Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12045
Revised: January 17, 2014
Accepted: April 8, 2014
Published online: September 14, 2014
Processing time: 325 Days and 22.5 Hours
The natural history of chronic hepatitis B is characterized by different phases of infection, and patients may evolve from one phase to another or may revert to a previous phase. The hepatitis B e antigen (HBeAg)-negative form is the predominant infection worldwide, which consists of individuals with a range of viral replication and liver disease severity. Although alanine transaminase (ALT) remains the most accessible test available to clinicians for monitoring the liver disease status, further evaluations are required for some patients to assess if treatment is warranted. Guidance from practice guidelines together with thorough investigations and classifications of patients ensure recognition of who needs which level of care. This article aims to assist physicians in the assessment of HBeAg-negative individuals using liver biopsy or non-invasive tools such as hepatitis B s antigen quantification and transient elastography in addition to ALT and hepatitis B virus DNA, to identify who will remain stable, who will reactivate or at risk of disease progression hence will benefit from timely initiation of anti-viral therapy.
Core tip: Hepatitis B e antigen (HBeAg)-negative, the predominant form of chronic hepatitis B infection worldwide, consist of individuals with varying levels of viral replication and liver disease. The dynamic nature of HBeAg-negative infection underscore the need for an appropriate classification and evaluation of this group of patients, including the use of additional tools such as transient elastography and hepatitis B s antigen quantification, with the aim of identifying potential candidates for treatment.
- Citation: Azmi AN, Tan SS, Mohamed R. Practical approach in hepatitis B e antigen-negative individuals to identify treatment candidates. World J Gastroenterol 2014; 20(34): 12045-12055
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12045.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12045
Chronic hepatitis B (CHB) remains a prevalent infection worldwide. The World Health Organization estimated that there are more than 2 billion people who had been exposed to hepatitis B and about 378 million with CHB globally[1]. The introduction of mass neonatal immunization against hepatitis B has resulted in a reduction of the number of new cases in some countries[2]. However, among the existing large pool of patients chronically infected, the hepatitis B virus (HBV) continues to confer a risk for cirrhosis and hepatocellular carcinoma (HCC) therefore the disease still poses a major health and economic burden particularly within the Asia-Pacific region[1].
There has been much progress in the understanding and management of hepatitis B in the past two decades. Covalently closed circular DNA (cccDNA) plays an important role in maintaining the chronicity of this viral infection. Active viral replication and liver disease inflammation can potentially lead to fibrosis, cirrhosis, end stage liver disease and HCC. Effective treatment has been shown to stop the progression of liver disease and decrease the risk of HCC.
The predominant CHB infection worldwide is hepatitis B e antigen (HBeAg) negative[3]. CHB is a heterogeneous disease therefore individuals who are HBeAg-negative can be further sub classified depending on their viral and liver disease activity. A recent systematic review on the liver histological changes from CHB patients revealed that nearly 50% of HBeAg-negative with slight increases in the level of alanine transaminases (ALT) have significant fibrosis[4]. Furthermore, the accuracy of international guidelines for identifying significant fibrosis in HBeAg-negative patients based on the ALT and HBV DNA levels have been questioned[5]. The characteristic features of fluctuations in the ALT and HBV DNA levels underscore the need for thorough repeated evaluation and long-term monitoring of all individuals who are HBeAg negative. This article summarizes the various international guidelines on the management of HBeAg-negative individuals and includes recent data on the use of non-invasive tools such as hepatitis B s antigen level (HBsAg) quantification and transient elastography (TE), which may help clinicians to better identify who warrants treatment or observation.
The natural history of hepatitis B acquired early in life can be divided into 4 phases: immune tolerant phase, immune clearance phase, “inactive carrier state” and reactivation phase. In immune tolerant phase, patients are positive for HBeAg with high HBV DNA levels but a normal serum ALT level. Liver biopsy usually shows no or minimal histological changes[6].
The next phase is the immune clearance phase and is characterized by liver disease activity with an increase in the ALT level. During this immune clearance phase, serum HBV DNA level will decrease and HBeAg may be cleared. The elevation of ALT is due to immune response against the infected hepatocytes, with resultant hepatocyte damage[7]. Thus, prolonged immune clearance phase may increase the risk of liver fibrosis and therefore disease progression[6].
Once HBeAg seroconversion from HBeAg to anti-HBe antibody is achieved, there is usually suppression of HBV DNA and normalization of ALT. Patients in this low replicative phase, referred to as the “inactive HBsAg carrier” or the immune control state, have favourable prognosis with low risk of liver disease progression. Documentation of repeatedly normal ALT based on serial ALT measurements 3-4 mo apart for at least a year is needed to determine whether a patient truly has normalization of ALT commonly referred as persistently normal ALT (PNALT)[8].
Following HBeAg loss, the majority of CHB patients remain stable with low level of viral replication[6]. However a proportion of HBeAg-negative patients may revert to a phase of significant viraemia with fluctuating ALT levels and at times borderline normal. This reactivated phase is part of the natural course of CHB, referred to as HBeAg-negative CHB, which occurs due to mutations in the precore or core promoter region of the HBV[9].
CHB is classically defined as having positive serum HBsAg for more than 6 mo. The term “hepatitis” in CHB refers to the nomenclature of the B virus rather than the presence of necroinflammatory disease of the liver. Two parameters are used to define Hepatitis B disease activity: the magnitude of viral replication and the liver disease status.
HBeAg-negative infection consists of individuals with varying levels of viral replication and liver disease status. As well described in the natural history of perinatally acquired CHB, a patient with normal ALT and negative HBeAg could be an inactive HBsAg carrier or a HBeAg negative individual with significant viral replication who at that point of testing has normal ALT. However it is crucial that the latter group of patients are identified and closely monitored as treatment may be indicated to prevent progression of fibrosis and cirrhosis.
The consensus definition for “Inactive HBsAg carrier state” adopted at the National Institutes of Health workshop on the “Management of Hepatitis B” is persistent HBV infection without significant necroinflammatory disease whereas “HBeAg-negative CHB” is a chronic necroinflammatory disease of the liver in those who are HBeAg-negative[10,11]. The diagnostic criteria for “inactive HBsAg carrier” and “HBeAg-negative CHB” exclude HBeAg negative individuals with significant viral replication, normal ALT levels, absent or minimal necroinflammation but presence of significant fibrosis and cirrhosis. It is well known that necroinflammation subsides with the advancing degrees of fibrosis. Similarly, the diagnostic criteria for “HBeAg positive CHB” currently used exclude HBeAg positive individuals with significant viral replication but with normal ALT levels in the “immune tolerant” phase.
In clinical practice, an individual who is HBsAg positive is initially assessed based on HBeAg status and serum ALT level in addition to other tests for liver profile. The term “chronic HBeAg positive infection” should be used for all chronic HBsAg positive individuals with detectable HBeAg irrespective of ALT level, liver disease or necroinflammatory activity. A proposed term for HBeAg positive individuals with active viral replication and active liver disease is “HBeAg-positive disease”. Similarly, chronic HBeAg-negative infection should encompass all CHB individuals who are HBeAg-negative and the term “HBeAg-negative disease”be reserved for HBeAg-negative individuals with significant viral replication and presence of liver injury. The proposed term of “HBeAg-negative disease” may help clinicians identify the disease state and suitable treatment candidates among individuals who are HBeAg negative.
CHB is a dynamic disease, the disease state and clinical manisfestations of the chronic evolving condition are dependent on the consequence of the interplay of the HBV and the host immune system. The progression from a low viremic “inactive HBsAg carrier” state to a high replication state indicates a transition from the immune control phase to an immune activation phase. Immune reactivation among HBeAg-negative patients usually represents a late phase of perinatally acquired hepatitis B infection with predominant precore and basal core promoter (BCP) mutants which are variants of the hepatitis B virus that are unable to express HBeAg antigen[12]. In general, these mutants are less efficient at viral production and patients who harbor HBeAg-negative mutants typically have lower HBV DNA levels than those with HBeAg-positive infection. However, HBeAg-negative mutants have been implicated in causing an increased risk of development of liver cirrhosis, hepatocellular carcinoma[13-16].
HBV DNA is the best measure of Hepatitis B replication. Although ALT remains the most accessible test to assess liver disease, ALT alone does not accurately reflect the extent of liver damage. In cirrhotic patients the serum ALT tends to be normal. Furthermore, liver enzymes and HBV DNA levels may fluctuate in individuals who are HBeAg-negative at times bordering to normal or high normal.
It is sometimes difficult to differentiate the reactivation phase from the immune control state among CHB individuals who are HBeAg-negative. Classically, the differentiation between these 2 groups is based on the level of HBV DNA and ALT. The reactivation phase is defined as HBV DNA > 2000 IU/mL and presence of elevated ALT, either persistently or intermittently. Therefore repeated testing of both ALT and HBV DNA over time are required for patients with negative HBeAg and normal ALT at first consultation. Factors which are able to predict those at risk of reactivation include HBV genotype C, male gender, ALT > 5 times upper limit normal (ULN) during the HBeAg-positive phase and age of HBeAg seroconversion ≥ 40 years[17].
Aspartate aminotransferase (AST)-to-platelet ratio index, AST/ALT ratio and platelet count are simple and useful markers of liver fibrosis and cirrhosis. Thrombocytopenia, possibly the earliest indication of cirrhosis in some patients, has been shown to reliably predict advanced fibrosis and cirrhosis[18,19].
The use of liver biopsy or non-invasive method to assess severity of liver damage is advocated in some patients. TE of the liver is one of the non-invasive methods, which is increasingly used to assess liver fibrosis. Several studies, including a meta-analysis, have reported that liver stiffness measurement (LSM) by this method shows good correlation with fibrosis stage in CHB patients[20-22]. Liver fibrosis assessment using TE is helpful in deciding the need for anti-viral therapy in patients with high viral load and normal or mildly raised ALT 1-2 times of ULN. However LSM is not widely available and cannot solely be relied on without considering the whole clinical picture and other relevant information[23].
There is also preliminary data using TE for monitoring liver fibrosis progression in HBeAg-positive and HBeAg-negative CHB[24,25]. A recent systematic review of HBeAg-negative CHB with PNALT revealed that it is rare to have significant liver disease based on liver biopsies in patients with stringent criteria for PNALT (defined by authors as: at least 3 ALT determinations at unspecified intervals over 6-12 mo or at least 3 ALT determinations at predefined intervals which are at least 2 mo apart over a minimum of 12 mo) and HBV DNA ≤ 20000 IU/mL. The rate of detection of mild inflammation and moderate fibrosis is even lower at 1.4% and 1% respectively if the HBV DNA levels were less than 2000 IU/mL compared to 7% and 10% if the HBV DNA levels were between 2000 to 20000 IU/mL. In this latter group of HBeAg-negative CHB with PNALT and repeatedly HBV DNA between 2000-20000 IU/mL, rapid and easily repeatable non-invasive liver fibrosis measurements like TE has a role in monitoring[8].
Studies on the natural history of CHB have shown the clinical benefits of HBsAg loss in reducing risk of hepatic decompensation and HCC as well as improving overall survival[26,27]. Clearance of HBsAg was associated with extremely low level of cccDNA in the nucleus of the infected hepatocyte[28,29]. HBsAg quantification has been used to predict HBsAg seroclearance following pegylated interferon (PEG-IFN)-α therapy[30]. Data has emerged on the role of HBsAg level to predict outcome of therapy and sustained response post NA treatment[31].
There is a wide variation in the HBsAg levels throughout different phases of CHB infection and across different HBV genotypes[32,33]. HBsAg levels could help distinguish active from inactive disease among HBeAg-negative individuals. In one study in HBeAg-negative genotype D, HBsAg levels were significantly lower in those with inactive disease (defined as serum HBV-DNA ≤ 2000 IU/mL) compared to those with significant viral replication[34]. The combined use of HBsAg < 1000 IU/mL and HBV-DNA ≤ 2000 IU/mL) at a single time point predicts those with inactive disease with a diagnostic accuracy of 94%, 91% sensitivity, 95% specificity, 88% positive predictive value and 97% negative predictive value.
The role of HBsAg quantification at one time point to differentiate active from inactive disease among HBeAg-negative individuals was also explored in HBV genotype C patients. Different thresholds of HBsAg levels > 850 IU/mL and HBV DNA > 850 IU/mL were applied with a diagnostic accuracy of 86% in predicting HBV reactivation[35].
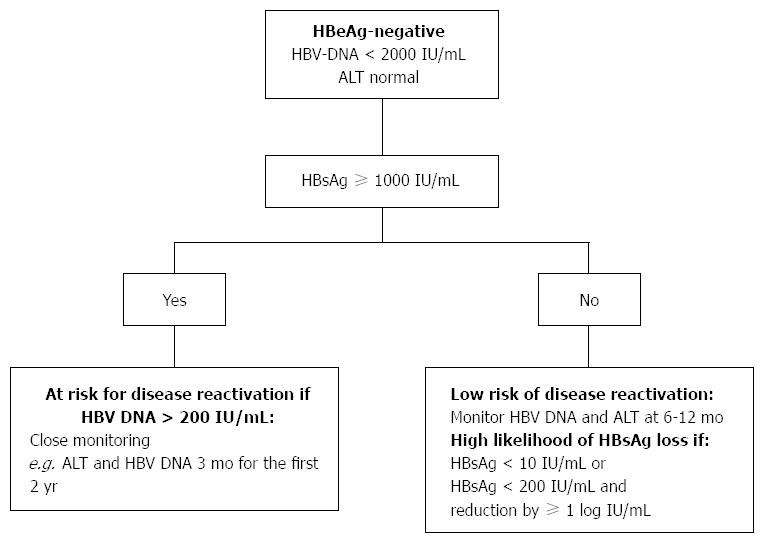
A recent study provides further evidence that HBsAg quantification can be used to predict disease reactivation in asymptomatic HBeAg-negative patients[36,37]. Patients with HBeAg-negative and persistently normal serum ALT levels for 1 year were recruited. The patients were followed-up 3 monthly for the first 2 years. Those with persistently normal ALT during the first two years were subsequently followed up every 6 monthly. Patients with HBsAg > 1000 IU/mL and HBV DNA > 200 IU/mL were more likely to experience disease reactivation, defined as HBV-DNA > 2000 IU/mL and increase in ALT level. The combined use of HBsAg > 1000 IU/mL and HBV DNA > 200 IU/mL is able to predict reactivated patients with a sensitivity of 92%, specificity of 51%, negative predictive value of 96%, and positive predictive value of 30%. Male gender was found to be an independent predictor of reactivation in HBeAg-negative disease. The authors recommended that HBsAg level be measured during follow up of HBeAg-negative patients to identify those at high risk of reactivation. Patients at high risk of reactivation require close monitoring of their ALT and HBV DNA levels every 3 monthly at least for the for the first 2 years of initial follow up (Figure 1).
HBsAg levels tend to reduce slowly over time in HBeAg-negative patients and spontaneous HBsAg seroclearance is more likely to occur in those with low HBsAg levels[38-40]. Quantification of HBsAg levels in inactive genotype B and C HBeAg-negative individuals may be able to predict spontaneous HBsAg loss with the highest HBsAg loss amongst those with HBsAg level of < 10 IU/mL[41-44]. The combined quantification of HBsAg < 200 IU/mL and a reduction of at least 1 log10 IU/mL in the preceding 2-years identified those who are likely to achieve HBsAg loss at 1 year with 97% positive predictive value and 100% negative predictive value.
Although HBsAg levels could help to predict disease activity and HBsAg loss among HBeAg-negative individuals, HBsAg quantification is not available worldwide, hence monitoring of ALT and HBV DNA level with other liver disease assessment remain the standard of care in these patients.
Patients with CHB with active viral replication are more likely to progress to cirrhosis, hepatic decompensation, and HCC. Hepatitis B viral load has been repeatedly shown to be the most important predictor of HCC such that the higher the viral load, the higher is the risk of HCC.
HBsAg seroconversion, which is the closest to a complete cure in hepatitis B, is rarely achieved with the current available therapy. Thus, the main aim of CHB treatment is the elimination of viral replication. Indicators of treatment response include maintained viral suppression on treatment and sustained viral suppression upon cessation of therapy.
For HBeAg-positive CHB, HBeAg loss with seroconversion to anti-HBe with undetectable HBV DNA is an important short-term goal of treatment. However, in HBeAg-negative CHB, the short term goal of therapy is unclear, hence, durable HBV DNA suppression to low or undetectable level is used as a surrogate marker of Hepatitis B control.
Prolonged viral suppression has been shown to improve long-term prognosis and delay the progression of cirrhosis, decompensation and HCC[45-47]. Clinical decompensation such as ascites, hepatic encephalopathy, jaundice or gastrointestinal bleeding can be prevented with treatment. There was a significant difference of 5-year HCC cumulative rates in patients who were cirrhotics where entecavir-treated patients showed lower cumulative rates of HCC compared to non-treated cirrhotics (13.8% vs 26.4%)[48].
Not all CHB patients require therapy. Treatment is indicated in those with active disease, evidence by active viral replication and liver damage.
All major clinical practice guidelines on the management of Hepatitis B from Asian Pacific Association for the Study of the Liver (APASL), European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Disease (AASLD) define treatment candidacy based on elevated HBV DNA and ALT levels[49-51]. However, the threshold for HBV DNA and ALT to select as candidates for treatment differs between guidelines (Table 1).
| AASLD (2009) | APASL (2012) | EASL (2012) | |
| Treatment candidacy | |||
| HBV DNA (IU/mL) | ≥ 20000 | ≥ 2000 | > 20000 |
| ALT | ≥ 2 × ULN | ≥ 2 × ULN | ≥ 2 × ULN |
| Other criteria | Treat if, HBV DNA > 2000, ALT > ULN and moderate to severe inflammation on liver biopsy and/or at least moderate fibrosis. | ||
| Liver biopsy (or noninvasive markers of fibrosis) to consider if | |||
| HBV DNA (IU/mL) | 2000-20000 | > 2000 | > 2000 |
| ALT | 1-2 × ULN | 1-2 × ULN | > ULN |
| Other criteria | ≥ 40 yr old | ||
| First-line treatment | |||
| PEG-IFN or Entecavir or Tenofovir | PEG-IFN or Entecavir or Tenofovir | PEG-IFN or Entecavir or Tenofovir | |
| Duration of treatment | |||
| IFN | 12 mo | 12 mo | 12 mo |
| Oral | > 1 yr | Unknown/long-term | Unknown/long-term |
| Stopping treatment strategy for NA | |||
| Until HBsAg clearance | Until HBsAg clearance, may consider stopping if treated for at least 2 yr with undetectable HBV DNA on three separate occasions 6 mo apart. | Until HBsAg clearance. | |
In HBeAg-negative disease, the APASL and EASL treatment guideline advocate initiation of treatment when HBV DNA > 2000 IU/mL whereas a higher threshold of > 20000 IU/mL is recommended by the AASLD guidelines (Table 1). The APASL and AASLD guidelines recommend commencement of treatment when ALT level is more than twice of the upper limit normal, or irrespective of the ALT level where there is evidence of advanced fibrosis or cirrhosis. EASL guidelines place a lower threshold and recommend initiation of treatment with any level of elevation of ALT when HBV DNA > 2000 IU/mL and liver biopsy shows moderate-severe necroinflammation and/or at least moderate fibrosis.
There is consensus by all major guidelines that individuals who are HBeAg-negative with HBV DNA of > 2000 IU/mL but persistently normal ALT should not be treated unless there is presence of significant liver damage. In general, patients with persistently normal serum ALT levels have no or minimal disease progression[52,53]. However, there is increasing evidence that significant fibrosis can occur in patients with normal ALT levels, but with high viral loads[54-57]. In addition, a significant proportion of patients with ALT just above the upper limit of normal have been shown to have presence of substantial liver fibrosis or cirrhosis[58]. In light of the emerging data, further assessment of liver disease by liver biopsy or TE are needed to better ascertain the treatment needs.
Patients who are 40 years old and above are recommended for liver biopsy or non-invasive liver fibrosis assessment[49]. Treatment is recommended if there is evidence of at least moderate inflammation or fibrosis.
Individuals with HBeAg-negative who do not require treatment need to be followed-up regularly every 3 mo for the first year then every 6-12 mo for the subsequent year[49].
Current available anti-HBV treatments are either immuno-modulators or oral nucleos(t)ide analogs (NA). Immuno-modulators available in most countries are the conventional IFN, PEG-IFN-α2a and PEG-IFN-α2b. Oral NA consist of the first generation drugs, lamivudine and adefovir, and the newer generations, entecavir, telbivudine and tenofovir. Evidence suggests that NA need to be given for a long duration as stopping NA therapy prematurely results in relapse of HBV in the majority of cases[59].
IFN based therapy is given for a finite duration. Although IFN-based therapy is associated with more side effects compared to NA, it has a higher likelihood of sustained off-treatment response. In HBeAg-negative patients, young age, female, high serum ALT levels, low serum HBV DNA were associated with a higher chance of achieving sustained response with PEG-IFN therapy[60].
IFN-α modulates the immune system and at the same time has an anti-viral effect. Conventional IFN-α is highly excreted by the kidneys, thus more frequent injection is needed for it to remain stable in the circulation. IFN-α treatment among European patients showed 60%-90% end-of-treatment biochemical and virological response whereas sustained response rate was only 10%-15% with 4-6 mo treatment and 22% with 12 mo treatment. Treatment response 6 mo after stopping therapy was seen in 30% of patients who received 6-10 mo of IFN treatment[17]. The usual recommended dose for conventional IFN is 5 MU daily or 10 MU three times weekly, but a lower dose is used for Asian patients at 5-6 MU three times weekly.
PEG-IFN-α has a longer half-life due to the addition of polyethylene glycol to the conventional IFN, thus allowing weekly injections. Use of PEG-IFN-α2a 180 μg weekly for 48 wk in one study showed 6 mo post-treatment response of ALT normalization in 59%, reduction of HBV DNA < 20000 copies/mL in 43%, HBV DNA drop < 400 copies/mL in 19% and HBsAg clearance in 3% of patients[61]. In comparison to PEG-IFN-α2b with or without lamivudine treatment for 48 wk, study showed ALT normalization in 40% and HBV DNA reduction to < 60 IU/mL in 43% of patients at 6 mo post-treatment[62]. In a long-term follow-up study of 230 patients, sustained virological response (HBV DNA < 10000 copies/mL) of 21% were observed 5 years post-treatment with PEG-IFN-α2a, and HBsAg clearance were 5% at 1 year post-treatment and 12% at 5 years post-treatment[63].
Combining Lamivudine to IFN or PEG-IFN therapy caused a better suppression of HBV DNA, but there were no differences in sustained off-treatment response[46]. Similarly, the combination of PEG-IFN with adefovir showed no difference in terms of sustained off-treatment response compared to PEG-IFN alone[64]. Sequential therapy with adefovir, entecavir or telbivudine followed by PEG-IFN was studied with promising results. A recent randomized controlled trial of sequential PEG-IFN and telbivudine for 48 wk in HBeAg-negative CHB showed that virological response rate at 24 wk post treatment was significantly higher (46.7% vs 13.3%) in patients treated with telbivudine followed by PEG-IFN than vice versa[64]. Further studies are required to clarify the role of sequential therapy in HBeAg-negative disease.
High rates of on-treatment virological suppression with good genetic barrier to resistance can be achieved with NA monotherapy, particularly with entecavir and tenofovir, thus, the preferred NAs. By 48 wk of treatment with entecavir or tenofovir, loss of serum HBV DNA (< 60-80 IU/mL) was observed up to 90% and 93% respectively[65,66]. Maintained viral suppression was seen in entecavir and tenofovir group up to 98% and 87% respectively at 3rd year of therapy. Sustained virological suppression is associated with histologic improvement and regression of fibrosis and cirrhosis[67,68]. Entecavir and tenofovir in a long-term study (5-6 years) has shown significant regression of fibrosis and cirrhosis with continuous histological improvement[68,69]. Several randomized clinical trials in HBeAg-negative CHB revealed less than 5% rate of sustained off-treatment virological response after 12 mo of NA therapy[61,65,70-72]. HBsAg loss at 12 mo of treatment was negligible for all the oral nucleos(t)ides analogues[61,65,66,70,73,74].
Since most HBeAg-negative CHB requires long-term treatment, viral resistance to NAs is a major concern. Cumulative incidence rate of resistance at 5 years for lamivudine and adefovir are 70% and 29% respectively[75,76]. While entecavir and tenofovir usage generally have very low long-term rate of resistance, which is 1.2% and 0% respectively after 5 years of treatment[68,77]. A rise in the HBV DNA level during therapy by at least 1 log10 IU/mL from nadir of initial response during therapy in a compliant patient suggests development of drug resistance. Resistance to one L-nucleoside analogue (lamuvidine, telbuvidine, emtricitibine) confers complete resistance to all other L-nucleoside analogues and compromises entecavir response, necessitating a higher entecavir dose. Patients on entecavir with prior lamivudine-resistant HBV have a high risk of resistance of up to 51% after 5 years of treatment[78]. One recent retrospective cohort study showed that a prior lamivudine exposure might increase entecavir resistance risk even though they had no detectable lamivudine resistance[79]. In patients with lamivudine-resistant HBV, tenofovir is the preferred NA as cross-resistance is generally unknown. In general, the most effective NA without cross resistance is recommended as a rescue therapy if drug resistance is suspected based on an increase of serum HBV DNA > 1 log10 IU/mL with or without genotypic analysis.
Combination therapy of two direct antiviral agents have not been shown to have better viral suppression nor higher rates of long-term efficacy compared to monotherapy, hence cannot be recommended as first-line therapy[80].
In HBeAg-negative CHB, it is unclear how long treatment with NA should be continued if HBsAg remain positive. The majority of patients will have an indefinite nucleos(t)ide treatment duration. Premature discontinuation of treatment will result in reactivation of Hepatitis B replication in the majority of cases. Long term oral NA raise the issue of drug resistance and high cost, hence stopping NA treatment could be a viable option in some patients.
AASLD 2009 and EASL 2012 guidelines recommend continuing NA treatment until HBsAg clearance has been achieved (Table 1). APASL 2008 guidelines on the management of hepatitis B proposed that cessation of treatment in HBeAg-negative CHB could be considered if HBV DNA is undetectable for three times, six months apart[23]. However, in one study of HBeAg-negative CHB patients who stopped therapy according to the guidelines, 47% had virological relapse, defined as HBV DNA > 1000 copies/mL[59]. The updated APASL 2012 guidelines state that in HBeAg-negative CHB, treatment duration is unknown unless HBsAg seroclearance has occurred, and decision to stop NA therapy has to take into consideration the clinical response and the extent of liver damage. However, treatment discontinuation can be considered if patients have been treated for at least 2 years with undetectable HBV DNA documented on three separate occasions 6 mo apart. Discontinuation can be considered after 2 years of treatment if HBV DNA was undetectable in three separate occasions, six months apart and this strategy has been shown to work especially in those with low baseline viral load at HBV DNA ≤ 20000 IU/mL.
A subsequent study on patients treated with entecavir suggests the risk of relapse is lower at 29% for those with low baseline viral load at HBV DNA ≤ 20000 IU/mL[81]. We would highlight that monitoring with ALT and HBV DNA after stopping is advisable and need to be at frequent intervals to detect relapse. It is recommended that, these to be done at monthly interval for the first 3 mo and then every 3 mo in the first year after therapy. Subsequently monitoring is at 3-6 mo, the shorter interval is recommended for cirrhotics.
HBsAg quantification may have a predictive role in successful control of HBV infection after cessation of NA therapy[82]. HBsAg level during treatment of ≤ 200 IU/mL and HBsAg reduction of > 1 log10 IU/mL from baseline has the highest prediction of sustained response off-treatment. Thus, cessation of therapy in this group may be considered because the risk of relapse is minimal. However in patients with either HBsAg reduction by > 1 log10 IU/mL from baseline or ≤ 200 IU/mL at the end of NA therapy, the sustained response will be approximately 50%.
Treatment in patients with advanced liver fibrosis or cirrhosis should not be stopped due to the risk of hepatitis flare and potential fatal liver decompensation.
We suggest stopping rules in CHB patients who are treated with IFN or Peg-IFN are based on the “week 12 stopping rule”. If no HBsAg level reduction and < 2 log10 HBV DNA level drop by week 12 of treatment in genotype A and D, treatment should be stopped or switched. For genotype B and C, treatment should be stopped if HBsAg titer remain > 20000 IU/mL by week 12. It was shown that these groups had a very low chance to develop a sustained response to treatment[83]. This suggestion is supported by EASL 2012 guideline. However AASLD 2009 guideline suggested patients who HBV DNA failed to reduce by 2 logs at 12 wk of treatment should switch or receive additional treatment. APASL 2012 guideline recommends continuing treatment for 12 mo regardless of the response during treatment.
All CHB patients need long term or lifelong follow-up. Those who are currently not candidates for treatment maybe suitable for treatment as the disease progress or new treatment modalities become available.
Those who are not on specific Hepatitis B therapy require regular follow up every 3 mo for the first year then every 3 monthly for HBeAg positive and 6 monthly for HBeAg-negative for the subsequent year. HBeAg-negative patients with HBV DNA > 20000 IU/mL and persistently normal ALT require 3 monthly follow up[49]. We would suggest patients with high risk of disease reactivation (Figure 1), for a close monitoring at least 3 monthly, looking specifically at the ALT and HBV DNA levels.
Moreover, some patients have indications for surveillance programs like surveillance for varices or hepatocellular carcinoma. Surveillance for hepatocellular carcinoma using ultrasound with or without alpha fetoprotein at 6 monthly intervals is recommended in males above age 40, females above 50 years old, presence of liver cirrhosis or family history of HCC[49]. HCC surveillance is still required in patients who already achieved virological response to hepatitis B treatment because studies showed that they are still at risk of developing HCC although the risk is lower[84]. Patients with cirrhosis are recommended to undergo upper gastrointestinal endoscopy for variceal surveillance. Those without varices would need to have reassessment 2-3 years later while those with significant oesophageal varices need primary prophylaxis with beta-blockers.
In real life clinical practice, clinicians of various levels of experience are managing CHB patients. Thorough evaluations of CHB patients especially in those with negative HBeAg using newer tools like TE and HBsAg quantifications will improve our management of CHB patients. Patients who are at risk of reactivation can be better identified and given treatment and/or the appropriate levels of monitoring.
There may be a need for an appraisal of the current terminology used to differentiate disease activity among HBeAg-negative individuals with the hope of not missing candidates who may benefit from treatment.
| 1. | WHO. Department of Communicable Diseases Surveillance and Response. Hepatitis B. Available from: http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf. |
| 2. | Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:74-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (6)] |
| 3. | Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002;9:52-61. [PubMed] |
| 4. | Nguyen LH, Chao D, Lim JK, Ayoub W, Nguyen MH. Histologic Changes in Liver Tissues from Patients with Chronic Hepatitis B and Minimal Increases in Levels of Alanine Aminotransferase: a Meta-analysis and Systematic Review. Clinical Gastroenterology and Hepatology. 2014;12:1262-1266. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Sanai FM, Babatin MA, Bzeizi KI, Alsohaibani F, Al-Hamoudi W, Alsaad KO, Al Mana H, Handoo FA, Al-Ashgar H, Alghamdi H. Accuracy of international guidelines for identifying significant fibrosis in hepatitis B e antigen--negative patients with chronic hepatitis. Clin Gastroenterol Hepatol. 2013;11:1493-1499.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1009] [Article Influence: 59.4] [Reference Citation Analysis (1)] |
| 7. | Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol. 2003;18:246-252. [PubMed] |
| 8. | Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012;57:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 363] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828-1853. [PubMed] |
| 11. | Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 441] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 12. | Buti M, Rodriguez-Frias F, Jardi R, Esteban R. Hepatitis B virus genome variability and disease progression: the impact of pre-core mutants and HBV genotypes. J Clin Virol. 2005;34 Suppl 1:S79-S82. [PubMed] |
| 13. | Chen YM, Wu SH, Qiu CN, Yu DJ, Wang XJ. Hepatitis B virus subgenotype C2- and B2-associated mutation patterns may be responsible for liver cirrhosis and hepatocellular carcinoma, respectively. Braz J Med Biol Res. 2013;46:614-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Sonneveld MJ, Rijckborst V, Zeuzem S, Heathcote EJ, Simon K, Senturk H, Pas SD, Hansen BE, Janssen HL. Presence of precore and core promoter mutants limits the probability of response to peginterferon in hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2012;56:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Tacke F, Gehrke C, Luedde T, Heim A, Manns MP, Trautwein C. Basal core promoter and precore mutations in the hepatitis B virus genome enhance replication efficacy of Lamivudine-resistant mutants. J Virol. 2004;78:8524-8535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Kim DW, Lee SA, Hwang ES, Kook YH, Kim BJ. Naturally occurring precore/core region mutations of hepatitis B virus genotype C related to hepatocellular carcinoma. PLoS One. 2012;7:e47372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Lampertico P, Del Ninno E, Viganò M, Romeo R, Donato MF, Sablon E, Morabito A, Colombo M. Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology. 2003;37:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Lin CS, Chang CS, Yang SS, Yeh HZ, Lin CW. Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C patients with hepatocellular carcinoma. Intern Med. 2008;47:569-575. [PubMed] |
| 19. | Tseng PL, Wang JH, Hung CH, Tung HD, Chen TM, Huang WS, Liu SL, Hu TH, Lee CM, Lu SN. Comparisons of noninvasive indices based on daily practice parameters for predicting liver cirrhosis in chronic hepatitis B and hepatitis C patients in hospital and community populations. Kaohsiung J Med Sci. 2013;29:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Wong GL, Wong VW, Choi PC, Chan AW, Chan HL. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2010;31:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH, Kim SU. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Mena Á, Pedreira JD, Castro Á, López S, Vázquez P, Poveda E. Metabolic syndrome association with fibrosis development in chronic hepatitis B virus inactive carriers. J Gastroenterol Hepatol. 2014;29:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 24. | Wong GL, Chan HL, Yu Z, Chan HY, Tse CH, Wong VW. Liver fibrosis progression in chronic hepatitis B patients positive for hepatitis B e antigen: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol. 2013;28:1762-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Wong GL, Chan HL, Yu Z, Chan HY, Tse CH, Wong VW. Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol. 2013;28:1842-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, Lau K, Yuen JC, Lai CL. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 27. | Fattovich G, Giustina G, Sanchez-Tapias J, Quero C, Mas A, Olivotto PG, Solinas A, Almasio P, Hadziyannis S, Degos F. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alpha therapy and disease prognosis. European Concerted Action on Viral Hepatitis (EUROHEP). Am J Gastroenterol. 1998;93:896-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Wong DK, Yuen MF, Poon RT, Yuen JC, Fung J, Lai CL. Quantification of hepatitis B virus covalently closed circular DNA in patients with hepatocellular carcinoma. J Hepatol. 2006;45:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Manesis EK, Papatheodoridis GV, Tiniakos DG, Hadziyannis ES, Agelopoulou OP, Syminelaki T, Papaioannou C, Nastos T, Karayiannis P. Hepatitis B surface antigen: relation to hepatitis B replication parameters in HBeAg-negative chronic hepatitis B. J Hepatol. 2011;55:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Moucari R, Korevaar A, Lada O, Martinot-Peignoux M, Boyer N, Mackiewicz V, Dauvergne A, Cardoso AC, Asselah T, Nicolas-Chanoine MH. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J Hepatol. 2009;50:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Chan HL, Wong GL, Chim AM, Chan HY, Chu SH, Wong VW. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther. 2011;16:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 33. | Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 34. | Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, Romagnoli V, Cherubini B, Moscato G, Maina AM. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 35. | Park H, Lee JM, Seo JH, Kim HS, Ahn SH, Kim do Y, Han KH, Chon CY, Park JY. Predictive value of HBsAg quantification for determining the clinical course of genotype C HBeAg-negative carriers. Liver Int. 2012;32:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Martinot-Peignoux M, Lapalus M, Laouénan C, Lada O, Netto-Cardoso AC, Boyer N, Ripault MP, Carvalho-Filho R, Asselah T, Marcellin P. Prediction of disease reactivation in asymptomatic hepatitis B e antigen-negative chronic hepatitis B patients using baseline serum measurements of HBsAg and HBV-DNA. J Clin Virol. 2013;58:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Martinot-Peignoux M, Lapalus M, Asselah T, Marcellin P. HBsAg quantification: useful for monitoring natural history and treatment outcome. Liver Int. 2014;34 Suppl 1:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Yu ML, Lee CM, Chuang WL, Lu SN, Dai CY, Huang JF, Lin ZY, Hu TH, Chen CH, Hung CH. HBsAg profiles in patients receiving peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. J Infect Dis. 2010;202:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 40. | Chan HL, Wong VW, Wong GL, Tse CH, Chan HY, Sung JJ. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology. 2010;52:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 41. | Tseng TC, Liu CJ, Su TH, Wang CC, Chen CL, Chen PJ, Chen DS, Kao JH. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology. 2011;141:517-25, 525.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Chan HL, Wong GL, Tse CH, Chan HY, Wong VW. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis. 2011;204:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology. 2012;55:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Chen YC, Jeng WJ, Chu CM, Liaw YF. Decreasing levels of HBsAg predict HBsAg seroclearance in patients with inactive chronic hepatitis B virus infection. Clin Gastroenterol Hepatol. 2012;10:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1749] [Article Influence: 79.5] [Reference Citation Analysis (1)] |
| 46. | Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, Tzourmakliotis D, Manesis E, Hadziyannis SJ. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Lee HW, Lee HJ, Hwang JS, Sohn JH, Jang JY, Han KJ, Park JY, Kim do Y, Ahn SH, Paik YH. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010;51:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 403] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 49. | Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, Gane E, Locarnini S, Lim S-G, Han KH. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561. [RCA] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 50. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2411] [Article Influence: 172.2] [Reference Citation Analysis (1)] |
| 51. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2176] [Article Influence: 128.0] [Reference Citation Analysis (2)] |
| 52. | Park BK, Park YN, Ahn SH, Lee KS, Chon CY, Moon YM, Park C, Han KH. Long-term outcome of chronic hepatitis B based on histological grade and stage. J Gastroenterol Hepatol. 2007;22:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology. 2009;49:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Park JY, Park YN, Kim DY, Paik YH, Lee KS, Moon BS, Han KH, Chon CY, Ahn SH. High prevalence of significant histology in asymptomatic chronic hepatitis B patients with genotype C and high serum HBV DNA levels. J Viral Hepat. 2008;15:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Evaluation of alanine transaminase and hepatitis B virus DNA to predict liver cirrhosis in hepatitis B e antigen-negative chronic hepatitis B using transient elastography. Am J Gastroenterol. 2008;103:3071-3081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Fung J, Lai CL, But D, Wong D, Cheung TK, Yuen MF. Prevalence of fibrosis and cirrhosis in chronic hepatitis B: implications for treatment and management. Am J Gastroenterol. 2008;103:1421-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 436] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 58. | Tsang PS, Trinh H, Garcia RT, Phan JT, Ha NB, Nguyen H, Nguyen K, Keeffe EB, Nguyen MH. Significant prevalence of histologic disease in patients with chronic hepatitis B and mildly elevated serum alanine aminotransferase levels. Clin Gastroenterol Hepatol. 2008;6:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, Xie R, Ge S, Hu J, Jiang Z. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment Pharmacol Ther. 2011;34:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Bonino F, Marcellin P, Lau GK, Hadziyannis S, Jin R, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut. 2007;56:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 61. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 862] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 62. | Papadopoulos VP, Chrysagis DN, Protopapas AN, Goulis IG, Dimitriadis GT, Mimidis KP. Peginterferon alfa-2b as monotherapy or in combination with lamivudine in patients with HBeAg-negative chronic hepatitis B: a randomised study. Med Sci Monit. 2009;15:CR56-CR61. [PubMed] |
| 63. | Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-2179.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 64. | Piccolo P, Lenci I, di Paolo D, Demelia L, Sorbello O, Nosotti L, Angelico M. A randomized controlled trial of sequential pegylated interferon-α and telbivudine or vice versa for 48 weeks in hepatitis B e antigen-negative chronic hepatitis B. Antivir Ther. 2013;18:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 912] [Article Influence: 45.6] [Reference Citation Analysis (1)] |
| 66. | Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 918] [Article Influence: 51.0] [Reference Citation Analysis (1)] |
| 67. | Marcellin P, Buti M, Gane EJ, Krastev Z, Flisiak R, Germanidis G, Washington MK, Barnes CN, Flaherty JF, Bornstein JD. Five years of treatment with tenofovir DF (TDF) for chronic hepatitis B (CHB) infection is associated with sustained viral suppression and significant regression of histological fibrosis and cirrhosis. Hepatology. 2011;54:1011A-1012A. |
| 68. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1410] [Article Influence: 108.5] [Reference Citation Analysis (1)] |
| 69. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 788] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 70. | Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, Hawley S, Barber J, Condreay L, Gray DF. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology. 1999;29:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 353] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 71. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 409] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 72. | Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, Han S, Kaymakoglu S, Tamez R, Yang J. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 602] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 74. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 735] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 75. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 681] [Article Influence: 34.1] [Reference Citation Analysis (2)] |
| 76. | Hadziyannis SJ, Papatheodoridis GV, Dimou E, Laras A, Papaioannou C. Efficacy of long-term lamivudine monotherapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2000;32:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 77. | Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 78. | Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 79. | Lee JH, Cho Y, Lee DH, Lee M, Yoo JJ, Choi WM, Cho YY, Lee YB, Yu SJ, Yoon JH. Prior exposure to lamivudine increases entecavir resistance risk in chronic hepatitis B Patients without detectable lamivudine resistance. Antimicrob Agents Chemother. 2014;58:1730-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Kumar M, Sarin SK. Systematic review: combination therapies for treatment-naïve chronic hepatitis B. Aliment Pharmacol Ther. 2008;27:1187-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, Liaw YF. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology. 2013;58:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 82. | Chan WK, Tan SS, Mohamed R. Stopping Therapy in HBeAg Negative Disease. Current Hepatitis Reports. 2013;12:pp 105-111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 83. | Rijckborst V, Hansen BE, Ferenci P, Brunetto MR, Tabak F, Cakaloglu Y, Lanza AG, Messina V, Iannacone C, Massetto B. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol. 2012;56:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 84. | Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, Vasiliadis T, Mimidis K, Gogos C, Ketikoglou I. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
P- Reviewer: Grasso A, Striker RT S- Editor: Ma YJ L- Editor: A E- Editor: Ma S