Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11871
Revised: March 27, 2014
Accepted: April 30, 2014
Published online: September 7, 2014
Processing time: 223 Days and 15.7 Hours
AIM: To investigate the relationship between low immediate postoperative platelet count and perioperative outcome after liver resection in patients with hepatocellular carcinoma (HCC).
METHODS: In a cohort of 565 consecutive hepatitis B-related HCC patients who underwent major liver resection, the characteristics and clinical outcomes after liver resection were compared between patients with immediate postoperative platelet count < 100 × 109/L and patients with platelet count ≥ 100 × 109/L. Risk factors for postoperative hepatic insufficiency were evaluated by multivariate analysis.
RESULTS: Patients with a low immediate postoperative platelet count (< 100 × 109/L) had more grade III-V complications (20.5% vs 12.4%, P = 0.016), and higher rates of postoperative liver failure (6.8% vs 2.6%, P = 0.02), hepatic insufficiency (31.5% vs 21.2%, P < 0.001) and mortality (6.8% vs 0.5%, P < 0.001), compared to patients with a platelet count ≥ 100 × 109/L. The alanine aminotransferase levels on postoperative days 3 and 5, and bilirubin on postoperative days 1, 3 and 5 were higher in patients with immediate postoperative low platelet count. Multivariate analysis revealed that immediate postoperative low platelet count, rather than preoperative low platelet count, was a significant independent risk factor for hepatic insufficiency.
CONCLUSION: A low immediate postoperative platelet count is an independent risk factor for hepatic insufficiency. Platelets can mediate liver regeneration in the cirrhotic liver.
Core tip: Recent animal experiments suggested that platelets not only have a role in hemostasis and thrombogenesis, but can also improve liver function by mediating liver regeneration. Our study found that patients with a low immediate postoperative platelet count < 100 × 109/L had more grade III-V complications, and higher rates of postoperative liver failure, hepatic insufficiency and mortality. In addition, these patients had worse liver function after liver resection, with higher alanine aminotransferase and bilirubin and lower albumin levels. This indicated that platelets could mediate liver regeneration in cirrhotic liver.
- Citation: Wang HQ, Yang J, Yang JY, Wang WT, Yan LN. Low immediate postoperative platelet count is associated with hepatic insufficiency after hepatectomy. World J Gastroenterol 2014; 20(33): 11871-11877
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11871.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11871
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common global cause of cancer-related deaths[1]. Liver resection is performed as first-line treatment in almost all HCC patients[2]. With the refinement of surgical techniques and perioperative management in liver surgery during the last few decades, outcomes after liver resection have improved substantially in recent years, with the operative mortality less than 5% in high-volume centers[3]. However, liver failure or hepatic insufficiency, which usually results in severe outcomes, is more common after liver resection, with an incidence ranging from 1.2% to 32%[4,5]. In Asia, about 80% of HCC cases occur in patients with cirrhosis derived from hepatitis B virus (HBV) infection[1]. Concomitant portal hypertension and thrombocytopenia usually increase the risk of postoperative liver failure and hepatic insufficiency[6,7]. Recent studies on animals demonstrated that platelets had a role not only in blood coagulation[8], but also in liver regeneration[9-12], tissue repair[13] and ischemia/reperfusion injury[14]. Several clinical studies[15-18] have also indicated that preoperative thrombocytopenia is a risk factor associated with postoperative complications and mortality. In addition, immediate postoperative low platelet count has recently been proved to be associated with delayed liver function recovery after partial liver resection for colorectal liver metastases, which indicated that platelets play a critical role in liver regeneration after liver resection[19]. However, the role of immediate postoperative low platelet count in underlying liver damage derived from HBV infection has not been investigated. Here, we report a cohort study to determine the relationship between immediate postoperative platelet count and outcome after partial liver resection in patients with HBV-related HCC.
Between January 2009 and March 2013, 574 consecutive HBV-related HCC patients who underwent major liver resection were included in this study. All included patients were diagnosed with HCC by histology and with HBV infection (or a history of HBV infection). All patients underwent surgery when Child-Turcotte-Pugh (CTP) class was A. Medical records containing patient demographics, comorbid conditions, laboratory values, intraoperative parameters and postoperative outcomes were obtained from the West China Hospital of Liver Cancer Registry Database. The protocol was approved by the West China Hospital Ethics Committee and written informed consent was obtained from all patients before inclusion. We excluded 9 patients due to synchronous biliary obstruction, splenectomy or platelet transfusion. This resulted in a total of 565 patients included in the study. All patients had major liver resection which was defined as the resection of more than three liver segments. The platelet count was obtained immediately after surgery (usually upon arrival at the intensive care unit or liver department after surgery, and referred to as the immediate postoperative platelet count), and the patients were stratified into the Low Platelet Group (platelet count < 100 × 109/L) and High Platelet Group (platelet count ≥ 100 × 109/L). The aim of this study was to evaluate whether immediate postoperative platelet count was associated with hepatic insufficiency and mortality.
All patients were managed by the same surgical team. The patients underwent a thorough history, physical examination and routine preoperative laboratory measurements. Routine preoperative imaging examination to evaluate the tumor included contrast computed tomography or magnetic resonance imaging of the abdomen. Echocardiography, chest radiography or computed tomography and pulmonary function tests were carried out if necessary. Patients were operated on under general anesthesia and were explored through an extended right subcostal incision, and intraoperative ultrasonography was used routinely. Hemihepatic vascular inflow occlusion[20] or the Pringle maneuver[21] was used according to the surgeon’s preference in most patients. Liver parenchymal transection was performed using the Hooking with ligation method[20] or an ultrasonic dissector with coagulator. Based on preoperative and intraoperative condition, patients were transferred to the intensive care unit for treatment if necessary.
The 50-50 criteria[22] defined as prothrombin time < 50% and serum bilirubin level > 50 μmol/L on day 5 after liver resection, were used to define liver failure. Based on the 50-50 criteria, hepatic insufficiency[19] was defined as bilirubin > 50 μmol/L or prothrombin time < 50% at any time point between postoperative day 1 and postoperative day 5. Hepatic insufficiency was used as a surrogate marker for poor liver regeneration[19]. The liver volume removed was calculated as follows: segment I: 2%, segment II: 8%, segment III: 8%, segment IV: 17%, segment V: 17.5%, segment VI: 15%, segment VII: 15%, segment VIII: 17.5%. Immediate postoperative platelet count indicated the platelet count obtained on the day of surgery. Mortality was defined as death within 30 d after surgery or death before discharge involving a hospital stay of more than 30 d. The Clavien-Dindo complication classification system[23] was used for postoperative complication grading, and grade III-V complications were defined as severe complications. Extrahepatic procedures included all other operations, except liver resection, such as bowel resection, adrenalectomy, diaphragm resection and adhesion separation due to reoperation.
All statistical analyses were performed using SPSS v17 software (SPSS Inc., Chicago, IL, United States), and statistical significance was set at P < 0.05. Continuous variables were reported as mean (SD) or median (range), and were compared using the Student t test for continuous variables with parametric distribution, and the Mann-Whitney U test or Kruskal-Wallis H test for those with nonparametric distribution. Categorical variables were reported as numbers and percentages, and compared using Pearson’s χ2 analysis or Fisher’s exact test. To identify risk factors for hepatic insufficiency, only significant factors associated with hepatic insufficiency in the univariate analysis were entered into the forward stepwise logistic regression analysis.
In our cohort, 146 (25.8%) patients had immediate low postoperative platelet count (platelet count < 100 × 109/L) (Table 1), of whom 79 (54.1%) had low preoperative platelet count. A total of 419 patients had postoperative platelet count > 100 × 109/L immediately after surgery. All these patients were in CTP class A when surgery was performed. Patients in the Low Platelet Group had a significantly increased rate of preoperative thrombocytopenia (P < 0.001) and diabetes mellitus (P = 0.001). The two groups had similar operative procedures (Table 2), except that the Low Platelet Group required more packed red blood cell transfusion (P = 0.001) because of greater blood loss (P = 0.036). There were no significant differences between the groups regarding the other analyzed parameters (Tables 1 and 2).
| Clinical characteristics | Low platelet group(n = 146) | High platelet group(n = 419) | P value |
| Male | 131 (89.7) | 353 (84.2) | 0.104 |
| Age > 65 yr | 23 (15.8) | 51 (12.2) | 0.269 |
| Preoperative platelet < 100 × 109/L | 79 (54.1) | 28 (6.7) | < 0.001 |
| ASA grade | 0.058 | ||
| I-II | 117 (80.1) | 369 (88.1) | |
| III | 24 (16.4) | 42 (10.0) | |
| IV | 5 (3.4) | 8 (1.9) | |
| BMI (kg/m2), mean (SD) | 22.93 (2.80) | 22.90 (2.83) | 0.941 |
| Esophageal varices | 22 (15.1) | 47 (11.2) | 0.221 |
| Hypertension | 30 (20.5) | 66 (15.8) | 0.184 |
| Cardiovascular disease | 3 (2.1) | 12 (2.9) | 0.822 |
| Pulmonary disease | 3 (2.1) | 9 (2.1) | 1.000 |
| Diabetes mellitus | 22 (15.1) | 26 (76.2) | 0.001 |
| HBsAg | 124 (84.9) | 330 (78.8) | 0.106 |
| HBeAg | 22 (15.1) | 58 (13.8) | 0.714 |
| HBV DNA > 2000 U/mL | 51 (34.9) | 135 (32.2) | 0.548 |
| AST (U/L) > ULN | 68 (46.6) | 194 (46.3) | 0.954 |
| ALT (U/L) > ULN | 49 (33.6) | 133 (31.7) | 0.685 |
| Total bilirubin (μmol/L), median (IQR) | 14.5 (10.3-18.6) | 12.9 (9.9-18.0) | 0.145 |
| Albumin (g/L), median (IQR) | 40.9 (43.1-37.6) | 40.5 (37.6-43.2) | 0.538 |
| Hemoglobin (g/L), median (IQR) | 141 (129-150) | 140 (127-152) | 0.913 |
| Intraoperative parameters | Low platelet group(n = 146) | High platelet group(n = 419) | P value |
| Liver volume removed | 0.140 | ||
| < 35% | 61 (41.8) | 215 (51.3) | |
| 35%-65% | 38 (26.0) | 91 (21.7) | |
| > 65% | 47 (32.2) | 113 (27.0) | |
| Extrahepatic procedures | 33 (22.6) | 90 (21.5) | 0.777 |
| Liver resection with | 0.883 | ||
| Hooking with ligation | 35 (24.0) | 103 (24.6) | |
| Ultrasonic dissection | 111 (76.0) | 316 (75.4) | |
| Inflow occlusion | 60 (41.4 ) | 191 (45.6) | 0.347 |
| Anatomic resection | 94 (64.4) | 293 (69.9) | 0.214 |
| Blood loss (mL), mean (SD) | 765 (1011) | 583 (436) | 0.036 |
| PRBCs transfusion | 58 (39.7) | 105 (25.1) | 0.001 |
| Total complication | 63 (43.2) | 150 (35.8) | 0.114 |
| III-V grade complication | 30 (20.5) | 52 (12.4) | 0.016 |
| Mortality | 10 (6.8) | 2 (0.5) | < 0.001 |
| Liver failure | 10 (6.8) | 11 (2.6) | 0.020 |
| Hepatic insufficiency | 46 (31.5) | 89 (21.2) | 0.012 |
| ICU stay (d), median (IQR) | 0 (0-1) | 0 (0-1) | 0.129 |
| Hospital stay (d), median (IQR) | 14 (12-18) | 13 (11-16) | 0.011 |
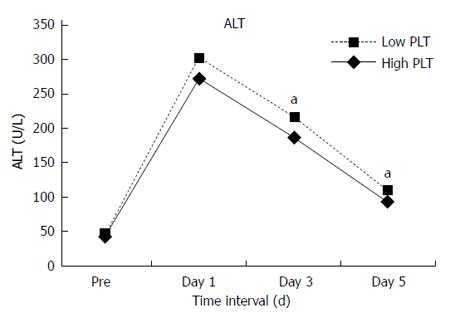
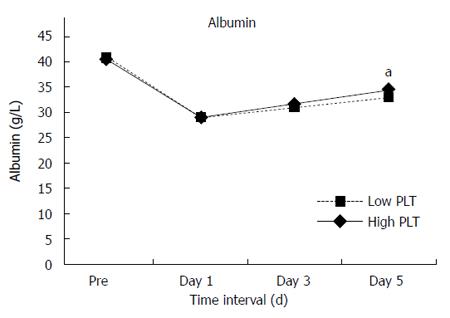
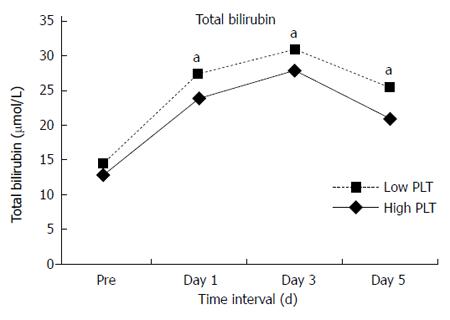
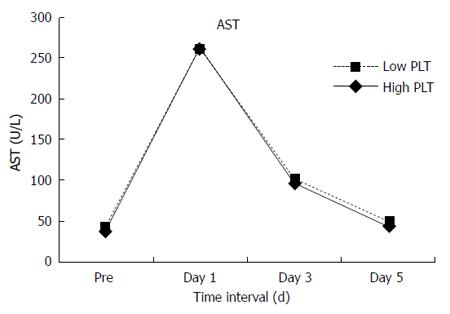
No significant differences were found in total complications between the two groups. However, compared with the High Platelet Group, the Low Platelet Group had more grade III-V complications (20.5% vs 12.4%, P = 0.016) and longer hospital stay (14 d vs 13 d, P = 0.011). In the whole study group of 565 patients, 21 (3.7%) patients developed irreversible postoperative liver failure and 135 (23.9%) had hepatic insufficiency. Postoperative liver failure (6.8% vs 2.6%, P = 0.02) and hepatic insufficiency (31.5% vs 21.2%, P < 0.001) occurred more frequently in the Low Platelet Group. Postoperative mortality within 30 d was 2.1% (12 patients). Mortality in the Low Platelet Group was 6.8%, which was almost 14 times higher than that in the High Platelet Group with a mortality rate of 0.5% (P < 0.001). In addition, more patients in the Low Platelet Group had delayed recovery of liver function. Statistically significant differences were noted in alanine aminotransferase (ALT) levels on postoperative day 3 and day 5 (Figure 1), albumin level on postoperative day 5 (Figure 2) and total bilirubin on postoperative day 1, day 3 and day 5 (Figures 3 and 4). No significant differences were found in the other perioperative parameters.
In order to identify the risk factors for postoperative hepatic insufficiency, a univariate analysis of patients with and without hepatic insufficiency was carried out. Univariate analysis (Table 3) showed that 11 variables were significantly associated with the occurrence of hepatic insufficiency. Both low preoperative platelet count and low postoperative platelet count were significant risk factors. The other 9 variables were male sex, esophageal varices, HBsAg, aspartate aminotransferase, ALT, total bilirubin, hemoglobin concentration, liver volume removed, and blood loss. These significantly different variables were included in a multivariate logistic regression model to identify whether low postoperative platelet count was an independent risk factor for hepatic insufficiency. The logistic regression analysis (Table 4) indicated that male sex, low postoperative platelet count, esophageal varices, total bilirubin, and liver volume removed were independent risk factors for hepatic insufficiency. Low platelet count remained a strong and independent risk factor for hepatic insufficiency, rather than low preoperative platelet count.
| Parameters | Liver dysfunction (n = 135) | No Liver dysfunction (n = 430) | OR (95%CI) | P value |
| Male | 127 (94.1) | 357 (83.0) | 2.87 (1.42-5.79) | 0.001 |
| Age > 65 yr | 12 (8.9) | 62 (14.4) | 0.58 (0.30-1.11) | 0.097 |
| Preoperative platelet < 100 × 109/L | 37 (27.4) | 70 (16.3) | 1.94 (1.23-3.07) | 0.004 |
| Postoperative platelet < 100 × 109/L | 46 (34.1) | 100 (23.3) | 1.71 (1.12-2.60) | 0.012 |
| BMI (kg/m2), mean (SD) | 22.9 (21.2-25.0) | 22.5 (20.9-24.5) | Not available | 0.147 |
| Esophageal varices | 36 (26.7) | 33 (7.7) | 4.38 (2.60-7.37) | 0.001 |
| Hypertension | 22 (16.3) | 74 (17.2) | 0.94 (0.56-1.58) | 0.805 |
| Cardiovascular disease | 4 (3.0) | 11 (2.6) | 1.16 (0.36-3.71) | 0.799 |
| Pulmonary disease | 2 (1.5) | 10 (2.3) | 0.63 (0.14-2.92) | 0.802 |
| Diabetes mellitus | 10 (7.4) | 38 (8.8) | 0.83 (0.4-1.70) | 0.603 |
| ASA III-IV | 17 (12.6) | 62 (14.4) | 0.86 (0.48-1.52) | 0.594 |
| HBsAg | 119 (88.1) | 335 (77.9) | 2.11 (1.19-3.73) | 0.009 |
| HBeAg | 24 (17.8) | 56 (13.0) | 1.44 (0.86-2.44) | 0.167 |
| HBV DNA > 2000 U/mL | 47 (34.8) | 139 (32.3) | 1.12 (0.74-1.68) | 0.591 |
| AST (U/L) > ULN | 75 (55.6) | 187 (43.5) | 1.62 (1.1-2.40) | 0.014 |
| ALT (U/L) > ULN | 55 (40.7) | 127 (29.5) | 1.64 (1.10-2.50) | 0.015 |
| Total bilirubin (μmol/L), median (IQR) | 16.1 (11.4-20.5) | 12.5 (9.6-17.4) | Not available | < 0.001 |
| Hemoglobin (g/L), median (IQR) | 146 (131-157) | 139 (127-150) | Not available | 0.001 |
| Liver volume removed | < 0.001 | |||
| < 35% | 15 (11.1) | 145 (33.7) | 1 (reference) | |
| 35%-65% | 32 (23.7) | 97 (22.6) | 3.19 (1.64-6.20) | |
| > 65% | 88 (65.2) | 188 (43.7) | 4.53 (2.51-8.15) | |
| Extrahepatic procedures | 37 (27.4) | 86 (20.0) | 1.51 (0.97-2.36) | 0.069 |
| Liver resection with | 0.248 | |||
| Hooking with ligation | 38 (28.1) | 100 (23.3) | 1.29 (0.84-2.0) | |
| Ultrasonic dissection | 97 (71.9) | 330 (76.7) | 0.77 (0.5-1.20) | |
| Inflow occlusion | 54 (40.0) | 197 (45.8) | 1.27 (0.86-1.88) | 0.236 |
| Anatomic resection | 98 (72.6) | 289 (67.2) | 1.29 (0.84-1.98) | 0.240 |
| Blood loss (mL), mean (SD) | 770 (1049) | 585 (433.0) | Not available | 0.003 |
| PRBCs transfusion | 47 (34.8) | 116 (27.0) | 1.45 (0.96-2.19) | 0.080 |
| Hospital stay (d), median (IQR) | 14 (11-19) | 13 (11-16) | Not available | 0.001 |
| Variables | Regression coefficient (β) | OR (95%CI) | P value |
| Male | -0.993 | 0.393 (0.179-0.864) | 0.020 |
| Postoperative platelet | 0.597 | 1.816 (1.138-2.899) | 0.012 |
| < 100 × 109/L | |||
| Esophageal varices | 1.446 | 4.244 (2.405-7.491) | < 0.001 |
| Total bilirubin | 0.072 | 1.075 (1.042-1.108) | < 0.001 |
| Liver volume removed (%) | 1 (Ref) | ||
| 35%-65% | 1.185 | 3.271 (1.601-6.683) | 0.001 |
| > 65% | 1.704 | 5.498 (2.908-10.394) | < 0.001 |
The perioperative outcomes after liver resection such as liver failure, hemorrhage and mortality are major concerns in hepatobiliary surgery, especially in HCC patients who usually have underlying liver diseases due to HBV infection. Many preoperative and intraoperative parameters affecting morbidity and mortality after hepatectomy have been evaluated, however, the effect of low postoperative platelet count on postoperative morbidity and mortality is not well known. We carried out a cohort study of 565 HCC patients undergoing major liver resection to assess whether immediate low postoperative platelet count affected postoperative outcomes. In our study, a low immediate postoperative platelet count was associated with liver failure, hepatic insufficiency and mortality in univariate and multivariate analyses. This demonstrated that low platelet count is an independent predictor of postoperative hepatic insufficiency.
To date, several studies have evaluated the role of platelets on morbidity and mortality. In addition, their short-term importance and positive significance have been confirmed. Three large high-volume studies[16-18] indicated that preoperative thrombocytopenia was associated with increased mortality or morbidity. Ishizawa et al[15] found that low platelet count was an independent risk factor for postoperative ascites. A study by Maithel et al[6] suggested that low preoperative platelet count may better serve with liver resection as a modified Child score which incorporates preoperative platelet count as a substitute for encephalopathy. However, with regard to the long-term effects of low platelet count, only one study has been carried out which suggested that the 3-year cumulative survival rate of HCC patients was comparable to those without thrombocytopenia[24]. Many studies have proved that a low preoperative platelet count was related to portal hypertension and its resulting hypersplenism and hepatic fibrosis[2,25]. Portal hypertension is considered a contraindication for liver resection because it significantly impairs liver function[2,7]. This is the reason why preoperative thrombocytopenia is associated with higher mortality and morbidity.
However, to our knowledge, few reports have been published on the effect of postoperative platelet count. Lesurtel[26] conducted a study in liver transplant patients with thrombocytopenia and found that a platelet count < 60 × 109/L on postoperative day 5 was an independent risk factor associated with severe complications and poor early graft and patient survival. Alkozai et al[19] reported a series of 216 patients with liver resection and demonstrated that a low immediate postoperative platelet count was an independent predictor of delayed postoperative liver function recovery and was associated with an increased risk of postoperative mortality. The study only included liver resection for colorectal liver metastases with normal liver parenchyma, however, the effect of low postoperative platelet count in HCC patients with underlying liver disease has not been studied.
The underlying mechanisms involved in the postoperative platelet count affecting postoperative liver function are not well understood. However, accumulating evidence from experimental and clinical studies indicated that platelets do not only have a role in hemostasis and thrombogenesis, but can also improve liver function by mediating liver regeneration[9]. Recent animal experiments suggested that platelets, or rather platelet-derived serotonin, contribute to cell cycle progression and metabolic pathways to prevent acute liver failure[9,11-13]. Other studies also proved that thrombopoietin[11] or platelets infused via the portal vein[10] can stimulate regeneration after hepatectomy in rats. This phenomenon in animal experiments was also confirmed in clinical practice. A retrospective study showed that transfused platelets were significantly associated with graft regeneration in liver donors[27].
The role of platelets in the promotion of liver regeneration has been clinically confirmed in patients undergoing liver resection for colorectal liver metastases[19]. In Asia, in contrast to the liver with metastatic tumors, the liver with HCC usually has cirrhosis or fibrosis and secondary hypersplenism due to HBV infection. There is not only the well-known feature of thrombocytopenia, but also decreased platelet function in chronic HBV liver diseases and cirrhosis[8]. It is not known whether postoperative thrombocytopenia also has an effect on liver function recovery after liver resection for HBV-related HCC. Therefore, in the present study, we selected low immediate postoperative platelet count instead of preoperative platelet count as the criterion for thrombocytopenia, as platelet count usually changes due to intraoperative blood loss or platelet transfusion. In addition, preoperative platelet count appeared to be a surrogate for the preoperative severity of a patient’s liver disease[26]. In our study, low immediate postoperative platelet count was associated with a greater likelihood of liver failure, hepatic insufficiency and mortality. Compared with patients with a high platelet count, the mortality rate was almost 14 times higher in patients with a low postoperative platelet count, and the rate of liver failure was 2.6 times higher. As there were a few cases involved, independent risk factors for liver failure and mortality were not analyzed by multivariate analysis. For hepatic insufficiency, multivariate analysis showed that liver volume removed was the strongest independent risk factor, followed by esophageal varices and platelet count. This differed from a previous study which found that low immediate postoperative platelet count was the strongest independent risk factor. A possible reasons for this difference was that patients in our study also had esophageal varices and underwent major liver resection. Portal hypertension (represented by esophageal varices) and major liver resection mainly contribute to hepatic insufficiency[5].
Our findings are instructive for surgeons to ensure that they take positive measures to increase platelet count to prevent hepatic insufficiency. These treatments should include platelet transfusion and administration of thrombopoietin and serotonin. One study[11] showed that administration of thrombopoietin reduces liver fibrosis and stimulates regeneration in the cirrhotic liver. This is suitable for HCC patients as most have different degrees of liver fibrosis which has a strong impact on morbidity. However, further research is needed before this treatment can be considered for use in the clinic.
Our study has several limitations mainly due to the retrospective analysis. It is important to point out that some of the patients in our study were highly selected for surgical safety. Secondly, the immediate postoperative platelet count may not be exact as it may have been affected by hemodilution or hemoconcentration after surgery. In addition, increased consumption of circulating platelets occurred due to subsequent bleeding and hemostasis after surgery. This was different in each patient and was not considered in our study.
In conclusion, our study found that a low immediate postoperative platelet count was associated with postoperative hepatic insufficiency, liver failure and mortality. A low immediate postoperative platelet count is an independent risk factor for hepatic insufficiency. These findings indicated that platelets can mediate liver regeneration in the cirrhotic liver.
Hepatocellular carcinoma (HCC) is common and is one of the most common causes of cancer-related deaths in the world. Liver resection is performed as first-line treatment in patients with HCC. However, postoperative liver failure and hepatic insufficiency are common after liver resection, with the incidence rate ranging from 1.2% to 32%, which usually results in severe outcomes.
Recent animal experiments suggested that platelets not only have a role in hemostasis and thrombogenesis, but can also improve liver function by mediating liver regeneration. Liver regeneration after liver resection can supply enough liver cells and increase the remnant liver volume to avoid liver failure and hepatic insufficiency. Several clinical studies have also indicated that a low preoperative platelet count is a risk factor associated with postoperative complications and mortality. In addition, a low immediate postoperative platelet count has been recently proved to be associated with delayed liver function recovery after partial liver resection for colorectal liver metastases.
This study found that patients with a low immediate postoperative platelet count < 100 × 109/L had more complications, and higher rates of postoperative liver failure, hepatic insufficiency and mortality. In addition, these patients had worse liver function after liver resection, with higher alanine aminotransferase, bilirubin and lower albumin levels.
A low immediate postoperative platelet count was an independent risk factor for hepatic insufficiency. These findings indicated that platelets can mediate liver regeneration in the cirrhotic liver.
Platelets are non-nucleated discoid particles which are essential for hemostasis, liver regeneration and thrombosis. Low platelet count, also known as thrombocytopenia, is usually defined as platelets < 100 × 109/L, and is associated with poor hemostasis and liver regeneration.
This paper brings the authors very interesting information about postoperative low platelet count associated with hepatic insufficiency after hepatectomy for hepatocellular carcinoma. The article is well redacted and its conclusions are very interesting for the international literature.
| 1. | Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 2. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4520] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 3. | Ramacciato G, D’Angelo F, Baldini R, Petrucciani N, Antolino L, Aurello P, Nigri G, Bellagamba R, Pezzoli F, Balesh A. Hepatocellular carcinomas and primary liver tumors as predictive factors for postoperative mortality after liver resection: a meta-analysis of more than 35,000 hepatic resections. Am Surg. 2012;78:456-467. [PubMed] |
| 4. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1821] [Article Influence: 121.4] [Reference Citation Analysis (1)] |
| 5. | Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC, McMasters KM, Cho CS, Winslow ER, Wood WC. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212:638-648; discussion 648-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Witters P, Freson K, Verslype C, Peerlinck K, Hoylaerts M, Nevens F, Van Geet C, Cassiman D. Review article: blood platelet number and function in chronic liver disease and cirrhosis. Aliment Pharmacol Ther. 2008;27:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-107. [PubMed] |
| 10. | Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg. 2008;248:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Parker RI. Etiology and significance of thrombocytopenia in critically ill patients. Crit Care Clin. 2012;28:399-411, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Ishizawa T, Hasegawa K, Kokudo N, Sano K, Imamura H, Beck Y, Sugawara Y, Makuuchi M. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 17. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698-708; discussion 708-10. [PubMed] |
| 18. | Taketomi A, Kitagawa D, Itoh S, Harimoto N, Yamashita Y, Gion T, Shirabe K, Shimada M, Maehara Y. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute’s experience with 625 patients. J Am Coll Surg. 2007;204:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Alkozai EM, Nijsten MW, de Jong KP, de Boer MT, Peeters PM, Slooff MJ, Porte RJ, Lisman T. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (3)] |
| 20. | Wen T, Chen Z, Yan L, Li B, Zeng Y, Wu G, Zheng G. Continuous normothermic hemihepatic vascular inflow occlusion over 60 min for hepatectomy in patients with cirrhosis caused by hepatitis B virus. Hepatol Res. 2007;37:346-352. [PubMed] |
| 21. | Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541-549. [PubMed] |
| 22. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-829. [PubMed] |
| 23. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 24. | Sugimachi K, Ikeda Y, Tomikawa M, Taketomi A, Tsukamoto S, Kawasaki K, Yamamura S, Korenaga D, Maehara Y, Takenaka K. Appraisal of hepatic resection in the treatment of hepatocellular carcinoma with severe thrombocytopenia. World J Surg. 2008;32:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 25. | Kaneko K, Shirai Y, Wakai T, Yokoyama N, Akazawa K, Hatakeyama K. Low preoperative platelet counts predict a high mortality after partial hepatectomy in patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:5888-5892. [PubMed] |
| 26. | Lesurtel M, Raptis DA, Melloul E, Schlegel A, Oberkofler C, El-Badry AM, Weber A, Mueller N, Dutkowski P, Clavien PA. Low platelet counts after liver transplantation predict early posttransplant survival: the 60-5 criterion. Liver Transpl. 2014;20:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010;34:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
P- Reviewer: Tisone G S- Editor: Qi Y L- Editor: Cant MR E- Editor: Ma S