis | 1 (2.6) | ||
| Buccal smears | |||
| Positive | 42/89 (47.2) | 28/38 (73.7) | 0.007 |
| C. albicans | 30 (71.4) | 17 (60.7) | |
| C. tropicalis | 1 (2.4) | 1 (3.6) | |
| C. dubliniensis | 2 (4.8) | 1 (3.6) | |
| C. glabrata | 3 (7.2) | 2 (7.2) | |
| Mixed cultures | 6 (14.3) | 7 (25.0) | |
| Stool samples | |||
| Positive | 24/62 (38.7) | 14/27 (51.9) | 0.489 |
| C. albicans | 14 (58.3) | 4 (28.6) | |
| C. tropicalis | 1 (4.2) | 1 (7.2) | |
| C. dubliniensis | 1 (4.2) | 1 (7.2) | |
| C. glabrata | 2 (8.3) | 4 (28.6) | |
| Other | 3 (12.5) | - |
Fungal species in blood culture analysis: None of the blood cultures, taken 4 h after the examination, provided evidence for candidemia.
Overall, no significant correlations between finding candida in swabs of the endoscope channel before the endoscopic examination and in buccal smears (P = 0.501), in bile (P = 0.088) or in stool samples (P = 1.000) were found.
After endoscopic examination, mycological analysis showed similar pathogens in bile and buccal smears, but not in stool samples (Table 4).
The overall presence of candida in buccal smears and bile samples correlated significantly (P = 0.007, Table 4), but subspecies analysis revealed no consistency between bile and buccal smears (e.g., C. albicans was found in buccal smears but C. glabrata in bile analysis (P = 0.700).
In three patients antifungal treatment was initiated outside the algorithm (one with candida sepsis, another with severe esophageal candidiasis). The third patient suffered from progressive colon cancer with liver metastasis and cholestasis; he was admitted in reduced general condition not explained by tumor disease alone. Here, invasive fungal infection was diagnosed by transpapillary biliary biopsy although fungal culture of the bile sample remained negative.
Eight patients were treated according to our suggested algorithm. Only one of these 8 patients had no proven biliary candidiasis and treatment decision was based on the prolonged course of the acute cholangitis despite antibiotic treatment and a high titer of serum candida antigen (302.1 pg/mL, Elisa test).
In the majority of cases (7/8 patients) the indication for antifungal treatment was based on the clinical aspect of missing response to antibiotic treatment alone. All of these patients suffered from severe chronic disease such as primary sclerosing cholangitis, cancer or retroperitoneal fibrosis. For example, one patient after hemihepatic resection due to rectal cancer suffered from persistent infection despite broad-spectrum antibiotic treatment. Mycological analysis revealed Candida albicans in the bile fluid and as well in a liver abscess with communication to the biliary tract. With antifungal treatment the patient recovered promptly and was discharged in good general condition.
In this study, nine patients where treated with echinocandins and two with fluconazole. The use of oral fluconazole in two cases was chosen as these patients preferred an oral medication to enable early discharge from hospital for private reasons.
Overall, patients treated with antimycotics had significantly higher cholestasis parameters at baseline compared to the initial blood analysis in the untreated group (Bilirubin: 6.9 ± 5.7 mg/dL vs 4.1 ± 7.4 mg/dL, P < 0.05; yGT: 908.4 ± 1031.8 mg/dL vs 443.2 ± 625.0 mg/dL, P < 0.05).
Serum Candida antigen levels do not correlate with positive fungal cultures of the bile (5.50 ± 40.73 vs 0.02 ± 0.11, P = 0.823), but interestingly, the serum candida antigen levels were significantly higher in the treatment group, compared to patients who did not receive antifungal treatment (37.83 ± 106.78 pg/mL vs 0.01 ± 0.05 pg/mL, P = 0.001).
There was no significant difference in length of stay (LOS) and time to readmission (TTR) between patients with and without biliary candidiasis (LOS: 9.4 ± 13.8 d vs 10.0 ± 14.4 d, P = 0.631 and TTR: 63.1 ± 46.6 vs 57.9 ± 67.9, P = 0.161).
Comparing the patients with positive fungal culture with and without antifungal treatment LOS was significantly longer and TTR significantly shorter in the treatment group (LOS: 21.9 ± 24.2 d vs 6.9 ± 8.9 d, P = 0.011 and TTR: 74.4 ± 73.3 vs 13.8 ± 13.7, P = 0.018).
Despite technical improvements of interventional endoscopy, little is known about the bile fluid and its microbial flora. Some data suggest that collecting bile during ERCP in patients with suspected cholangitis may be useful[2,3,6,7]. However, this is not routinely practiced. Microbial bile analysis helps to tailor an individual therapy and identifies the bacterial spectrum of each center to improve empiric antibiotic therapy[6]. Some groups also find high percentages of Candida in the bile fluid (Kaya et al[38]: 10%, Negm et al[6]: 10%[6], Lenz et al[17]: 44%). The present study showed a high percentage of fungal species in the bile fluid-even in a multicenter setting including three centers of tertiary care. The distribution of fungal species was comparable with our previous study with Candida albicans as predominant species, followed by C. glabrata and tropicalis[17]. The study was conducted in a high-risk collective of patients which may have biased the microbiological results. The diagnostic algorithm seems, therefore, especially relevant for other tertiary centers or treatment of critically ill patients.
As this study was conducted at three institution of tertiary care, most of the enrolled patients have already had previous ERCP-procedures. Previous EST was identified as independent risk factor for biliary candidiasis. On might speculate that an altered biliary sphincter after EST might facilitate colonization of the bile fluid with fungal species and therefore predispose for ascending infections. Our conclusions would be more clear cut if we had used rather untreated, naïve patients. Nevertheless, cholangitis also occurs in patients with “virgin” papilla and analysis of bile samples may therefore also be useful in those patients.
Blood cultures after the examination were negative in all patients. This is in agreement with Diebel et al[22] who found that patients with biliary Candida infection had no evidence of candidemia. Furthermore, the sensitivity of blood cultures to detect Candida is 50%-75% and lower sensitivity rates have been reported[13,39,40].
Especially in immunosuppressed patients, an early diagnosis and adequate treatment of invasive fungal infection is crucial[41]. Any delay in the initiation of antimycotic therapy in candidemia may result in a decrease of overall survival[42,43]. Microbial analysis of bile fluid and implementation of the suggested diagnostic algorithm might accelerate early diagnosis and pave the way for adequate treatment.
Concordant with our pilot study in 2009[17], high percentages of complicated and potentially azole-resistant Candida subspecies were identified (36.7%). The proportion of non-albicans Candida species in bile and buccal smears or stool samples was not significantly different in patients with and without biliary candidiasis (P = 0.316 and P = 0.582 respectively). Comparing buccal smears and stool samples we found a significant difference (P = 0.034). The influence of different Candida subspecies on the individual patient cannot be answered by this study and may be answered be future analysis, especially regarding the human gut microbiome[44-46].
The swabs of the endoscope channel do not exclude contamination during the introduction process of the endoscope via the oral route. The recessed position of the elevator from the endoscope tip, the promptly delivery of bile samples for mycological analysis and the inconsistency between the species found in buccal smears and bile may speak against contamination artifacts.
When positive fungal cultures of the bile are obtained it remains controversial under what circumstances antimycotic treatment is indicated. Moreover it is still an open question whether the billary tract is a sterile compartment or not (according to Ascioglu et al[32]). Positive fungal cultures of the bile have to be regarded different from positive findings in stool and buccal smears, as the biliary tract might be primarily regarded as sterile compartment. This assumption is underlined by the fact that previous endoscopic sphincterotomy is an independent risk factor for biliary candidiasis. The question whether the simple presence of candida in bile is part of a systemic infection, colonization or simply contamination could not be answered by our study. Nevertheless, we hope to provide the clinician a direction under which circumstances a positive fungal culture of a bile specimen should be treated.
Most of our study patients were treated by antimycotics after insufficient clinical response to antibiotic treatment alone. This study may also contribute to the awareness of increasing fungal involvement in biliary tract infections and support antifungal treatment in case of unsuccessful antibiotic therapy. However, the treatment arm of our study was not randomized and should be regarded as a feasibility approach.
In our study population, Candida hyphae were found in only one patient, and that patient lacked a positive fungal culture from bile. In patients with relevant risk factors for biliary tract infection (e.g., primary sclerosing cholangitis, liver transplant recipients), physicians should ask the pathologist to look for signs of invasive fungal infections of the biliary tract.
Candida antigen testing is not routinely recommended[34]. Nevertheless, serological diagnostic methods seem to be useful in screening high-risk patients and, in combination with risk stratification, may allow early diagnosis of invasive fungal infections[47]. Candida antigen testing may, therefore, be regarded as an additional factor to guide therapeutic decisions and monitor treatment response.
Given the background of our previous published studies[17,24] combined with the experiences gained by the present trial, we suggest a modified diagnostic algorithm for biliary candidiasis to implicate therapeutic decisions (Figure 1). Following Cornely et al[48], positive fungal cultures of bile samples may justify pre-emptive treatment decisions and will be useful in identifying patients, who need antifungal treatment.
In all patients treated with echinocandins, no adverse events in regard to drug treatment were observed underscoring the safety of this drug-even in a high-risk population. The reason for prolonged hospital stay and shorter TTR in the treatment group may rather lay in the severity of the individual disease than in less effective treatment. Regarding overall survival, three patients without and two patients with biliary candidiasis died within a 2-mo follow-up period (P = 0.635). The high-risk collective at three institutions of maximum care may explain the mortality rate of 4% within the study.
In conclusion, this study underlines the diagnostic utility of microbial bile analysis. Physicians should be aware of possible fungal involvement in cholangitis and other biliary diseases. Especially in patients with risk factors, such as age, previous EST, immunosuppression, and previous long-term antibiotic therapy the suggested algorithm facilitates efficient clinical management.
Thanks to Professor Gary Powell for careful language editing of the manuscript and true friendship for over 25 years. We gratefully thank Dr. Borowski M, Department of Medical Informatics and Biomathematics, for revision and optimization of the statistics in the manuscript.
Candida and other fungal species play an increasing role in nosocomial infections, not only in pneumonia and blood stream infections, but also in cholangitis and cholangiosepsis. Bile aspiration during endoscopic retrograde cholangiopancreatography and microbiological analysis is a safe and useful diagnostic tool.
The authors undertook a prospective multicenter trial to evaluate the clinical impact of microbial analysis of bile fluid in diagnosing biliary candidiasis. Additionally a diagnostic algorithm is suggested to facilitate the clinical management and to improve antimicrobial therapy in patients with cholangitis and involvement of fungal species.
Candida and other fungal species were frequently detected in patients with biliary disorders. In tertiary centers, high percentages of azole-resistant species should be taken into account when initiating empiric antifungal treatment. Patients’ age and previous endoscopic sphincterotomy were identified as independent risk factors for biliary candidiasis. Additionally, patients with immunosuppression and recent long-term antibiotic therapy tend to be at risk for biliary candidiasis. When fungal species are found in bile samples in case of cholangitis antimicrobial therapy should be expanded to antimycotics, when antibiotics alone do not relieve patient´s symptoms.
By establishing antimicrobial analysis of bile fluid in patients with cholangitis, antimicrobial therapy can be individually tailored and may positively impact patient´s outcome.
The suggested diagnostic algorithm for biliary candidiasis facilitates the clinical management of patients who were diagnosed with biliary candidiasis.
This is a prospective multicenter study. The author reported the distribution of Candida spp. in bile, buccal, and stool specimens. They also suggested algorithm facilitating the clinical management. This is an important study.
| 1. | Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Chang WT, Lee KT, Wang SR, Chuang SC, Kuo KK, Chen JS, Sheen PC. Bacteriology and antimicrobial susceptibility in biliary tract disease: an audit of 10-year’s experience. Kaohsiung J Med Sci. 2002;18:221-228. [PubMed] |
| 3. | Karpel E, Madej A, Bułdak Ł, Duława-Bułdak A, Nowakowska-Duława E, Łabuzek K, Haberka M, Stojko R, Okopień B. Bile bacterial flora and its in vitro resistance pattern in patients with acute cholangitis resulting from choledocholithiasis. Scand J Gastroenterol. 2011;46:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Maluenda F, Csendes A, Burdiles P, Diaz J. Bacteriological study of choledochal bile in patients with common bile duct stones, with or without acute suppurative cholangitis. Hepatogastroenterology. 1989;36:132-135. [PubMed] |
| 5. | Melzer M, Toner R, Lacey S, Bettany E, Rait G. Biliary tract infection and bacteraemia: presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad Med J. 2007;83:773-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 6. | Negm AA, Schott A, Vonberg RP, Weismueller TJ, Schneider AS, Kubicka S, Strassburg CP, Manns MP, Suerbaum S, Wedemeyer J. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest Endosc. 2010;72:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 7. | Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest Endosc. 2002;56:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3023] [Cited by in RCA: 3176] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 9. | Chow JK, Golan Y, Ruthazer R, Karchmer AW, Carmeli Y, Lichtenberg DA, Chawla V, Young JA, Hadley S. Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med. 2008;36:1993-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Playford EG, Marriott D, Nguyen Q, Chen S, Ellis D, Slavin M, Sorrell TC. Candidemia in nonneutropenic critically ill patients: risk factors for non-albicans Candida spp. Crit Care Med. 2008;36:2034-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Playford EG, Nimmo GR, Tilse M, Sorrell TC. Increasing incidence of candidaemia: long-term epidemiological trends, Queensland, Australia, 1999-2008. J Hosp Infect. 2010;76:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 610] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 13. | Arendrup MC, Fuursted K, Gahrn-Hansen B, Schønheyder HC, Knudsen JD, Jensen IM, Bruun B, Christensen JJ, Johansen HK. Semi-national surveillance of fungaemia in Denmark 2004-2006: increasing incidence of fungaemia and numbers of isolates with reduced azole susceptibility. Clin Microbiol Infect. 2008;14:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 2940] [Article Influence: 154.7] [Reference Citation Analysis (0)] |
| 15. | George J, Baillie J. Contemporary Management of Biliary Tract Infections. Curr Infect Dis Rep. 2005;7:108-114. [PubMed] |
| 16. | Singh N, Wagener MM, Marino IR, Gayowski T. Trends in invasive fungal infections in liver transplant recipients: correlation with evolution in transplantation practices. Transplantation. 2002;73:63-67. [PubMed] |
| 17. | Lenz P, Conrad B, Kucharzik T, Hilker E, Fegeler W, Ullerich H, Heinecke A, Domschke W, Domagk D. Prevalence, associations, and trends of biliary-tract candidiasis: a prospective observational study. Gastrointest Endosc. 2009;70:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Aabakken L. Endoscopic retrograde cholangiopancreatography. Endoscopy. 2012;44:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Cotton PB. Cannulation of the papilla of Vater by endoscopy and retrograde cholangiopancreatography (ERCP). Gut. 1972;13:1014-1025. [PubMed] |
| 20. | Lunel FM, Donnelly JP, van der Lee HA, Blijlevens NM, Verweij PE. Performance of the new Platelia Candida Plus assays for the diagnosis of invasive Candida infection in patients undergoing myeloablative therapy. Med Mycol. 2011;49:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Fegeler W. [Aspects in the diagnosis of deep-seated opportunistic mycoses]. Mycoses. 1994;37 Suppl 2:8-19. [PubMed] |
| 22. | Diebel LN, Raafat AM, Dulchavsky SA, Brown WJ. Gallbladder and biliary tract candidiasis. Surgery. 1996;120:760-764; discussion 764-5. [PubMed] |
| 23. | Domagk D, Bisping G, Poremba C, Fegeler W, Domschke W, Menzel J. Common bile duct obstruction due to candidiasis. Scand J Gastroenterol. 2001;36:444-446. [PubMed] |
| 24. | Domagk D, Fegeler W, Conrad B, Menzel J, Domschke W, Kucharzik T. Biliary tract candidiasis: diagnostic and therapeutic approaches in a case series. Am J Gastroenterol. 2006;101:2530-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kulaksiz H, Rudolph G, Kloeters-Plachky P, Sauer P, Geiss H, Stiehl A. Biliary candida infections in primary sclerosing cholangitis. J Hepatol. 2006;45:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Morris AB, Sands ML, Shiraki M, Brown RB, Ryczak M. Gallbladder and biliary tract candidiasis: nine cases and review. Rev Infect Dis. 1990;12:483-489. [PubMed] |
| 27. | Noack KB, Osmon DR, Batts KP, Wilhelm M, Krom RA, Lindor KD. Successful orthotopic liver transplantation in a patient with refractory biliary candidiasis. Gastroenterology. 1991;101:1728-1730. [PubMed] |
| 28. | Wig JD, Singh K, Chawla YK, Vaiphei K. Cholangitis due to candidiasis of the extra-hepatic biliary tract. HPB Surg. 1998;11:51-54. [PubMed] |
| 29. | Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, Pitt HA, Gouma DJ, Garden OJ, Büchler MW. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:578-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Freydiere AM, Guinet R, Boiron P. Yeast identification in the clinical microbiology laboratory: phenotypical methods. Med Mycol. 2001;39:9-33. [PubMed] |
| 31. | Espinel-Ingroff A, Pfaller M, Erwin ME, Jones RN. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J Clin Microbiol. 1996;34:848-852. [PubMed] |
| 32. | Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1840] [Cited by in RCA: 1803] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 33. | Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2308] [Cited by in RCA: 2063] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 34. | Ruhnke M, Böhme A, Buchheidt D, Cornely O, Donhuijsen K, Einsele H, Enzensberger R, Hebart H, Heussel CP, Horger M. Diagnosis of invasive fungal infections in hematology and oncology--guidelines from the Infectious Diseases Working Party in Haematology and Oncology of the German Society for Haematology and Oncology (AGIHO). Ann Oncol. 2012;23:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Goicoechea M, Fierer J, Johns S. Treatment of candidal cholangitis with caspofungin therapy in a patient with a liver transplant: documentation of biliary excretion of caspofungin. Clin Infect Dis. 2004;38:1040-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Bennett JE. Echinocandins for candidemia in adults without neutropenia. N Engl J Med. 2006;355:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Kullberg BJ, Verweij PE, Akova M, Arendrup MC, Bille J, Calandra T, Cuenca-Estrella M, Herbrecht R, Jacobs F, Kalin M. European expert opinion on the management of invasive candidiasis in adults. Clin Microbiol Infect. 2011;17 Suppl 5:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Kaya M, Beştaş R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol. 2012;18:3585-3589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Arendrup MC, Bruun B, Christensen JJ, Fuursted K, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Møller J, Nielsen L. National surveillance of fungemia in Denmark (2004 to 2009). J Clin Microbiol. 2011;49:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit Care Med. 2009;37:1612-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 389] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 41. | Pacholczyk M, Lagiewska B, Lisik W, Wasiak D, Chmura A. Invasive fungal infections following liver transplantation - risk factors, incidence and outcome. Ann Transplant. 2011;16:14-16. [PubMed] |
| 42. | Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 893] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 43. | Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 832] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 44. | Takagi Y, Hattori H, Adachi H, Takakura S, Horii T, Chindamporn A, Kitai H, Tanaka R, Yaguchi T, Fukano H. Genotypes of Candida albicans involved in development of candidiasis and their distribution in oral cavity of non-candidiasis individuals. Med Mycol J. 2011;52:315-324. [PubMed] |
| 45. | Gong YB, Zheng JL, Jin B, Zhuo DX, Huang ZQ, Qi H, Zhang W, Duan W, Fu JT, Wang CJ. Particular Candida albicans strains in the digestive tract of dyspeptic patients, identified by multilocus sequence typing. PLoS One. 2012;7:e35311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2392] [Article Influence: 170.9] [Reference Citation Analysis (0)] |
| 47. | Kedzierska A, Kochan P, Pietrzyk A, Kedzierska J. Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: galactomannan, mannan and (1--> 3)-beta-D-glucan antigens. Eur J Clin Microbiol Infect Dis. 2007;26:755-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 917] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
P- Reviewer: Lo HJ, Souza JLS, Schrittwieser R, Teoh AYB S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN