Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.11000
Revised: March 25, 2014
Accepted: May 23, 2014
Published online: August 21, 2014
Processing time: 191 Days and 4.1 Hours
AIM: To evaluate the clinical efficacy and safety of acupuncture and moxibustion for the treatment of active Crohn’s disease (CD).
METHODS: Ninety-two patients were equally and randomly divided into the treatment group and received herb-partitioned moxibustion combined with acupuncture, and the control group received wheat bran-partitioned moxibustion combined with superficial acupuncture. The patients received three treatment sessions per week for 12 wk and were followed up for 24 wk. The main outcome was evaluated using the CD Activity Index (CDAI) score, and the secondary outcomes were evaluated using laboratory indicators such as hemoglobin (HGB), C-reactive protein (CRP), erythrocyte sedimentation rate, quality-of-life, endoscopic ratings, and intestinal histology scores.
RESULTS: The CDAI scores of both the treatment and control groups were significantly reduced after treatment compared with those measured before treatment. However, the degree of improvement in the treatment group was significantly greater than that of the control group. The improvement in symptoms in patients of the treatment group was sustained at follow-up, whereas that of the control group was not. The overall efficacy of the treatment was significantly greater than that of the control. Both groups demonstrated significant improvements in quality-of-life ratings after treatment, but the improvement was significantly greater in the treatment group than in the control group. In addition, the patients in the treatment group showed significantly increased HGB and significantly decreased CRP levels and histopathological scores at the end of treatment, whereas the control group did not exhibit significant changes.
CONCLUSION: Moxibustion with acupuncture provided significant therapeutic benefits in patients with active CD beyond the placebo effect and is therefore an effective and safe treatment for active CD.
Core tip: Acupuncture treatment has been widely used in the clinical treatment of various diseases, particularly gastrointestinal diseases. Crohn’s disease (CD) is a type of inflammatory bowel disease, and its incidence increases each year in China. However, there are limited numbers of reports on the efficacy of acupuncture treatment for CD. In the present study, we found that acupuncture provided significant therapeutic benefits to patients with mild to moderate CD and is therefore an effective and safe treatment.
- Citation: Bao CH, Zhao JM, Liu HR, Lu Y, Zhu YF, Shi Y, Weng ZJ, Feng H, Guan X, Li J, Chen WF, Wu LY, Jin XM, Dou CZ, Wu HG. Randomized controlled trial: Moxibustion and acupuncture for the treatment of Crohn’s disease. World J Gastroenterol 2014; 20(31): 11000-11011
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/11000.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.11000
Crohn’s disease (CD) is a type of inflammatory bowel disease and is a recurrent systemic inflammatory disease that mainly affects the gastrointestinal tract, with extraintestinal manifestations and related immune diseases[1]. With a steadily increasing incidence, CD has become a common digestive disease in Asia, especially in China[2]. CD has a significant impact on the productivity of society and on personal quality of life[3-6], and places a major burden on public health care resources[7]. Current medicinal treatments for CD primarily utilize salicylic acid-based drugs, corticosteroids, immunosuppressives, and biological agents. However, the poor efficacy and significant adverse effects of these treatments and the susceptibility to recurrence after dose reduction or discontinuation limit the long-term clinical application of these drugs[8].
Acupuncture has a history of more than 4000 years and is popular in China and many other countries. Acupuncture has been widely used for the clinical treatment of various diseases, particularly gastrointestinal diseases such as CD[9,10], ulcerative colitis[11,12], irritable bowel syndrome[13,14], and functional dyspepsia[15,16]. However, there are a limited number of reports on the clinical study of acupuncture in the treatment of CD[17], and its efficacy is therefore not fully established. In particular, the effects of acupuncture on endoscopic findings and the intestinal histopathological changes of CD have not been reported. Therefore, it is necessary to perform an objective evaluation of the efficacy of acupuncture in CD.
Our research team has performed clinical and basic research on acupuncture and moxibustion for the treatment of inflammatory bowel disease for over 30 years. Herb-partitioned moxibustion combined with acupuncture therapy is commonly used for the treatment of CD. Herb-partitioned moxibustion, a critical component of moxibustion therapy, is performed by placing a cake of herbs (dispensing a traditional Chinese medicinal (TCM) formula) on the patient’s acupoints, followed by the placement and ignition of moxa cones, which are composed of refined mugwort floss, on the herbal cake to treat diseases. Acupuncture is a collection of procedures involving penetration of the skin with needles to stimulate certain points on the body. Here, we conducted a randomized controlled clinical trial of herb-partitioned moxibustion combined with acupuncture for the treatment of active CD with the goal of evaluating its clinical efficacy and safety.
From January 2010 to April 2013, CD patients treated at the acupuncture outpatient center for inflammatory bowel disease of the Shanghai Institute of Acupuncture and Meridian, the Endoscopy Center of Zhongshan Hospital at Fudan University, the Department of Acupuncture-Moxibustion of Shuguang Hospital affiliated with the Shanghai University of Traditional Chinese Medicine, and the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated with the Shanghai University of Traditional Chinese Medicine were recruited as subjects for this study. The diagnosis of CD was confirmed in all patients by clinical manifestation evaluation, imaging analysis, and endoscopic and histopathological examinations.
This clinical trial was approved by the Ethics Committee of the Yueyang Hospital of Integrated Chinese and Western Medicine affiliated with the Shanghai University of TCM. All subjects provided informed consent prior to enrollment into the trial. The study was registered in the Clinical Trials Registry at http://clinicaltrials.gov/(NCT01697761).
Patients who had a confirmed diagnosis of mild or moderate CD (CD Activity Index (CDAI) values ranging from 151 to 350), had not taken medications such as salicylic acid drugs and/or prednisone (at a dose ≤ 15 mg) for at least 1 mo, and had not taken immunosuppressants or used anti-TNF-α biological agents for 3 mo prior to enrollment in the study were included.
Pregnant or lactating patients; patients with serious diseases of the heart, brain, liver, kidney, or hematopoietic system; patients with mental illness; and patients with other severe diseases were excluded.
After enrollment, the patients who were using CD medications maintained their drug dosage unchanged. If their conditions deteriorated during the treatment period or if the patients needed to increase their dose or take other medications, these subjects were withdrawn from the study. During the follow-up period, patients were allowed to adjust their dose of Western medicine after recording each adjustment. If patients increased their dose, became sicker, or took other drugs, these subjects were also withdrawn.
We used a simple random sampling method by generating a random number table using the SPSS 16.0 software. The first number in the random number table was used as a starting point to create random assignment cards, which were then sealed in envelopes. The envelopes were numbered (the same number as the sequence number of the card inside) and kept secure by a dedicated person. When a qualified participant was enrolled into the trial, researchers then asked this dedicated person for a random number by phone or text message. According to the order in which the patient was enrolled, the person in charge opened the envelope with the same sequence number and informed the researchers of the assigned information by phone or text message. The odd random numbers were assigned to the treatment group, and even numbers were assigned to the control group. The participants were randomly divided into the treatment and control groups at a ratio of 1:1.
All patients were blinded during the trial and were therefore unaware of the specific treatment they received. All subjects in each treatment session were treated in a private room to avoid potential communication and comparison among subjects. In addition, a blinded evaluation was conducted in which a third researcher who was unaware of the group assignments assessed the treatment outcomes. Blinded statistical data analysis was also conducted in which the researchers, operators, and statisticians were separated from one another.
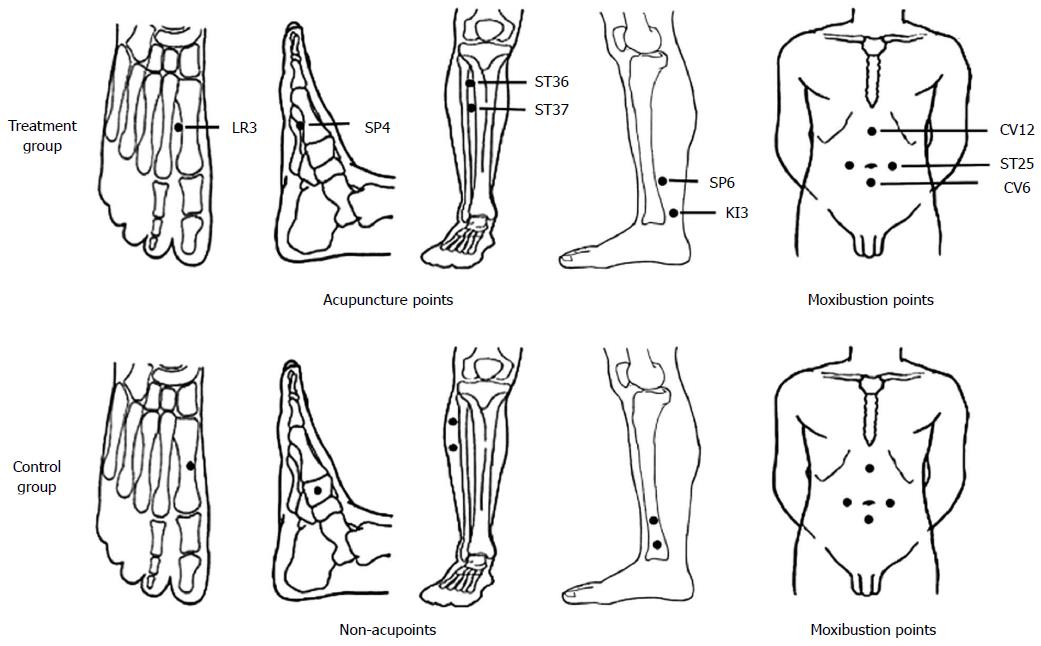
The treatment group received herb-partitioned moxibustion combined with acupuncture. The acupoints are listed in Table 1 and Figure 1; these acupoints were selected based on TCM principles according to the clinical manifestations of the patients. All of these acupoints were shown to be effective for the treatment of CD in our previous report[10].
| Treatment group | Control group | |
| Acupuncture points | Zusanli (ST36) | 20 mm away from the posterior of ST36 |
| Shangjuxu (ST37) | 20 mm away from the posterior of ST37 | |
| Gongsun (SP4) | On the medial aspect of the 1st cuneiform bone, between LR4 and SP4 | |
| Sanyinjiao (SP6) | 15 mm away from the anterior of SP6, on the medial aspect of the tibia | |
| Taixi (KI3) | 15 mm away from the anterior of KI3, on the medial aspect of the tibia | |
| Taichong (LR3) | On the dorsal aspect of the 1st metatarsal bone between LR3 and SP3 | |
| Moxibustion points | Tianshu (ST25), Qihai (CV6), Zhongwan (CV12) | |
The herbal cake recipe used for moxibustion in the treatment group included Coptis chinensis, Radix Aconiti Lateralis, Cortex Cinnamomi, Radix Aucklandiae, Flos Carthami, Salvia miltiorrhiza, and Angelica sinensis as the main ingredients[10]. These herbs were ground into fine powders, which were then passed through a 100-mesh sieve and stored for future use. During treatment, 2.8 g of the herbal powder was mixed with maltose and made into a thick paste by adding warm water. The paste was then pressed into a mold to make herbal cakes with a diameter of 28 mm and a thickness of 5 mm. For moxibustion, pure refined moxa sticks (“Hanyi”, Nanyang, China, size: 17 mm × 200 mm) were cut into segments of 16 mm in height that weighed approximately 1.8 g. In each session, each acupoint was treated with two segments of moxa sticks.
Positioning of the acupuncture points was based on the “Nomenclature and location of acupuncture points” (Chinese National Standard (GB/T12346-2006)). Sterile disposable stainless steel needles (with a diameter of 0.30 mm and length of 40 mm or 25 mm, “Huatuo”, Suzhou, China) were used. The needles were directly inserted 20-30 mm into the skin and elicited a de-qi sensation. The needle was kept in position for 30 min. Herb-partitioned moxibustion and acupuncture were performed at the same time once every other day (three times per week) for a total of 36 sessions (12 wk). Subjects who received at least 80% of the required number of treatment sessions (29 or more) were considered to have completed the entire treatment plan.
The control group received wheat bran-partitioned moxibustion combined with superficial acupuncture. Wheat bran-partitioned moxibustion is often used by our research team as a placebo control method, and previous studies have shown that wheat bran-partitioned moxibustion differs significantly from herb-partitioned moxibustion[12]. The acupoints used in wheat bran-partitioned moxibustion were the same as those used for the treatment group. The procedure of superficial needling at non-acupoints was based on previous studies[15]. Non-meridian and non-acupoint zones located 1-2 cm away from the acupoints of the treatment group were selected for the control group, and an equal number of points were used in each group (Figure 1).
Wheat bran was used instead of herbs for the control group partition cake, and the treatment methods were the same as those of the treatment group. Needles with the same diameter and length as those used in the treatment group were used for needling; however, the needles were directly inserted only 1-2 mm into the skin, without eliciting a de-qi sensation. Wheat bran-partitioned moxibustion and acupuncture were performed at the same time, and the sessions were the same as those of the treatment group.
In this study, the acupuncture practitioners were all clinicians who were qualified TCM practitioners and had at least 2 years of clinical experience.
The CDAI[18] was used as the main measurement of outcome. This index consists of eight factors, with each factor totaled after adjustment with a weighting factor. CDAI evaluations were performed on the day of enrollment, in the 12th week after enrollment, and at follow-up (24th week). The changes in CDAI score and total treatment efficacy were evaluated. The total treatment efficacy evaluation standards were as follows: after treatment, a CDAI score measuring less than 150 indicated clinical remission, a CDAI score decreased by more than 70 indicated an improvement, and a CDAI score decreased by less than 70 or an increased CDAI score indicated an ineffective treatment. The effective rate = (the number of patients who demonstrated clinical remission + the number of patients who demonstrated an improvement with treatment)/the total number of patients.
Six parameters were used as secondary outcome measures. The quality of life of the patients was evaluated using the inflammatory bowel disease questionnaire (IBDQ), which is specific for patients with inflammatory bowel disease[19,20]. The level of C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) were used to assess the level of intestinal inflammation, and hemoglobin (HGB) measurements were used to assess the presence and severity of anemia. Fifteen patients from each group were selected to receive enteroscopic examinations and mucosal biopsies. The CD endoscopic index of severity (CDEIS)[21] and the D’Haens-Geboes intestinal histology scoring system[22] were used to evaluate endoscopic mucosal manifestations and the pathomorphological and inflammatory conditions of the patients, respectively. Intestinal mucosa samples obtained from biopsy were immediately placed in 10% formalin for storage. After hematoxylin and eosin (HE) staining, the tissues were imaged, and the pathological changes were scored. All secondary outcomes were measured on the day of enrollment and during the 12th week after enrollment in the trial.
All patients were followed-up by phone, email, or other methods in the 24th week regarding changes in disease and medication conditions. During the follow-up period (weeks 12-24), patients who had aggravated symptoms with a CDAI score greater than 150 and a CDAI score increase greater than 70 points were defined as patients with recurrence.
The safety evaluation included the following three aspects: vital sign monitoring including post-treatment temperature, respiration, heart rate, blood pressure, and liver and kidney function; acupuncture abnormalities including bleeding, hematoma, fainting during acupuncture treatment, pain in the acupuncture sites, increased blood pressure, and other adverse reactions; and moxibustion abnormalities including skin burns, blistering, or other discomfort caused by moxibustion.
According to the literature, the effective rate of acupuncture and moxibustion in the treatment of CD is 56%[9]. Our group previously used a non-randomized concurrent control method to conduct a pilot study that demonstrated an effective rate of herb-partitioned moxibustion and acupuncture in the treatment of CD of 86.67%[10]. Therefore, the current study established an expected effective rate value of 85%. The study consisted of two groups, with a significance level of α = 0.05 (one-sided) and a test power of 1 - β = 0.9. The formula for sample size estimation in comparing two sample rates was as follows:
n = (Uα + Uβ)2 (1 + 1/k) P (1 - P)/(Pe - Pc)2
Based on this calculation, the required sample size for each group was equal to 42 (n = 42 patients). With the addition of a 10% dropout rate (four patients), the two groups needed to include no less than 92 patients.
Statistical analyses of the baseline information and disease-related information were performed using the SPSS 16.0 software package (SPSS Inc., Chicago, IL, United States). The primary efficacy indicator, CDAI, was analyzed using per-protocol (PP) analysis and intention-to-treat (ITT) analysis, and the secondary efficacy indicator and subgroup analyses were performed using PP analysis. Normally distributed measurement data were analyzed using the t-test, and data that did not pass the normality test were analyzed using a non-parametric test (Mann-Whitney test). Count data were analyzed using the χ2 test. All tests were two-sided, and P < 0.05 was considered statistically significant.
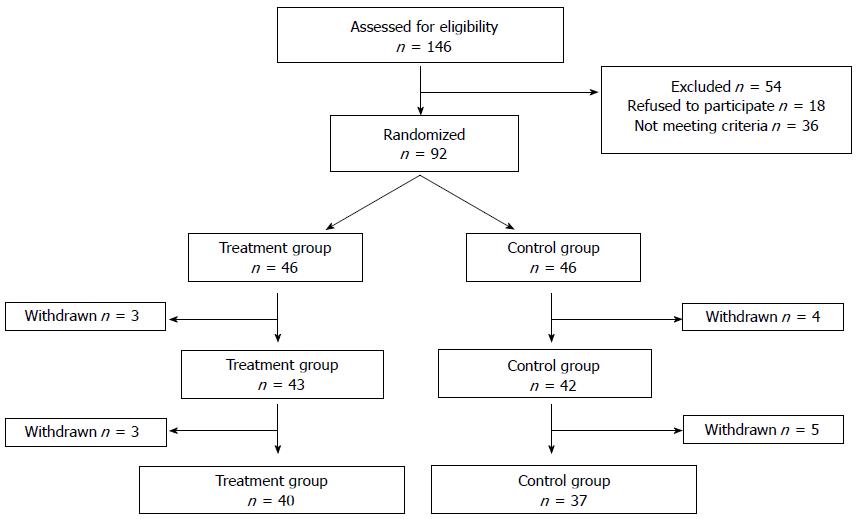
Of 146 screened patients, 26 could not be included due to CDAI scores that were too low (n = 22) or too high (n = 4), refusal to participate (n = 18), or other reasons including the use of immunosuppressive therapy or traditional Chinese drugs (n = 10). A total of 92 patients were assigned to the treatment (n = 46) and control (n = 46) groups. A total of 85 patients completed the trial: of the patients in the treatment group, 43 completed the treatment, and three did not (two patients reported work obligations and one patient traveled abroad); of the patients in the control group, 42 completed the treatment and four did not (two patients presented deteriorating conditions and received prednisone at doses > 15 mg/d, one patient became pregnant, and one quit for unknown reasons). A total of 77 patients completed the follow-up study, including 40 in the treatment group and 37 in the control group (Figure 2).
There were no statistically significant differences between the treatment group and control group in baseline data including patient age, gender, height, weight, disease duration, surgical history, disease severity, CDAI, quality of life, and Montreal classification (age at diagnosis, lesion location, and lesion type) (Table 2).
| Characteristics | Treatment group | Control group | P value1 |
| Age (yr); mean ± SD | 36.98 ± 14.46 | 32.38 ± 11.83 | 0.113 |
| Gender (male/female) | 27/16 | 25/17 | 0.757 |
| Height (cm) | 167.47 ± 9.20 | 166.71 ± 6.79 | 0.670 |
| Weight (kg) | 53.69 ± 11.73 | 53.44 ± 11.73 | 0.924 |
| Duration of disease (yr) | 4.64 ± 3.75 | 4.67 ± 4.51 | 0.978 |
| Prior resection (yes/no) | 8/35 | 4/38 | 0.229 |
| Severity (mild/moderate) | 24/19 | 30/12 | 0.135 |
| CDAI | 210.84 ± 48.03 | 201.04 ± 57.13 | 0.394 |
| IBDQ | 157.67 ± 30.43 | 157.36 ± 33.19 | 0.963 |
| HGB | 118.05 ± 17.79 | 124.79 ± 20.70 | 0.111 |
| ESR | 21.80 ± 16.86 | 24.64 ± 22.28 | 0.625 |
| CRP | 18.52 ± 24.35 | 12.32 ± 13.43 | 0.164 |
| Concomitant medication | 27 | 24 | 0.398 |
| Aminosalicylates | 16 | 11 | |
| Corticosteroids | 2 | 3 | |
| Aminosalicylates and corticosteroids | 9 | 10 | |
| Montreal classification | |||
| Age at diagnosis | |||
| A1 | 2 | 3 | 0.373 |
| A2 | 32 | 33 | |
| A3 | 9 | 6 | |
| Location | |||
| L1 | 6 | 8 | 0.251 |
| L2 | 8 | 11 | |
| L3 | 29 | 23 | |
| L4 | 0 | 0 | |
| Behavior | |||
| B1 | 12 | 9 | 0.853 |
| B2 | 2 | 2 | |
| B3 | 10 | 12 | |
| B1P | 7 | 10 | |
| B2P | 3 | 1 | |
| B3P | 9 | 8 |
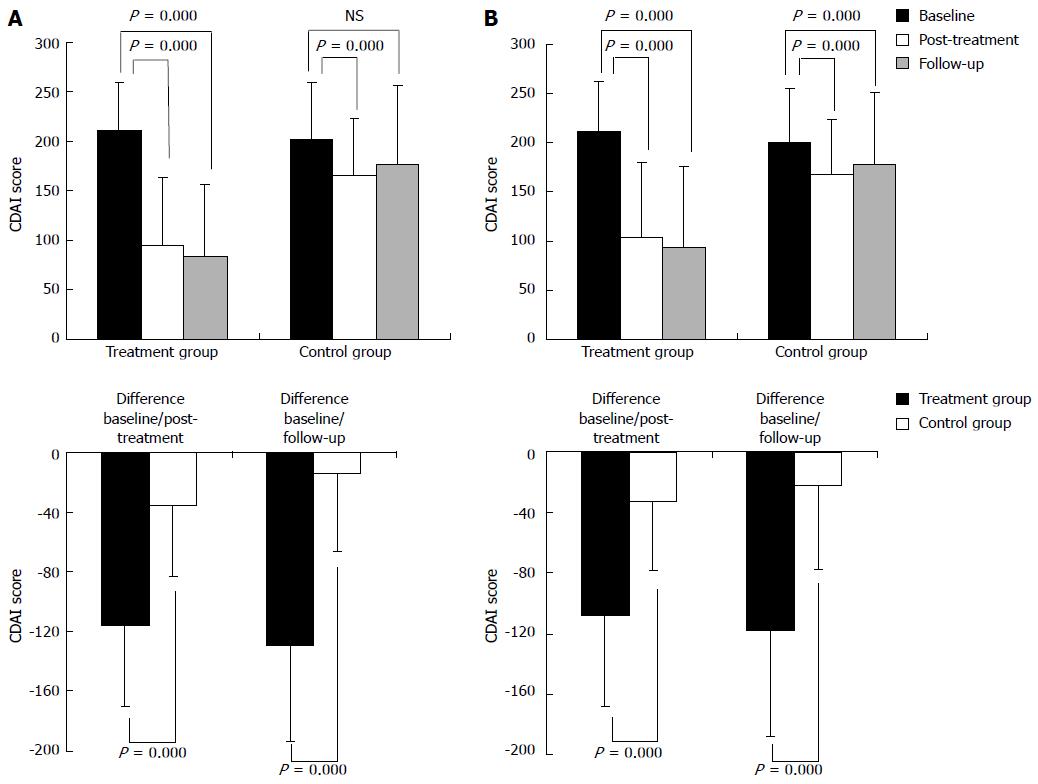
PP analysis demonstrated that the post-treatment CDAI scores of patients in the treatment group and the control group were significantly reduced compared with those measured at baseline (P = 0.000). Patients in the treatment group showed a significantly larger decrease in CDAI scores than the control group (P = 0.000). Furthermore, in the treatment group, the CDAI score during the follow-up period was significantly lower than the pre-treatment baseline (P = 0.000). Although the CDAI score of the control group was decreased during the follow-up period, the change was not statistically significant (P = 0.056). However, the difference in CDAI scores during the follow-up period between the two groups was statistically significant (P = 0.000). In both groups, changes in CDAI scores between the follow-up period and the post-treatment time were not significant (treatment group P = 0.094, control group P = 0.064) (Table 3, Figure 3A).
| Analysis set | Group | n | Baseline | Changes from baseline to post-treatment | P value1 | n | Changes from baseline to follow up | P value1 |
| PP analysis | Treatment | 43 | 210.84 ± 48.03 | -115.35 ± 55.05b | 0.000 | 40 | -128.93 ± 64.46b | 0.000 |
| Control | 42 | 201.04 ± 57.13 | -35.68 ± 46.91b | 37 | -14.32 ± 52.09 | |||
| ITT analysis | Treatment | 46 | 211.81 ± 50.97 | -107.83 ± 60.47b | 0.000 | 46 | -117.85 ± 70.10b | 0.000 |
| Control | 46 | 200.20 ± 54.91 | -32.58 ± 45.91b | 46 | -22.19 ± 55.31b |
The ITT analysis results were generally consistent with those of the PP analysis, with the exception that the ITT analysis showed a significant difference between the CDAI score of the control group during follow up and the baseline level before treatment (Table 3, Figure 3B).
Based on the principle that only a value equal to or greater than the minimal clinically important difference (MCID) has clinical significance, the MCID was determined to be 70 points on the CDAI scale[23], demonstrating that changes in the treatment group were clinically significant both after treatment and in the follow-up period, whereas changes in the control group were not clinically significant after treatment or in the follow-up period.
The PP analysis indicated that the total treatment efficacies of the treatment and control groups were 83.72% and 40.48%, respectively, with a statistically significant difference (P = 0.000). The ITT analysis indicated that the total treatment efficacies of the treatment and control groups were 78.26% and 36.96%, respectively, with a statistically significant difference (P = 0.000) (Table 4).
| Analysis set | Group | n | Clinical remission | Improved | Ineffective | P value |
| PP analysis | Treatment | 43 | 32 | 4 | 7 | 0.000 |
| Control | 42 | 15 | 2 | 25 | ||
| ITT analysis | Treatment | 46 | 32 | 4 | 10 | 0.000 |
| Control | 46 | 15 | 2 | 29 |
All post-treatment IBDQ scores of patients in the treatment and control groups were increased from the pre-treatment baseline scores, with both groups showing statistically significant intragroup differences (P = 0.000). The patients in the treatment group showed a larger increase in IBDQ score than the patients in the control group, and this difference between the groups was statistically significant (P = 0.017) (Table 5).
| Outcome measures | Group | Baseline | Post-treatment | Changes from baseline to post-treatment | P value |
| IBDQ | Treatment | 157.67 ± 30.43 | 182.23 ± 33.07b | 24.56 ± 34.15 | 0.017 |
| Control | 157.36 ± 33.19 | 167.29 ± 29.85b | 9.93 ± 19.13 | ||
| HGB | Treatment | 118.05 ± 17.79 | 123.14 ± 20.87a | 5.09 ± 14.45 | 0.029 |
| (g/L) | Control | 124.79 ± 20.70 | 123.86 ± 21.23 | -0.93 ± 10.07 | |
| ESR | Treatment | 21.80 ± 16.86 | 17.85 ± 14.14 | -3.77 ± 13.00 | 0.163 |
| (mm/h) | Control | 24.64 ± 22.28 | 24.41 ± 22.10 | -0.21 ± 10.12 | |
| CRP | Treatment | 18.52 ± 24.35 | 8.71 ± 8.94b | -8.67 ± 20.04 | 0.008 |
| (mg/L) | Control | 12.32 ± 13.43 | 13.60 ± 14.81 | 1.16 ± 12.11 | |
| CDEIS | Treatment | 9.92 ± 8.94 | 7.64 ± 6.84 | -2.28 ± 5.52 | 0.380 |
| Control | 6.15 ± 3.90 | 5.54 ± 4.79 | -0.60 ± 4.75 | ||
| HS | Treatment | 11.2 ± 1.47 | 9.00 ± 2.11b | -2.2 ± 2.21 | 0.029 |
| Control | 11.07 ± 1.44 | 10.53 ± 2.13 | -0.53 ± 1.73 |
Compared with the baseline values obtained prior to treatment, the HGB levels of the patients in the treatment group were significantly increased after treatment (P = 0.026), whereas those of the control group were not. Furthermore, the difference in HGB level between the two groups following treatment was statistically significant (P = 0.029) (Table 5).
Compared with the baseline values before treatment, the CRP levels of the patients in the treatment group were significantly reduced after treatment (P = 0.007). Moreover, the CRP levels of the control group did not change significantly, although the difference between the two groups was statistically significant (P = 0.008) (Table 5).
Compared with the baseline values before treatment, the ESR levels of the patients in the treatment and control groups were similarly decreased. However, there was no significant difference between the two groups (Table 5).
The treatment and control groups both showed reduced CDEIS scores following treatment when compared with pre-treatment scores; however, these differences were not statistically significant. Likewise, there was no significant difference between the two groups (Table 5).
Compared with pre-treatment assessment, the treatment and control groups both showed reduced histopathological scores following treatment. However, only the treatment group showed a statistically significant difference (P = 0.002), and the difference between the two groups was also statistically significant (P = 0.029) (Table 5).
We also performed subgroup analysis to assess changes in the CDAI score, which served as the primary efficacy indicator, between the two groups of patients after treatment based on the subcategories of gender (male/female), disease severity (mild/moderate), the use of corticosteroids, and lesion position classification (L1-Ileal; L2-Colonic; L3-Ileocolonic). The results showed that in all subcategories, patients in the treatment and control groups did not differ significantly in terms of pre-treatment baseline values and were thus comparable. However, the subgroup analysis comparing the patient values after treatment with the pre-treatment values demonstrated that the patients in the treatment group differed significantly from the control group in terms of CDAI score changes in all subcategories, including gender (male/female), disease severity (mild/moderate), the use of corticosteroids, and lesion position classification (L1/L2/L3) (Table 6).
| Subgroup (treatment group/control group) | Treatment group | Control group | P value1 |
| Sex | |||
| Male (27/25) | -118.84 ± 47.29 | -48.97 ± 51.95 | 0.001 |
| Female (16/17) | -109.48 ± 67.45 | -16.14 ± 30.12 | 0.001 |
| CDAI at baseline | |||
| CDAI ≤ 220 (24/30) | -110.18 ± 49.51 | -26.87 ± 43.11 | 0.001 |
| CDAI > 220 (19/12) | -121.89 ± 62.10 | -57.70 ± 50.59 | 0.005 |
| Treatment with corticosteroids | |||
| Yes (11/13) | -131.30 ± 34.25 | -28.86 ± 32.35 | 0.001 |
| No (32/29) | -109.87 ± 60.05 | -38.73 ± 52.36 | 0.001 |
| Location | |||
| L1-Ileal (6/8) | -96.77 ± 55.42 | -19.70 ± 51.42 | 0.020 |
| L2-Colonic (8/11) | -114.31 ± 34.93 | -50.29 ± 46.47 | 0.005 |
| L3-Ileocolonic (29/23) | -119.49 ± 60.00 | -34.25 ± 45.56 | 0.001 |
A total of 77 patients, including 40 patients in the treatment group and 37 patients in the control group, completed the follow-up period. Five patients (12.5%) in the treatment group relapsed, and 12 patients (32.4%) in the control group relapsed. This difference between the two groups was statistically significant (P = 0.035).
A total of two adverse events were reported (2/92) during this clinical trial: one patient in the treatment group experienced pain or subcutaneous hematoma during acupuncture, and one patient in the control group received a mild burn during moxibustion. There were no serious adverse events.
This randomized controlled study evaluated the treatment efficacy of acupuncture (herb-partitioned moxibustion combined with acupuncture) for the treatment of mild to moderate CD, using placebo acupuncture (wheat bran-partitioned moxibustion combined with superficial acupuncture) as the control. The results showed that both acupuncture and placebo acupuncture significantly reduced the CDAI score of CD patients and improved their quality of life. In addition, the efficacy of acupuncture was significantly superior to placebo acupuncture, indicating that herb-partitioned moxibustion combined with acupuncture has significant therapeutic effects in addition to the placebo effect.
After 12 wk of treatment, 74% of patients in the treatment group and 36% of patients in the control group entered remission (CDAI score < 150). After treatment, 70% of patients in the treatment group and 14% of patients in the control group showed CDAI score reductions greater than 100 points. In addition, 79% of patients in the treatment group and 21% of the control group patients demonstrated a CDAI score reduction greater than 70 points. ITT analysis of follow-up observations showed that both acupuncture and placebo acupuncture had fairly good long-term efficacies and significantly reduced CDAI scores compared with the baseline level prior to treatment. Additionally, these results revealed that the conditions of the patients were maintained at a level comparable to that immediately following treatment. The PP analysis results were similar to those of the ITT analysis, except that the PP analysis showed no significant differences in the CDAI scores of the control group measured at follow-up vs baseline, suggesting that the long-term efficacy of placebo acupuncture might not be stable. Joos et al[9] also demonstrated that both acupuncture and placebo acupuncture reduced the CDAI score of patients with mild to moderate CD and that the effect of acupuncture was significantly superior to placebo acupuncture. In addition, follow-up observations indicated that acupuncture had more stable long-term efficacy, as the placebo acupuncture group demonstrated no significant difference compared with observations recorded before treatment, consistent with the results of our study. The above results suggest that acupuncture has stable short-term and long-term effects on controlling the degree of disease activity in patients with mild to moderate CD and that these effects are clearly advantageous in comparison with placebo acupuncture.
Subgroup analysis further confirmed that acupuncture treatment was significantly more effective than placebo acupuncture in the four subgroups of gender, disease severity (mild/moderate), the use of corticosteroids, and lesion position (L1/L2/L3), suggesting that acupuncture has a broad application scope for the treatment of CD and fairly good clinical efficacy for patients in all subcategories. The results of Joos et al[9] are generally consistent with our results, with the exception of a subgroup analysis based on CDAI score. In their study, the results were negative for patients with CDAI scores < 250 and positive for patients with CDAI scores ≥ 250. In contrast, we used 220 points as the cut-off score, as this score is commonly used to classify the severity of the disease as mild or moderate. It is possible that the negative results detected in the study by Joos et al[9] may be due to this different classification.
In addition, the study by Joos et al[9] also showed that acupuncture and placebo acupuncture significantly improved patient quality of life; however, there was no significant difference between the two groups, which may have been caused by the small sample size (fewer than 60 cases) and a shorter treatment course (4 wk). In addition, different acupuncture techniques might explain why the results differ between the two studies. The present study used herb-partitioned moxibustion combined with acupuncture, whereas the study by Joos et al[9] mainly applied acupuncture with needles, and only patients exhibiting yang deficiency and chill symptoms were supplemented with moxibustion treatment. Despite the fact that both studies used acupuncture combined with moxibustion for CD treatment, the differences in moxibustion methods and acupuncture points likely resulted in different treatment effects. Anemia is a common clinical manifestation in patients with CD. We chose HGB level to reflect the impact of acupuncture on peripheral red blood cell volume in patients with CD. Our results showed that acupuncture significantly increased the HGB level of CD patients, whereas placebo acupuncture had no such effects. In addition, during the active stage, CD patients have elevated CRP and ESR levels, and elevated CRP levels, in particular, often directly reflect the degree of bowel inflammatory activity[24,25]. Our results showed that acupuncture significantly reduced CRP levels in patients with CD, whereas placebo acupuncture had no such effects. The ESR levels of patients in the treatment group showed a downward trend after treatment compared with the baseline levels before treatment, but this difference was not statistically significant. In contrast, the ESR levels in patients of the control group did not change significantly. The above results suggest that acupuncture effectively controls the inflammatory response and eases intestinal inflammation. The study by Joos et al[9] also showed that CRP levels were reduced after acupuncture treatment, but their observed difference was not statistically significant.
We also assessed the effects of acupuncture on endoscopic findings and intestinal tissue histopathological scores in patients with CD. Neither the treatment group nor the control group showed significant changes in endoscopic findings after treatment, and longer observations may be required to observe any improvements in mucosal integrity. However, the patients in the treatment group showed significant reductions in intestinal tissue histology scores after treatment, and the difference between the treatment and control groups was statistically significant. The above results suggest that acupuncture may have certain beneficial effects on improving intestinal inflammation.
TCM theory suggests that weak spleen and dominant dampness are the common pathogenic mechanisms of CD patients, who exhibit varying degrees of kidney weakness and liver stagnation. Therefore, this study mainly utilized spleen enhancement and dampness reduction supplemented with kidney augmentation, soothing the liver, and qi regulation as the main treatment principle. Herb-partitioned moxibustion is a treatment method that has been used for many years by our research team to treat diseases such as CD[10,26-28], ulcerative colitis[12,29-31], and irritable bowel syndrome[32,33]. The TCM ingredients in herbal cakes gently augment the spleen and kidney, remove dampness, and regulate qi. Thermal stimulation generated during moxa stick combustion strengthens the action of this effect. The acupuncture points used in the current treatment were based on the principles of Chinese medicine and the common pathogenesis of patients with CD. The control group in this study received wheat bran-partitioned moxibustion combined with superficial acupuncture to maintain patient blinding. In addition, the same acupoints were used in both wheat bran-partitioned moxibustion and herb-partitioned moxibustion treatments. Because the abdomen contains a relatively large number of meridians and acupoints, the local warm sensation produced by moxibustion often affects a large area of the abdomen. Therefore, although we chose a non-meridian and non-acupoint zone 1-2 cm away for the placebo group, the warm sensation likely spread to nearby acupoints, which may have been detrimental to the placebo moxibustion control. In addition, our group previously performed clinical studies on herb-partitioned moxibustion for the treatment of ulcerative colitis[12], using wheat bran-partitioned separated moxibustion as the placebo-control and the same acupoints for the two groups. The results showed that there were significant differences in the clinical efficacy between the herb-partitioned moxibustion treatment and the wheat bran-partitioned moxibustion treatment, suggesting that wheat bran-partitioned moxibustion is a fairly appropriate model for placebo moxibustion. Although both treatment methods produce thermal stimulation, different ingredients in the herbal cakes are the reasons underlying the differences in efficacy between the two groups. Moreover, we cannot rule out the effect of superficial acupuncture in the control group; this may be an important factor contributing to the effects produced in the control group in addition to the placebo effect. Although there is controversy regarding the use of superficial acupuncture as a placebo control because superficial acupuncture may produce treatment effects[34], a large number of studies have used this method[9,11,35-38]. Our study demonstrated positive safety features and high patient compliance; only two patients experienced mild adverse events, which can likely be avoided in future studies. In addition, acupuncture is highly cost-effective, representing a clear advantage in comparison with other medications for the treatment of CD[39-41].
Although our study and studies from other groups have shown that acupuncture has a positive effect as a treatment for CD, the mechanism of how acupuncture achieves its treatment efficacy is not fully understood. Our team previously conducted studies on the mechanisms of acupuncture for the treatment of CD. The results suggested that acupuncture and/or moxibustion might inhibit the abnormal expression of the inflammatory cytokines, TNF-α and TNFR1, in the colonic mucosa and peripheral blood of a rat model of CD. These changes may subsequently reduce colonic epithelial cell apoptosis, improve colonic epithelial barrier structure and function, and increase the expression of the colonic epithelial tight junction proteins occludin, claudin-1, and ZO-1 via the TNF-α/TNFR1 pathway, thereby reducing intestinal inflammation in CD model rats, restoring/protecting the colonic epithelial barrier, and ultimately achieving the goal of alleviating chronic bowel inflammation in CD[26-28].
The present study did have certain limitations. During the follow-up period, only the primary efficacy indicator, CDAI, was evaluated; the secondary efficacy indicators, such as IBDQ and laboratory parameters, were not recorded. Moreover, the patients’ anxiety and depression scale scores were not measured in the present study. Thus, future trials should consider the above limitations.
In summary, the clinical efficacy of herb-partitioned moxibustion combined with acupuncture in mild to moderate CD was significantly better than placebo acupuncture. In addition to the placebo effect, the treatment provided significant additional therapeutic effects. Thus, herb-partitioned moxibustion combined with acupuncture represents an effective and safe therapy for the clinical treatment of mild to moderate CD.
In Asia, particularly in China, the incidence of Crohn’s disease (CD) has increased steadily. Although certain medicinal treatments have been administered for CD, several disadvantages have limited their long-term clinical application. Therefore, increasing numbers of patients are seeking alternative medical therapies, particularly those involving acupuncture. However, the efficacy of acupuncture in the treatment of CD has not been fully established.
This research team has performed clinical and basic research on acupuncture and moxibustion for the treatment of inflammatory bowel diseases, including CD and ulcerative colitis, for more than 30 years. Although previous studies have demonstrated that this treatment can relieve the symptoms of CD, more observation is required to confirm its efficacy on peripheral inflammation levels, the capacity of blood cells, and intestinal endoscopic and histopathologic changes.
Herb-partitioned moxibustion and acupuncture not only reduced the disease activity index and improved quality of life, but also alleviated intestinal inflammation and improved hemoglobin levels in CD patients. Most importantly, few side effects were observed. The authors found that herb-partitioned moxibustion combined with acupuncture is an effective and safe treatment for mild to moderate CD. In addition to the placebo effect, it also provided significant therapeutic effects.
The results of the present study suggest that herb-partitioned moxibustion combined with acupuncture has the potential to be a very useful adjunct therapy for mild to moderate CD.
Herb-partitioned moxibustion is a critical component of moxibustion therapy for disease treatment. It is performed by placing a cake of herbs (dispensing a traditional Chinese medicinal formula) on the patient’s acupoints, followed by the placement and ignition of moxa cones, composed of refined mugwort floss, on the herbal cake.
This is a well-conducted study that evaluates the clinical efficacy and safety of herb-partitioned moxibustion combined with acupuncture for the treatment of active CD. The authors show the overall efficacy of the treatment was significantly greater than that of the control. In addition, the patients in the treatment group showed significantly increased hemoglobin and significantly decreased C-reactive protein levels and histopathological scores at the end of treatment, whereas the control group did not exhibit significant changes. Herb-partitioned moxibustion combined with acupuncture is therefore an effective and safe treatment method for mild and moderate CD.
| 1. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1577] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 2. | Zheng JJ, Zhu XS, Huangfu Z, Shi XH, Guo ZR. Prevalence and incidence rates of Crohn’s disease in mainland China: a meta-analysis of 55 years of research. J Dig Dis. 2010;11:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 3. | Gray WN, Denson LA, Baldassano RN, Hommel KA. Disease activity, behavioral dysfunction, and health-related quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Iglesias M, Vázquez I, Barreiro-de Acosta M, Figueiras A, Nieto L, Piñeiro M, Gómez R, Lorenzo A, Domínguez Muñoz JE. Health related quality of life in patients with Cohn´s disease in remission. Rev Esp Enferm Dig. 2010;102:624-630. [PubMed] |
| 5. | Canavan C, Abrams KR, Hawthorne B, Drossman D, Mayberry JF. Long-term prognosis in Crohn’s disease: factors that affect quality of life. Aliment Pharmacol Ther. 2006;23:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Casellas F, Vivancos JL, Sampedro M, Malagelada JR. Relevance of the phenotypic characteristics of Crohn’s disease in patient perception of health-related quality of life. Am J Gastroenterol. 2005;100:2737-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 426] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 8. | Clark M, Colombel JF, Feagan BC, Fedorak RN, Hanauer SB, Kamm MA, Mayer L, Regueiro C, Rutgeerts P, Sandborn WJ. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21-23, 2006. Gastroenterology. 2007;133:312-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 9. | Joos S, Brinkhaus B, Maluche C, Maupai N, Kohnen R, Kraehmer N, Hahn EG, Schuppan D. Acupuncture and moxibustion in the treatment of active Crohn’s disease: a randomized controlled study. Digestion. 2004;69:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 10. | Shi Y, Bao CH, Wu HG, Chen WF, Qin XD, Zhang R, Wu LY. Effects of herbs-partitioned moxibustion on the expressions of intestinal mucosa TNF-α, TNFR1, TNFR2 and apoptosis of intestinal epithelial cells in Crohn’s disease patients. Shanghai Zhongyiyao Zazhi. 2011;45:46-50. |
| 11. | Joos S, Wildau N, Kohnen R, Szecsenyi J, Schuppan D, Willich SN, Hahn EG, Brinkhaus B. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Zhou EH, Liu HR, Wu HG, Shi Z, Zhang W, Zhu Y, Shi DR, Zhou S. Down-regulation of protein and mRNA expression of IL-8 and ICAM-1 in colon tissue of ulcerative colitis patients by partition-herb moxibustion. Dig Dis Sci. 2009;54:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 13. | Park JW, Lee BH, Lee H. Moxibustion in the management of irritable bowel syndrome: systematic review and meta-analysis. BMC Complement Altern Med. 2013;13:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Anastasi JK, McMahon DJ, Kim GH. Symptom management for irritable bowel syndrome: a pilot randomized controlled trial of acupuncture/moxibustion. Gastroenterol Nurs. 2009;32:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 15. | Park YC, Kang W, Choi SM, Son CG. Evaluation of manual acupuncture at classical and nondefined points for treatment of functional dyspepsia: a randomized-controlled trial. J Altern Complement Med. 2009;15:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ma TT, Yu SY, Li Y, Liang FR, Tian XP, Zheng H, Yan J, Sun GJ, Chang XR, Zhao L. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Schneider A, Streitberger K, Joos S. Acupuncture treatment in gastrointestinal diseases: a systematic review. World J Gastroenterol. 2007;13:3417-3424. [PubMed] |
| 18. | Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology. 1979;77:843-846. [PubMed] |
| 19. | Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A, Kinnear D, Saibil F, McDonald JW. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287-296. [PubMed] |
| 20. | Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804-810. [PubMed] |
| 21. | Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 683] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 22. | D’Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 615] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 23. | Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, Present DH, Rutgeerts P, Schölmerich J, Stange EF. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Colombel JF, Solem CA, Sandborn WJ, Booya F, Loftus EV, Harmsen WS, Zinsmeister AR, Bodily KD, Fletcher JG. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Bao CH, Wu LY, Wu HG, Shi Y, Liu HR, Zhang R, Yu LQ, Wang JH. Moxibustion inhibits apoptosis and tumor necrosis factor-alpha/tumor necrosis factor receptor 1 in the colonic epithelium of Crohn’s disease model rats. Dig Dis Sci. 2012;57:2286-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Bao CH, Wu LY, Shi Y, Wu HG, Liu HR, Zhang R, Yu LQ, Wang JH. Moxibustion down-regulates colonic epithelial cell apoptosis and repairs tight junctions in rats with Crohn’s disease. World J Gastroenterol. 2011;17:4960-4970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Shi Z, Ma XP, Wu HG, Qin XD, Qian QL, Zhang W. Effect of acupuncture-moxibustion on TNF-α, sTNFR-I and sTNFR-II of rats with Crohn’s disease. J Acupunct Tuina Sci. 2009;7:29-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Wu HG, Liu HR, Tan LY, Gong YJ, Shi Y, Zhao TP, Yi Y, Yang Y. Electroacupuncture and moxibustion promote neutrophil apoptosis and improve ulcerative colitis in rats. Dig Dis Sci. 2007;52:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Wang XM, Lu Y, Wu LY, Yu SG, Zhao BX, Hu HY, Wu HG, Bao CH, Liu HR, Wang JH. Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis. World J Gastroenterol. 2012;18:6819-6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Wang X, Zhou S, Yao W, Wan H, Wu H, Wu L, Liu H, Hua X, Shi P. Effects of Moxibustion Stimulation on the Intensity of Infrared Radiation of Tianshu (ST25) Acupoints in Rats with Ulcerative Colitis. Evid Based Complement Alternat Med. 2012;2012:704584. [PubMed] |
| 32. | Zhou EH, Liu HR, Wu HG, Shi Y, Wang XM, Yao LQ, Zhong YS, Yang Y. Herb-partition moxibustion relieves chronic visceral hyperalgesia and 5-HT concentration in colon mucosa of rats. Neurol Res. 2009;31:734-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Liu HR, Yang Y, Wu HG. Clinical study on acupuncture in treating diarrhea-predominant irritable bowel syndrome. J Acupunct Tuina Sci. 2008;6:360-362. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Vincent C, Lewith G. Placebo controls for acupuncture studies. J R Soc Med. 1995;88:199-202. [PubMed] |
| 35. | Avis NE, Legault C, Coeytaux RR, Pian-Smith M, Shifren JL, Chen W, Valaskatgis P. A randomized, controlled pilot study of acupuncture treatment for menopausal hot flashes. Menopause. 2008;15:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Yeung WF, Chung KF, Tso KC, Zhang SP, Zhang ZJ, Ho LM. Electroacupuncture for residual insomnia associated with major depressive disorder: a randomized controlled trial. Sleep. 2011;34:807-815. [PubMed] |
| 37. | Haake M, Müller HH, Schade-Brittinger C, Basler HD, Schäfer H, Maier C, Endres HG, Trampisch HJ, Molsberger A. German Acupuncture Trials (GERAC) for chronic low back pain: randomized, multicenter, blinded, parallel-group trial with 3 groups. Arch Intern Med. 2007;167:1892-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 411] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 38. | Linde K, Witt CM, Streng A, Weidenhammer W, Wagenpfeil S, Brinkhaus B, Willich SN, Melchart D. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 402] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 39. | Smith JP, Bingaman SI, Ruggiero F, Mauger DT, Mukherjee A, McGovern CO, Zagon IS. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial. Dig Dis Sci. 2011;56:2088-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2447] [Article Influence: 152.9] [Reference Citation Analysis (1)] |
| 41. | D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 957] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
P- Reviewer: Arankalle DV, Liu S, Yim YK S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN