Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11305
Revised: July 18, 2014
Accepted: July 29, 2014
Published online: August 28, 2014
Processing time: 82 Days and 1.3 Hours
AIM: To evaluate the role of the 13C-methacetin breath test (13C-MBT) in the assessment of acute liver injury in a rat model.
METHODS: Acute liver injury in rats was induced by a single intraperitoneal injection of D-galactosamine (D-GalN). Forty-eight male Sprague-Dawley rats were randomly assigned to a control group (n = 8) and five model groups (each n = 8), and acute liver injury was assessed at different time points (6, 12, 24, 48 and 72 h) after D-GalN injection. The 13C-MBT, biochemical tests, 15-min retention rate of indocyanine green (ICGR15), and liver biopsy were performed and compared between the control and model groups. Correlations between parameters of the 13C-MBT (Tmax, MVmax, CUM120 and DOBmax), biochemical tests, ICGR15 and liver necrosis score were also analyzed using Spearman’s correlation analysis.
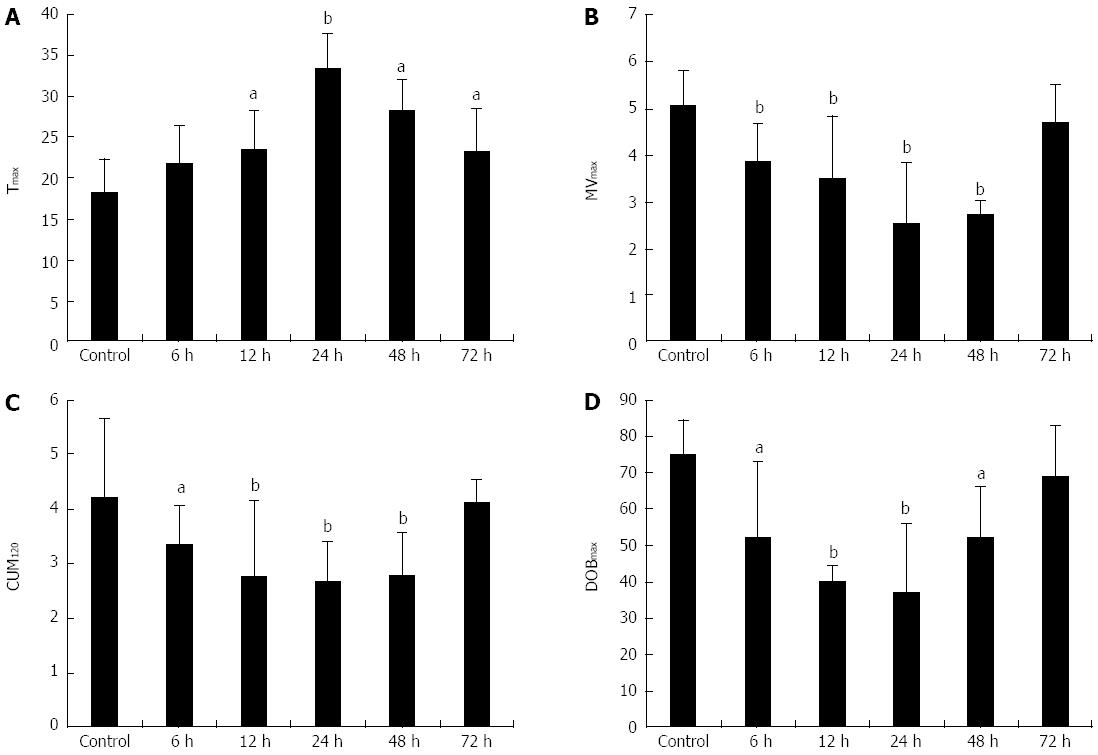
RESULTS: Tmax, MVmax, CUM120 and DOBmax, as well as most of the traditional methods, correlated with the liver necrosis score (r = 0.493, P < 0.05; r = -0.731, P < 0.01; r = -0.618, P < 0.01; r = -0.592, P < 0.01, respectively). MVmax, CUM120 and DOBmax rapidly decreased and were lower than those in the controls as early as 6 h after D-GalN injection (3.84 ± 0.84 vs 5.06 ± 0.78, P < 0.01; 3.35 ± 0.72 vs 4.21 ± 1.44, P < 0.05; 52.3 ± 20.58 vs 75.1 ± 9.57, P < 0.05, respectively) and reached the lowest point 24 h after D-GalN injection. MVmax, CUM120 and DOBmax returned to normal levels 72 h after D-GalN injection and preceded most of the traditional methods, including liver biopsy.
CONCLUSION: The 13C-MBT is a sensitive tool for the timely detection of acute liver injury and early prediction of recovery in a rat model. Further clinical studies are warranted to validate its role in patients with acute liver injury.
Core tip: The 13C-methacetin breath test (13C-MBT) is a promising tool for the assessment of metabolic liver function. Previous studies have mainly been conducted in the setting of chronic liver disease; however, evidence on acute liver injury is scanty. The present study evaluated the role of 13C-MBT in the assessment of acute liver injury in an animal model and showed that 13C-MBT was sensitive for the timely detection of acute liver injury and for early prediction of liver function recovery.
- Citation: Zhu D, Zhang H, Mao JY, Wang HY, Li X, Xu YQ. Role of the 13C-methacetin breath test in the assessment of acute liver injury in a rat model. World J Gastroenterol 2014; 20(32): 11305-11312
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11305
Acute liver disease may progress to fulminant hepatic failure, as a result of which, a high proportion of patients will die unless liver transplantation is performed[1]. Therefore, timely and accurate assessment of the degree of liver damage in acute liver disease outweighs specific disease etiologies with regard to treatment and prognosis[2]. However, current methods of disease severity assessment in patients with acute liver disease are far from optimal. Conventional biochemical tests, such as transaminase, bilirubin, albumin (ALB) plasma levels and prothrombin time (PT), provide information on a mixture of injury and function, but none of these are regarded as a sensitive and reliable marker of the severity of liver injury[3]. Although the 15-min retention rate of indocyanine green (ICGR15) is considered to be a reliable method for assessing liver function impairment[4-6], several drawbacks, such as cumbersome processing, potential allergic reaction, and its invasive nature limit its use in clinical settings. Many patients with liver disease, acute or chronic, undergo a liver biopsy to guide therapeutic decisions[7]. However, liver biopsy is highly invasive and impractical as a follow-up test. Thus, a simple, noninvasive and powerful alternative to liver biopsy in the management of liver disease is required.
Non-invasive breath tests using carbon isotope 13C have been proposed as a measure of metabolic liver function. 13C can be incorporated into organic substances and metabolized by the liver. The by-product of this metabolism is 13CO2, which changes the 13CO2/12CO2 isotope ratio of the patient’s exhaled breath. Measuring the 13CO2/12CO2 ratio in exhaled air enables quantification of microsomal function[8]. Thus, the breath test can be used early to evaluate quantitatively liver function damage or hepatic reserve function at hepatocyte metabolic level. The substances used in breath tests include aminopyrine, methacetin, caffeine, galactose and others[9]. Methacetin is widely used for breath tests because of its low toxicity in small doses. The 13C-methacetin breath test (13C-MBT) is considered a safe, simple and repeatable method for monitoring dynamic liver function.
The 13C-MBT could accurately identify liver fibrosis and differentiate the grades of fibrosis in patients with chronic hepatitis C infection[7,8,10]. In patients with acute liver disease, preliminary evidence indicated that clinical improvement and normalization of biochemical parameters were accompanied by progressive improvement of the 13C-MBT scores[11]. Detection of improvement using 13C-MBT occurred 1-3 d earlier than any of the other clinical and laboratory parameters, suggesting that the 13C-MBT might serve as a more sensitive decision-making tool for the follow-up of these patients in the setting of severe acute liver disease[12].
The present study was designed to preliminarily evaluate the role of the 13C-MBT in the assessment of acute liver injury in an animal model. Parameters of the 13C-MBT, as well as ICGR15 and biochemical variables, were tested and compared between the control and model groups (at different time points during the study); their associations with liver biopsy were also analyzed.
Forty-eight male Sprague-Dawley (SD) rats of similar age and weight (200 ± 20 g) were obtained from Shanghai Laboratory Animal Center and were fed a Purina laboratory chow, ad libitum, in an internal animal breeding house at Beijing Tiantan Hospital. The rats were individually housed in a temperature and humidity controlled environment with a 12-h light-dark cycle and were free to drink tap water until the week before the study began. All animals received human care in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Academy Press[13]. The study was approved by local ethic committee of Beijing Tiantan Hospital, Capital Medical University. D-Galactosamine (D-GalN) was purchased from Sigma Chemical Company (St. Louis, MO, United States). Indocyanine green was purchased from Dandong Yichuang Pharmaceutical Co., Ltd. (Dandong, China). 13C-methacetin powder and the Infra-Red Isotope Analyzer (IRIS) were purchased from WAGNER Analysen Technik (Bremen, Germany). A UV-vis spectrophotometer was purchased from Beijing Purkinje General Instrument Co., Ltd. (Beijing, China).
The 48 rats were randomly assigned to six groups, including a control group and five acute liver injury model groups (M6h, M12h, M24h, M48h and M72h). The variables in the subscript after the capital letter “M” denote the time points after intraperitoneal injection. Acute liver injury was induced by a single intraperitoneal injection of D-GalN (450 mg/kg), which was dissolved in saline (0.6 mL/100 g of body weight)[14]. The rats in the control group received the same volume of saline as a substitute for D-GalN. ICGR15 and biochemical analyses were conducted at predetermined time points (6, 12, 24, 48 and 72 h) for each model group after D-GalN injection, while the 13C-MBT was conducted 2 h ahead of the predetermined time point because of the time taken to perform the test. The rats were then sacrificed under anesthesia and liver tissues were harvested and used for histopathological analysis. The rats in the control group underwent the same procedure within 6 h after intraperitoneal injection of saline.
13C-methacetin powder was completely dissolved in distilled water to a final concentration of 4 g/L. According to the experimental design, each rat was given 13C-methacetin (200 mg/100 g of body weight) by gavage after 10 h fasting. For the 13C-MBT, a home-made device was used to collect expiratory air; Shirin et al[9] describe the details of the device structure. Breath samples for 13CO2 measurement were collected for 15 s continuously by an aspirator pump and were collected at baseline and 10, 20, 30, 40, 50, 60, 80, 100 and 120 min after gavage. Each sample was then transferred into a special aluminum covered plastic bag (100 mL capacity). The bag was closed with a plastic cork immediately at the end of exhalation and prepared for analysis. After connecting the bag to the IRIS, the machine analyzed the breath samples automatically and provided several valuable parameters such as DOBmax, Tmax, MVmax and CUM120. DOBmax is the peak value of the delta over baseline (DOB) curve, which reflects the change in the natural 13CO2/12CO2 ratio exhaled to the ingestion of a labeled substrate during the test period. Tmax reflects the time at which the peak amplitude of DOB curve appears. MVmax reflects the largest metabolic rate of 13CO2, and CUM120 reflects cumulative percentage of administered dose of 13CO2 recovered over time at 120 min[15,16].
The ICG standard curve was drawn according to the manufacturer’s instructions. Indocyanine green was completely dissolved in distilled water and prepared to a final concentration of 0.1 mg/mL. According to the experimental design, each rat was given a caudal intravenous injection of indocyanine green at 50 mg/100 g of body weight. Abdominal aortic puncture was performed to collect blood in a heparinized tube at 15 min after injection of ICG, followed by isolation of plasma with centrifugation at 1500 ×g for 15 min. Plasma (0.25 mL) was extracted and diluted with 1.25 mL saline. Absorbance value of the plasma sample at 805 nm was determined by A UV-vis spectrophotometry determined the absorbance of the plasma sample at 805 nm, which were compared with the value for normal plasma. The standard curve was used to calculate the serum concentration of ICG. An automatic biochemical analyzer detected the biochemical parameters, including alanine transaminase (ALT), total bilirubin (TBIL), ALB and PT, from a further 0.6-1.0 mL of extracted plasma.
Rats were sacrificed after the 13C-MBT, ICGR15 and blood collection were completed. Their livers were harvested and midsections of the left lobes were fixed with 10% formalin solution and processed for staining with hematoxylin and eosin (HE) and then studied by light microscopy. The extent of liver necrosis was determined by counting the number of necrotic foci under low-power field (LPF) magnification, using a × 10 objective. A necrotic area of 3600 μm2 measured by a 0.01 mm microscope micrometer was defined as a necrotic focus. Ten LPFs were chosen randomly along with a W-shaped sampling path for each slice. The liver necrosis score was obtained by counting the total number of necrotic foci in 10 LPFs.
Data analysis was performed using SPSS 17.0 statistical analysis software (SPSS, Chicago, IL, United States). Continuous variables were described as mean ± SD and were analyzed by the Student’s two-independent-sample t test (normal distribution) or Mann-Whitney non-parametric U test (skewed distribution). Normality of distribution was determined using the Kolmogorov-Smirnov test (cut-off at P = 0.01). Associations between the liver necrosis score and biochemical parameters, ICGR15 and the 13C-MBT were described using Spearman’s correlation analysis. All tests were two-sided and considered significant at P < 0.05.
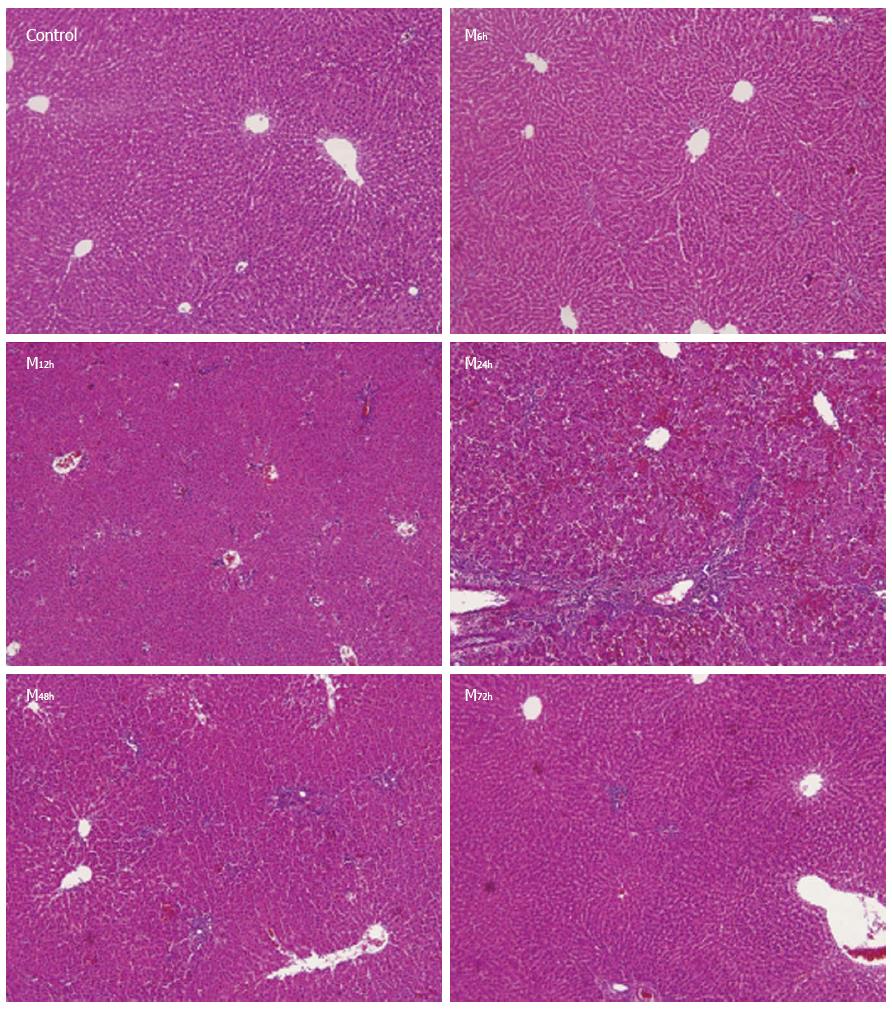
In the control group, the hepatic architecture was normal, with little necrosis and inflammatory cell infiltration. At 6 h after intraperitoneal injection of D-GalN, the structure of the hepatic lobules was largely normal with spotty, focal necrosis and little inflammatory cell infiltration. At 12 h, the structure of hepatic lobules was slightly damaged, with focal necrosis and moderate inflammatory cell infiltration. At 24 h, there was an extensive area of central-central and central-portal bridging necrosis, containing many red cells trapped in the collapsed reticulin network, and significant inflammatory cell infiltration was observed. At 48 h, hepatic necrosis and inflammatory cell infiltration gradually reduced and at 72 h, hepatic lobular structure had returned to normal with very little inflammatory cell infiltration (Figure 1). Thus, the severity of liver necrosis increased with time after D-GalN injection, and reached a peak between 24 and 48 h, and then gradually returned to normal. The liver necrosis scores in the control group and model groups at 6, 12, 24, 48 and 72 h were 0.4 ± 0.2, 28.6 ± 18.5, 59.5 ± 9.5, 80.3 ± 4.2, 36.0 ± 3.4, and 21.1 ± 4.1, respectively. The liver necrosis scores in the model groups at 6, 12, 24, 48 and 72 h were significantly higher than those in the control group (Table 1).
| Group | Tmax (min) | MVmax | CUM120 | DOBmax | Liver necrosis score |
| Control | 18.3 ± 4.1 | 5.06% ± 0.78% | 4.21% ± 1.44% | 75.1% ± 9.57% | 0.4 ± 0.2 |
| M6h | 21.7 ± 4.7 | 3.84% ± 0.84%b | 3.35% ± 0.72%a | 52.3% ± 20.58%a | 28.6 ± 18.5b |
| M12h | 23.3 ± 5.2a | 3.52% ± 1.32%b | 2.77% ± 1.40%b | 40.1% ± 4.13%b | 59.5 ± 9.5b |
| M24h | 33.3 ± 4.3b | 2.55% ± 1.31%b | 2.67% ± 0.71%b | 37.3% ± 18.50%b | 80.3 ± 4.2b |
| M48h | 28.3 ± 3.8a | 2.72% ± 0.31%b | 2.77% ± 0.81%b | 52.23% ± 14.16%a | 36.0 ± 3.4b |
| M72h | 23.3 ± 5.2a | 4.72% ± 0.81% | 4.12% ± 0.44% | 69.1% ± 13.96% | 21.1 ± 4.1a |
The mean value of Tmax (min) in the control group and model groups at 6, 12, 24, 48 and 72 h were 18.3 ± 4.1, 21.7 ± 4.7, 23.3 ± 5.16, 33.3 ± 4.3, 28.3 ± 3.83, and 23.3 ± 5.16, respectively. Compared with the control group, the Tmax in the model groups at 12 h, 24 h, 48 h and 72 h were significantly higher. The MVmax (%), CUM120 (%) and DOBmax (%) in the control group were 5.06 ± 0.78, 4.21 ± 1.44, and 75.1 ± 9.57, respectively. These three parameters followed a similar pattern and decreased simultaneously, reaching the lowest value at 24 h, and then returning to normal. MVmax, CUM120 and DOBmax in the model groups at 6, 12, 24 and 48 h were significantly lower than those in the control group. At 72 h, the differences in these three parameters between the control group and model groups were no longer significant (Table 1, Figure 2). Correlation analysis of the parameters of the 13C-MBT and liver necrosis score revealed that Tmax was positively correlated with liver necrosis score (r = 0.493, P < 0.05), while MVmax, CUM120 and DOBmax were negatively correlated with liver necrosis score (r = -0.731, P < 0.01; r = -0.618, P < 0.01; r = -0.592, P < 0.01, respectively) (Table 1).
The mean level of ALT in the control group was 45.5 ± 7.0 U/L. After injection of D-GalN, ALT level increased rapidly and reached a peak (994.3 ± 427.5 U/L) at 24 h, and then gradually decreased. ALT levels in the model groups at 12, 24 and 48 h were significantly higher than that in the control group (444.1 ± 230.4 U/L, P < 0.01; 994.3 ± 427.5 U/L, P < 0.01; 436.8 ± 103.7 U/L, P < 0.01, respectively). Correlation analysis revealed that ALT was positively correlated with liver necrosis score (r = 0.768,P < 0.01). The changes in TBIL following D-GalN injection were similar to those of ALT. Levels of TBIL (μmol/L) in the model groups at 12, 24, 48 and 72 h were significantly higher than that in the control group (2.41 ± 0.98, P < 0.05; 13.74 ± 0.82, P < 0.001; 12.99 ± 1.67, P < 0.001; 12.81 ± 0.71, P < 0.001, respectively). Correlation analysis revealed that TBIL was positively correlated with liver necrosis score (r = 0.368, P = 0.01). The mean level of ALB in the control group was 34.52 ± 2.69 g/L. After injection of D-GalN, ALB level decreased and remained low at 72 h. ALB levels (g/L) in the model groups at 24, 48 and 72 h were significantly lower than that in the control group (32.08 ± 4.04, P < 0.05; 31.48 ± 1.57, P < 0.05; 28.79 ± 1.73, P < 0.001, respectively). Correlation analysis revealed that ALB was not correlated with liver necrosis score (r = 0.009, P > 0.05). PT values in the model groups at each time point were significantly longer than that in the control group (P < 0.01), and correlation analysis revealed that PT was positively correlated with liver necrosis score (r = 0.627, P < 0.01) (Table 2). ICGR15 (%) in the control group and model groups at 6, 12, 24, 48 and 72 h were 2.8 ± 0.4, 3.1 ± 0.6, 6.8 ± 1.4, 17.0 ± 2.3, 11.5 ± 1.6 and 5.7 ± 1.2, respectively. Compared with the control group, ICGR15 in the model groups at 12, 24 and 48 h were significantly higher (P < 0.05; P < 0.001; P < 0.001, respectively). Correlation analysis revealed that ICGR15 was positively correlated with liver necrosis score (r = 0.604, P < 0.01) (Table 2).
| Group | ALT (U/L) | TBIL (μmol/L) | PT (s) | ALB (g/L) | ICG15 |
| Control | 45.5 ± 7.0 | 1.36 ± 0.61 | 13.55 ± 0.55 | 34.52 ± 2.69 | 2.8% ± 0.4% |
| M6h | 166.8 ± 40.7 | 1.56 ± 0.72 | 18.89 ± 3.38b | 33.93 ± 1.27 | 3.1% ± 0.6% |
| M12h | 444.1 ± 230.4b | 2.41 ± 0.98a | 23.99 ± 3.09b | 33.65 ± 1.57 | 6.8% ± 1.4%a |
| M24h | 994.3 ± 427.5b | 13.74 ± 0.82b | 36.36 ± 2.45b | 32.08 ± 4.04a | 17.0% ± 2.3%b |
| M48h | 436.8 ± 103.7b | 12.99 ± 1.67b | 33.05 ± 3.22b | 31.48 ± 1.57a | 11.5% ± 1.6%b |
| M72h | 238.7 ± 70.7 | 12.81 ± 0.71b | 29.13 ± 2.94b | 28.79 ± 1.73b | 5.7% ± 1.2% |
In recent years, the role of the 13C-MBT to assess liver function has been investigated in patients with chronic liver disease. Matsumoto et al[11] found that the 13C-MBT value (the 13C recovery over 30 min) was significantly reduced in patients with chronic aggressive hepatitis and in those with liver cirrhosis, but not in patients with chronic persistent hepatitis or healthy controls. Patients with either advanced cirrhosis or hepatocellular carcinoma showed significantly lower values than those with well-compensated cirrhosis, indicating that the 13C-MBT[11] can effectively evaluate the severity of liver damage. Goetze et al[10] tested 100 patients with untreated chronic HCV infection, and 100 age- and sex-matched healthy volunteers using the 13C-MBT following ingestion of 75 mg methacetin. They found that the 13C-MBT was an accurate tool for measuring the degree of inflammation and fibrosis in patients with chronic HCV infection and normal serum alanine aminotransferase (NALT)[10]. Further studies have supported the role of the 13C-MBT in the assessment of chronic liver disease[17-19]. A few studies have focused on the assessment of the degree of hepatic damage in acute liver disease. A recent study showed that the 13C-MBT was a sensitive test and may be a useful tool to evaluate functional liver mass in animal models of acute liver failure and cirrhosis[9].
The present study was designed to explore the role of the 13C-MBT in assessing acute liver injury using a rat model. The histopathological analysis showed that the severity of liver necrosis increased with time after intraperitoneal injection of D-GalN, reached a peak between 24 and 48 h, and then gradually returned to normal. Correlation analysis revealed that parameters of the 13C-MBT, as well as most of the traditional methods, correlated with liver necrosis score, which was in accordance with the morphologic findings. The changes in MVmax, CUM120 and DOBmax reflected the severity of liver injury after D-GalN injection. Liver function abnormalities were recognized by the 13C-MBT as early as 6 h after initiation of liver damage, which was earlier than most of the traditional methods, including ALT, TBIL, ALB, ICGR15 and liver biopsy. In addition, the 13C-MBT reflected the recovery of liver injury after D-GalN injection. The parameters of the 13C-MBT gradually returned to baseline levels after 24 h, which was in accordance with the liver necrosis score. The differences in the parameters of the 13C-MBT between the model groups and control group at 72 h were no longer significant, while most of the other methods, including liver histopathology are not sufficiently informative, indicating that the 13C-MBT may be a sensitive tool for predicting recovery from acute liver injury.
Some limitations in the present study should be acknowledged. Firstly, the limited number of rats in each group may have lowered the statistical power and led to false positive or false negative results. This could be minimized by enlarging the sample size in future studies. Secondly, we used a self-defined liver necrosis score to evaluate the severity of liver injury, according to previously published literature, as no consensus standardized method or scoring system is available in the setting of acute liver injury[20]. We found that the liver necrosis score reflected the severity of liver injury following corroboration of the results with other methods. Thirdly, whether the results of animal experiments are applicable to humans is unknown, and further studies on patients to validate the results of our study are warranted. Lastly, we used liver biopsy as the standard method to evaluate the role of the 13C-MBT in acute liver injury. Although liver biopsy has been widely regarded as the “gold standard” for defining liver disease status, it also has drawbacks that have prompted questions about its value[21,22]. However, there are currently no other tools that can serve as alternative methods. Although the Child-Turcotte-Pugh score and the model for end-stage liver disease score were reported to be valuable to predict liver disease progression[23-25], their suitability for use in animal models is unknown.
In conclusion, the results of the present study suggest that the 13C-MBT may be a valuable tool to assess liver function in acute liver injury in a rat model. The 13C-MBT is sensitive for timely detection of acute liver injury and early prediction of liver function recovery. To validate its role in patients, further clinical studies are warranted.
We thank David B Nwinee from International School of Capital Medical University for his help with the manuscript.
The 13C-methacetin breath test (13C-MBT) has been proposed as a promising tool for the assessment of metabolic liver function in the setting of chronic liver disease. However, evidence of its role in acute liver injury is scanty and anecdotal.
Recent studies have shown that the 13C-MBT is an accurate tool to identify liver fibrosis and differentiating grades of fibrosis in patients with chronic hepatitis C infection. In patients with acute liver disease, preliminary evidence indicated that clinical improvement and normalization of biochemical parameters were accompanied by progressive improvements in 13C-MBT scores. The 13C-MBT detected improvement 1-3 d earlier than the other clinical and laboratory parameters, suggesting that the 13C-MBT might serve as a more sensitive decision-making tool for the follow-up of patients with severe acute liver disease.
Although the 13C-MBT has been studied in the clinical setting for several years, most of these studies were case reports or case series, and were related to chronic liver disease. There are few studies focusing on patients with acute liver injury, probably because of concerns for the safety of patients with serious conditions. This study evaluated the role of the 13C-MBT in the assessment of acute liver injury in an animal model. Parameters of the 13C-MBT, as well as ICGR15 and biochemical variables, were tested and compared between the control and model groups (at different time points during the study); their associations with liver biopsy were also analyzed.
The 13C-MBT is a sensitive tool for timely detection of liver injury and early prediction of liver function recovery in the setting of acute liver disease.
13C-MBT: 13C can be incorporated into organic substances and metabolized by the liver. The by-product of this metabolism is 13CO2, which changes the 13CO2/12CO2 isotope ratio of the patient’s exhaled breath. Measuring the 13CO2/12CO2 ratio in exhaled air enables quantification of microsomal function. Methacetin is widely used for breath tests because of its low toxicity in small doses.
Zhu et al presented the results of their study on the role of 13C-MBT in the assessment of acute liver injury induced by D-galactoamine in rats. It has to be noted that there are already some studies concerning the role of 13C-MBT in the evaluation of hepatic damage/reserve during both chronic and acute liver damage. However, to date, the 13C-MBT has not been evaluated significantly or comprehensively in the acute setting. Overall, the study is well-designed, results are somewhat interesting and the paper is well-written.
| 1. | Emond JC, Aran PP, Whitington PF, Broelsch CE, Baker AL. Liver transplantation in the management of fulminant hepatic failure. Gastroenterology. 1989;96:1583-1588. [PubMed] |
| 2. | Lalazar G, Ilan Y. Assessment of liver function in acute or chronic liver disease by the methacetin breath test: a tool for decision making in clinical hepatology. J Breath Res. 2009;3:047001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Armuzzi A, Candelli M, Zocco MA, Andreoli A, De Lorenzo A, Nista EC, Miele L, Cremonini F, Cazzato IA, Grieco A. Review article: breath testing for human liver function assessment. Aliment Pharmacol Ther. 2002;16:1977-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Faybik P, Hetz H. Plasma disappearance rate of indocyanine green in liver dysfunction. Transplant Proc. 2006;38:801-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Erdogan D, Heijnen BH, Bennink RJ, Kok M, Dinant S, Straatsburg IH, Gouma DJ, van Gulik TM. Preoperative assessment of liver function: a comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004;24:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Gupta S, Chawla Y, Kaur J, Saxena R, Duseja A, Dhiman RK, Choudhary NS. Indocyanine green clearance test (using spectrophotometry) and its correlation with model for end stage liver disease (MELD) score in Indian patients with cirrhosis of liver. Trop Gastroenterol. 2012;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Lalazar G, Pappo O, Hershcovici T, Hadjaj T, Shubi M, Ohana H, Hemed N, Ilan Y. A continuous 13C methacetin breath test for noninvasive assessment of intrahepatic inflammation and fibrosis in patients with chronic HCV infection and normal ALT. J Viral Hepat. 2008;15:716-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Vranova J, Hendrichova M, Kolarova H, Kratka K, Rosina J, Horak J. ¹³C-methacetin breath test in the evaluation of disease severity in patients with liver cirrhosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Shirin H, Aeed H, Shalev T, Sorin V, Stavinski S, Shahmurov M, Ilan Y, Avni Y. Utility of a 13C-methacetin breath test in evaluating hepatic injury in rats. J Gastroenterol Hepatol. 2008;23:1762-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Goetze O, Selzner N, Fruehauf H, Fried M, Gerlach T, Mullhaupt B. 13C-methacetin breath test as a quantitative liver function test in patients with chronic hepatitis C infection: continuous automatic molecular correlation spectroscopy compared to isotopic ratio mass spectrometry. Aliment Pharmacol Ther. 2007;26:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Matsumoto K, Suehiro M, Iio M, Kawabe T, Shiratori Y, Okano K, Sugimoto T. [13C]methacetin breath test for evaluation of liver damage. Dig Dis Sci. 1987;32:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Lalazar G, Adar T, Ilan Y. Point-of-care continuous (13)C-methacetin breath test improves decision making in acute liver disease: results of a pilot clinical trial. World J Gastroenterol. 2009;15:966-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. ILAR J. 1997;38:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 413] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Galun E, Zeira E, Pappo O, Peters M, Rose-John S. Liver regeneration induced by a designer human IL-6/sIL-6R fusion protein reverses severe hepatocellular injury. FASEB J. 2000;14:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Giannini E, Romagnoli P, Fasoli A, Chiarbonello B, Malfatti F, Botta F, Risso D, Lantieri PB, Savarino V, Testa R. Influence of Helicobacter pylori eradication therapy on 13C aminopyrine breath test: comparison among omeprazole-, lansoprazole-, or pantoprazole-containing regimens. Am J Gastroenterol. 2000;95:2762-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Desta Z, Modak A, Nguyen PD, Lemler SM, Kurogi Y, Li L, Flockhart DA. Rapid identification of the hepatic cytochrome P450 2C19 activity using a novel and noninvasive [13C]pantoprazole breath test. J Pharmacol Exp Ther. 2009;329:297-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Razlan H, Marzuki NM, Tai ML, Shamsul AS, Ong TZ, Mahadeva S. Diagnostic value of the C methacetin breath test in various stages of chronic liver disease. Gastroenterol Res Pract. 2011;2011:235796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Festi D, Capodicasa S, Sandri L, Colaiocco-Ferrante L, Staniscia T, Vitacolonna E, Vestito A, Simoni P, Mazzella G, Portincasa P. Measurement of hepatic functional mass by means of 13C-methacetin and 13C-phenylalanine breath tests in chronic liver disease: comparison with Child-Pugh score and serum bile acid levels. World J Gastroenterol. 2005;11:142-148. [PubMed] |
| 19. | Kasicka-Jonderko A, Nita A, Jonderko K, Kamińska M, Błońska-Fajfrowska B. C-methacetin breath test reproducibility study reveals persistent CYP1A2 stimulation on repeat examinations. World J Gastroenterol. 2011;17:4979-4986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Uehara T, Kosyk O, Jeannot E, Bradford BU, Tech K, Macdonald JM, Boorman GA, Chatterjee S, Mason RP, Melnyk SB. Acetaminophen-induced acute liver injury in HCV transgenic mice. Toxicol Appl Pharmacol. 2013;266:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2245] [Article Influence: 132.1] [Reference Citation Analysis (2)] |
| 22. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1636] [Article Influence: 96.2] [Reference Citation Analysis (2)] |
| 23. | Bazarah SM, Peltekian KM, McAlister VC, Bitter-Suermann H, MacDonald AS. Utility of MELD and Child-Turcotte-Pugh scores and the Canadian waitlisting algorithm in predicting short-term survival after liver transplant. Clin Invest Med. 2004;27:162-167. [PubMed] |
| 24. | Huo TI, Lin HC, Wu JC, Lee FY, Hou MC, Lee PC, Chang FY, Lee SD. Proposal of a modified Child-Turcotte-Pugh scoring system and comparison with the model for end-stage liver disease for outcome prediction in patients with cirrhosis. Liver Transpl. 2006;12:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Bie CQ, Yang DH, Tang SH, Huang W. The value of model for end-stage liver disease and Child-Turcotte-Pugh scores over time in evaluating the prognosis of patients with decompensated cirrhosis: experience in the Chinese mainland. Hepatol Res. 2009;39:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
P- Reviewer: Vespasiani-Gentilucci U S- Editor: Qi Y L- Editor: Stewart G E- Editor: Zhang DN