Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10440
Revised: March 28, 2014
Accepted: April 28, 2014
Published online: August 14, 2014
Processing time: 225 Days and 6.3 Hours
AIM: To investigate the effects of osteopontin (OPN) gene expression knockdown on colon cancer Lovo cells in vitro.
METHODS: Four candidate small interfering RNA (siRNA) constructs targeting the OPN gene and a scrambled control sequence (NC-siRNA) were synthesized and inserted into a pGPU6/GFP/Neo expression vector. After confirmation by restriction enzyme digestion and DNA sequencing, the recombinant plasmids were subsequently transfected into a human colon cancer cell line (Lovo) using a liposome transfection method. Stably transfected cells were maintained with G418 selection and referred to as Lovo-OPN-1, -2, -3, -4, and Lovo-NC cells. Knockdown efficiency of each of the four siRNA constructs was determined by real-time reverse transcription polymerase chain reaction assays and western blotting, and the construct with the most effective silencing was used for subsequent experiments. Cell proliferation, adhesion, and Matrigel invasion assays were performed to analyze the effects of OPN knockdown in stably transfected Lovo cells. The levels of four angiogenic factors, namely vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-2, MMP-9 and urokinase plasminogen activator were detected by enzyme-linked immunosorbent assays (ELISA).
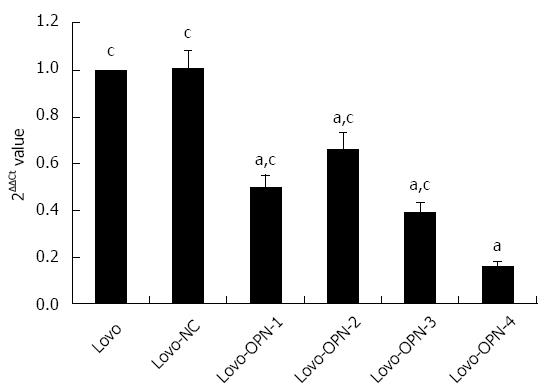
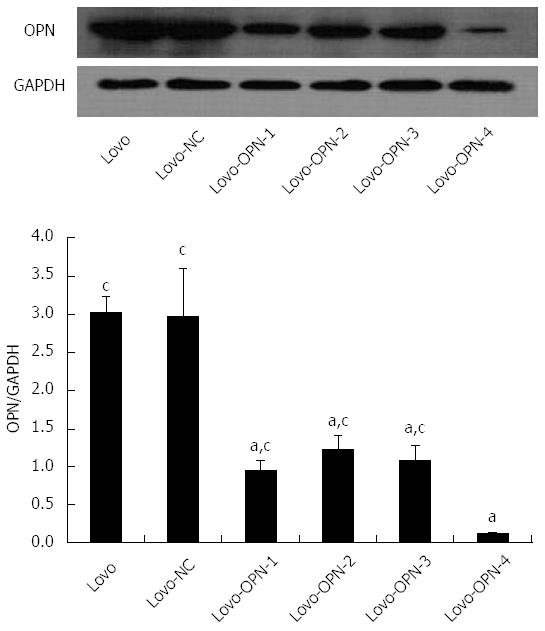
RESULTS: Recombinant vectors containing OPN-specific and scrambled siRNA sequences were successfully constructed and stably transfected into Lovo cells. Compared with the control Lovo and Lovo-NC cells, the levels of OPN mRNA and protein expression in Lovo-OPN-1, -2, -3, and -4 were significantly reduced (all P < 0.05), with the most efficient reduction observed in Lovo-OPN-4 cells (P < 0.05). Relative to untransfected Lovo cells, OPN mRNA expression levels in Lovo-NC and Lovo-OPN-4 cells were 1.008 ± 0.067 and 0.160 ± 0.023, respectively. The relative OPN protein expression levels in Lovo, Lovo-NC, and Lovo-OPN-4 cells were 3.024 ± 0.211, 2.974 ± 0.630, and 0.121 ± 0.008, respectively. Moreover, transfection with the scrambled sequence had no effect on the expression of OPN. After 24, 48, 72, and 96 h of cultivation, absorption values at 450 nm to assess proliferation of Lovo-OPN-4 cells were 0.210 ± 0.017, 0.247 ± 0.024, 0.314 ± 0.037, and 0.359 ± 0.043, respectively, which were significantly lower than those of Lovo (0.244 ± 0.031, 0.313 ± 0.024, 0.513 ± 0.048 and 0.783 ± 0.051) and Lovo-NC cells (0.241 ± 0.029, 0.309 ± 0.022, 0.563 ± 0.023, and 0.735 ± 0.067) (all P < 0.05). The absorption values at 595 nm, which were measured in a cell adhesion assay, showed that adhesion of Lovo-OPN-4 cells (0.215 ± 0.036) was significantly decreased compared to Lovo (0.490 ± 0.037) and Lovo-NC cells (0.462 ± 0.043) (P < 0.05). The number of invasive Lovo-OPN-4 cells (16.1 ± 1.9) was also significantly decreased compared to Lovo (49.9 ± 5.4) and Lovo-NC cells (48.8 ± 4.5) (P < 0.05). ELISA assays showed significant reductions in Lovo-OPN-4 cells compared to Lovo and Lovo-NC cells with regard to the expression of VEGF (1687.85 ± 167.84 ng/L vs 2348.54 ± 143.80 ng/L and 2284.39 ± 138.62 ng/L, respectively), MMP-2 (2966.07 ± 177.36 μg/L vs 4084.74 ± 349.54 μg/L and 4011.41 ± 424.48 μg/L, respectively), MMP-9 (3782.89 ± 300.64 μg/L vs 5062.90 ± 303.02 μg/L and 4986.38 ± 300.75 μg/L, respectively) and uPA (1152.69 ± 120.79 μg/L vs 1380.90 ± 147.25 μg/L and 1449.80 ± 189.92 μg/L, respectively) (all P < 0.05).
CONCLUSION: Knockdown of OPN gene expression suppresses colon cancer cell growth, adherence, invasion, and expression of angiogenic factors.
Core tip: This study investigated the anti-tumor effects of osteopontin (OPN) knockdown in human colon cancer (Lovo) cells. Despite recent evidence that shows OPN expression is closely related to the development of colorectal cancer, the mechanism remains elusive. The results of the present study showed that siRNA-mediated downregulation of OPN inhibited the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, MMP-9 and urokinase plasminogen activator in Lovo cells, which may lead to decreased invasion and angiogenesis of colon cancer.
- Citation: Wu XL, Lin KJ, Bai AP, Wang WX, Meng XK, Su XL, Hou MX, Dong PD, Zhang JJ, Wang ZY, Shi L. Osteopontin knockdown suppresses the growth and angiogenesis of colon cancer cells. World J Gastroenterol 2014; 20(30): 10440-10448
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10440
The increasing incidence of colorectal cancer has made it the third most commonly diagnosed cancer in the world, with over 1.2 million newly diagnosed cases and 608700 deaths in 2008[1]. Colon cancer presents a clinical challenge due to its asymptomatic progression, metastasis at early disease stages, and poor prognosis. Therefore, clarification of the specific mechanisms of colon cancer invasion and metastasis at the gene expression level in addition to the identification of early tumor molecular markers for screening will potentially be of important diagnostic and therapeutic value.
Osteopontin (OPN) is a secreted phosphoprotein and matricellular component involved in a variety of physical and pathologic conditions, including the progression of inflammation, angiogenesis, and carcinogenesis[2]. OPN is multi-functional, regulating both adhesion molecule expression, and cytokine secretion[3,4]. For example, when combined with integrins and CD44 cell surface receptors, OPN can reduce tumor cell adhesion, promote the hydrolysis of the extracellular matrix, and enhance tumor cell movement. Thus, OPN plays a pivotal role in cancer progression[5,6]. Although studies show that OPN expression is closely related to invasion, metastasis, angiogenesis, and prognosis of colorectal, breast, gastric, and lung cancers, the mechanisms by which OPN exerts these effects remain to be elucidated[7-11]. The aim of the present study was to evaluate the influence of OPN on carcinoma cell behavior and assess its effects on the expression of several angiogenesis-related soluble factors using Lovo cells, which constitutively express OPN[12], and small interfering RNA (siRNA)-mediated OPN knockdown.
Human colon carcinoma Lovo cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Fetal bovine serum (FBS), RPMI-1640 medium, and Dulbecco’s phosphate-buffered saline (DPBS) were obtained from HyClone of Thermo Fisher Scientific (Waltham, MA, United States). The pGPU6/GFP/Neo plasmid was purchased from GenePharma Co. (Shanghai, China), and Lipofectamine™ 2000 and TRIzol reagents were purchased from Invitrogen (of Thermo Fisher Scientific). The enzymes BbsI, BamHI, PstI, T4 DNA Ligase and the Lambda DNA/Eco130I Marker were purchased from Fermentas (of Thermo Fisher Scientific). DNA gel extraction and real-time RT-PCR kits were obtained from TIANGEN Co. (Beijing, China). Kanamycin was purchased from Sangon Co. (Shanghai, China). Plasmid DNA extraction kits were obtained from QIAGEN (Venlo, Limburg, Netherlands). G418 was purchased from AMRESCO (Solon, OH, United States). Anti-OPN antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, United States). The Cell Counting Kit-8 (CCK-8) and fibronectin (Fn) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Transwell inserts (6.5 mm polycarbonate membrane, 8.0 μm pore) were purchased from Corning Inc. (Corning, NY, United States). Matrigel was purchased from BD Biosciences (Franklin Lakes, NJ, United States). The enzyme-linked immunosorbent assay (ELISA) kits for vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-2, MMP-9, and urokinase-type plasminogen activator (uPA) were purchased from RD Systems (Minneapolis, MN, United States).
The DNA sequence of OPN was obtained from GenBank (NM_000582.2) and four candidate potential target sequences for RNA interference were scanned with the siRNA Target Finder and Design Tool available at the Ambion, Inc. website (http://www.ambion.com/techlib/misc/siRNA_finder.html). The four sequences identified (OPN-siRNA-1, -2, -3, and -4 corresponding to nucleotides 356-376, 406-426, 532-552, and 1113-1133, respectively) and control-siRNA (NC-siRNA) were designed and synthesized as follows: OPN-siRNA-1, (5’-CACCGAGTTCAATTCCAGTTGAACATTCAAGAGATGTTCAACTGGAATTGAACTCTTTTTTG-3’), OPN-siRNA-2 (5’-CACCGGGTCACTGCAATTAGACTGCTTCAAGAGAGCAGTCTAATTGCAGTGACCCTTTTTTG-3’), OPN-siRNA-3 (5’-CACCGCTGTGTCCTCTGAAGAAACCTTCAAGAGAGGTTTCTTCAGAGGACACAGCTTTTTTG-3’), OPN-siRNA-4 (5’-CACCGAGCAATGAGCATTCCGATGTTTCAAGAGAACATCGGAATGCTCATTGCTCTTTTTTG-3’), NC-siRNA (5’-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3’). All of the above sequences were inserted between the BbsI and BamHI sites of the pGPU6/GFP/Neo plasmid and are referred to as p-OPN-siRNA-1, -2, -3, -4, and p-NC-siRNA.
The human colon carcinoma Lovo cells were cultured in RPMI-1640 medium supplemented with 10% FBS and kept at 37 °C in 5% CO2 in a humidified incubator. After reaching 90%-95% confluency, Lovo cells were transfected with p-OPN-siRNA-1, -2, -3, -4, or p-NC-siRNA, referred to as Lovo-OPN-1, -2, -3, -4, and Lovo-NC, respectively, using Lipofectamine™ 2000 following the manufacturer’s instructions. Forty-eight hours later, G418 (500 mg/L) was added to the culture medium for selection of transfected cells. Single or mixed clonal populations of stably transfected cells were grown under G418 selection for at least six weeks. The expression levels of OPN in G418-resistant clones (Lovo-OPN-1, -2, -3, -4, and Lovo-NC) and normal Lovo cells (Lovo) were thereafter evaluated by real-time reverse transcription-polymerase chain reaction (RT-PCR) and western blot analysis.
Total RNA was extracted from the cells with TRIzol and reverse transcribed using real-time RT-PCR kits with primers for OPN (forward, 5’-CCTGACATCCAGTACCCTGA-3’; reverse, 5’-TCATCCAGCTGACTCGTTTC-3’; 167 bp product), and GAPDH (forward, 5’-GGGTGTGAACCATGAGAAGT-3’; reverse, 5’-GGCATGGACTGTGGTCATGA-3’; 142 bp product). Real-time PCR was performed using an Applied Biosystems 7500 system (of Thermo Fisher Scientific) with the following conditions: an initial denaturation at 94 °C for 2 min, followed by 40 cycles of denaturation at 94 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. Duplicate Ct values from experiments performed in triplicate were analyzed in Microsoft Excel (Redmond, WA, United States) using the comparative Ct (ΔΔCt) method. The expression of OPN was normalized to the levels of GAPDH and expressed relative to levels in control Lovo cells.
Cells were lysed in a buffer containing 50 mmol/L Tris-Cl, pH = 8.0, 150 mmol/L NaCl2, 0.025% NaN3, 0.1% sodium dodecyl sulfate (SDS), 100 μg/mL phenylmethanesulfonyl fluoride, 1 μg/mL aprotinin, 0.5% deoxysodium cholate, and 0.1% nonidet P-40, and centrifuged at 10000 r/min for 10 min at 4 °C. The protein concentration was determined using a BCA protein assay reagent kit (Pierce of Thermo Fisher Scientific). The cell lysates (40 μg/lane) were separated by 10% SDS-polyacrylamide gel electrophoresis, and the products were electro-transferred to polyvinylidene difluoride membranes. Blotting was performed using anti-OPN (1:200) and anti-GAPDH (1:1000) antibodies, followed by goat anti-mouse IgG-HRP (1:1000). Immunodetection was performed using SuperSignal Chemiluminescent Substrate (Pierce), and appropriate bands were quantified from exposed photographic films using ImageJ software (National Institutes of Health, Bethesda, Maryland, United States). The relative protein levels of OPN were calculated and compared with GAPDH. The experiment was performed in triplicate.
Lovo-OPN-4, Lovo-NC, and Lovo cells were seeded into 96-well culture plates at a density of 3 × 103 cells/well. Absorption values from one of four culture plates were initially determined by CCK-8 at 450 nm (A450) after 24 h cultivation, and from all plates over the following three days. The experiment was performed in triplicate.
Culture plates (96-well) were precoated with Fn (5 μg/well) and 2% bovine serum albumin (to avoid non-specific binding) in phosphate-buffered saline overnight at 4 °C. Lovo-OPN-4, Lovo-NC, and Lovo cells were cultured with serum-free medium after the cells reached 80% confluency. Twenty-four hours later, the cells were harvested and plated into the precoated culture plates (5 wells per group) at a density of 5 × 104 cells/well with serum-free medium, and allowed to incubate for 2 h at 37 °C. The medium containing dead cells was removed and adherent cells were fixed with 4% formaldehyde and stained with 0.1% crystal violet. The dye was solubilized with 33% acetic acid, and the absorption value was determined at 595 nm (A595). The experiment was performed in triplicate.
The cell invasion assay was performed using Transwell inserts coated with 50 μL of Matrigel. Lovo-OPN-4, Lovo-NC, and Lovo cells were plated (5 wells per group) into the upper compartment of the chamber at a density of 1 × 105 cells/well. The lower chamber was filled with 600 μL RPMI-1640 medium supplemented with 10% FBS. After reaching 70%-80% confluency in serum-free medium, the cells on the top surface of the membranes were removed with cotton swabs, and those that remained on the membranes were then fixed with 4% formaldehyde and stained with 0.1% crystal violet. The invasive cells which were attached to the bottom surface of the membranes were photographed and counted under a microscope at × 200 magnification. Invasive cells were counted in three fields randomly selected from each membrane. The experiment was performed in triplicate.
After reaching 70%-80% confluency, Lovo-OPN-4, Lovo-NC, and Lovo cells were harvested and plated into 6-well culture plates at a density of 4 × 105 cells/well (6 wells per group). After 24 h, the culture medium was replaced with an equivalent volume of fresh serum-free medium. Cell culture supernatants were collected 48 h later and centrifuged at 3000 r/min for 10 min, and levels of VEGF, MMP-2, MMP-9, and μPA were determined using ELISA kits. The experiment was performed in triplicate.
Statistical analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, United States). Values are presented as mean ± SD. One-way analysis of variance (ANOVA) or Kruskal-Wallis tests were used to compare mean values. Bonferroni or Mann-Whitney U tests were used to compare the differences between two groups. A probability level of 0.05 was chosen for statistical significance.
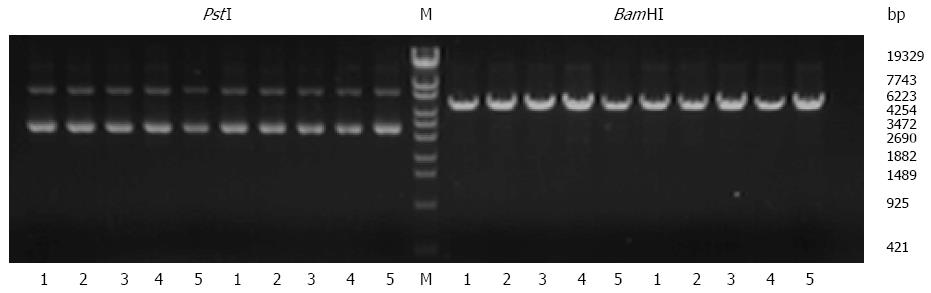
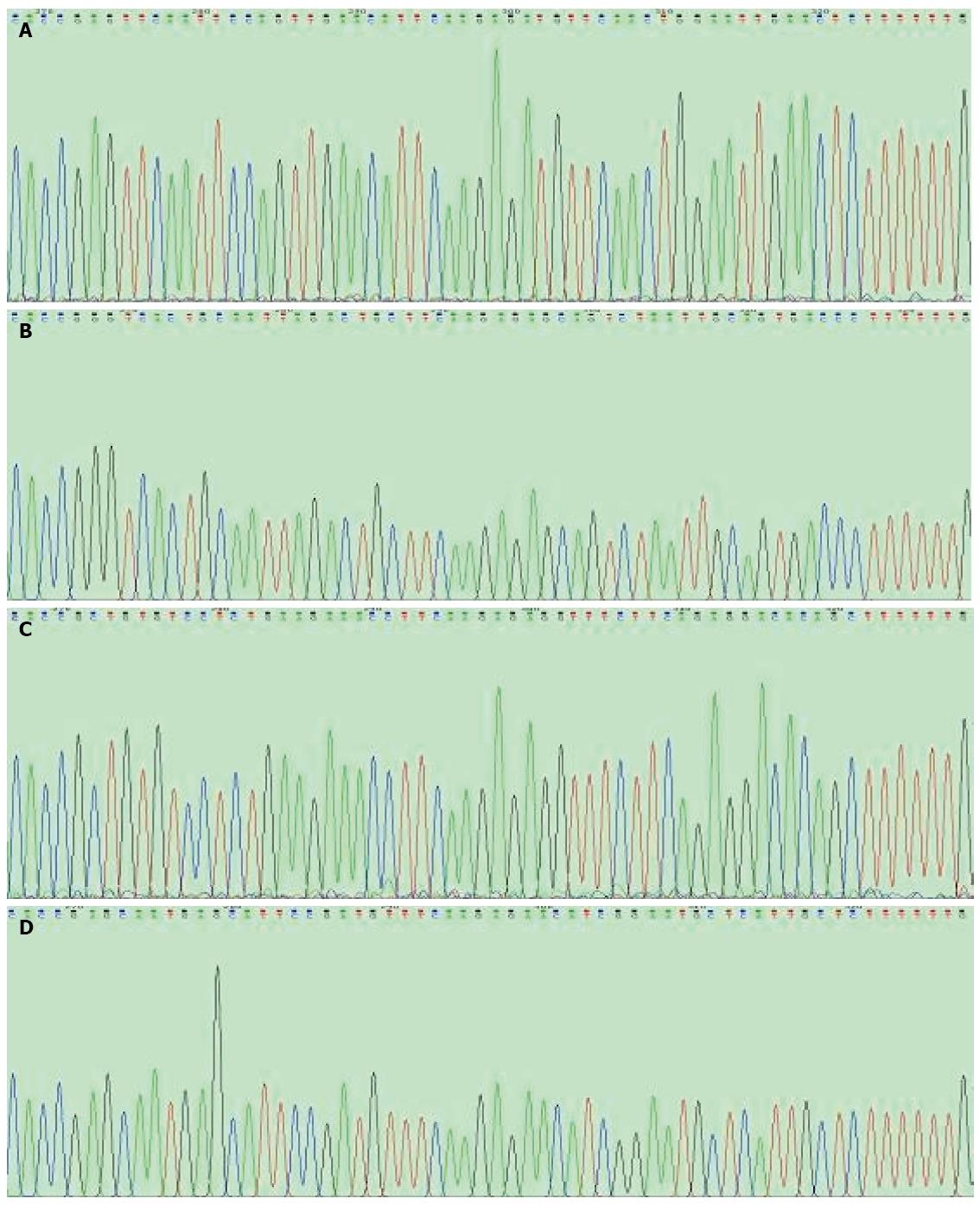
Enzyme digestion (Figure 1) and sequencing results (Figure 2) showed that all plasmids were positive for the recombinant vector and the coding sequence was inserted into the correct position with no mutations. Both real-time RT-PCR (Figure 3) and western blot (Figure 4) results showed that compared with Lovo and Lovo-NC cells, the expression levels of OPN mRNA and protein were significantly reduced in Lovo-OPN-1, -2, -3, and -4 cells (all P < 0.05), with the largest reduction observed in Lovo-OPN-4 (all P < 0.05 for RT-PCR and western blot). Relative to mRNA levels in Lovo cells, OPN levels were 1.008 ± 0.067 in Lovo-NC and 0.160 ± 0.023 in Lovo-OPN-4 cells. The relative protein expression levels in Lovo, Lovo-NC, and Lovo-OPN-4 cells were 3.024 ± 0.211, 2.974 ± 0.630, and 0.121 ± 0.008, respectively. Importantly, transfection with the scrambled sequence had no effect on OPN expression, with no significant differences in levels between Lovo-NC and Lovo cells.
The proliferation of Lovo cells was evaluated by CCK-8 analysis. The results showed that A450 values of Lovo-OPN-4 after 24, 48, 72, and 96 h of cultivation (0.210 ± 0.017, 0.247 ± 0.024, 0.314 ± 0.037, and 0.359 ± 0.043, respectively) were significantly lower than those of Lovo (0.244 ± 0.031, 0.313 ± 0.024, 0.513 ± 0.048, and 0.783 ± 0.051, respectively) and Lovo-NC cells (0.241 ± 0.029, 0.309 ± 0.022, 0.563 ± 0.023, and 0.735 ± 0.067, respectively) at each time point (all P < 0.05 compared with Lovo and Lovo-NC). In addition, there were no significant differences between Lovo-NC and Lovo.
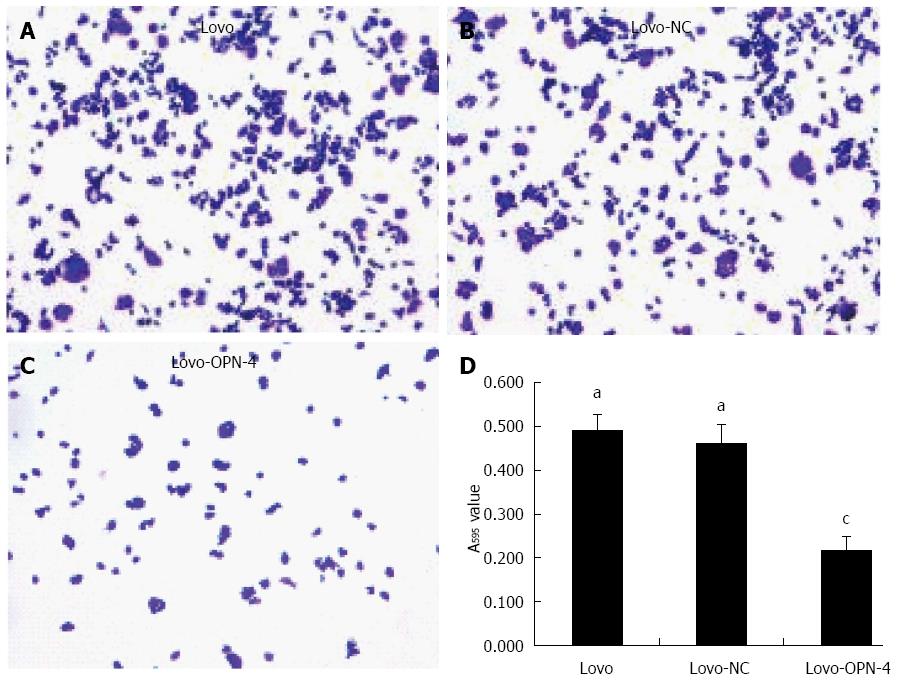
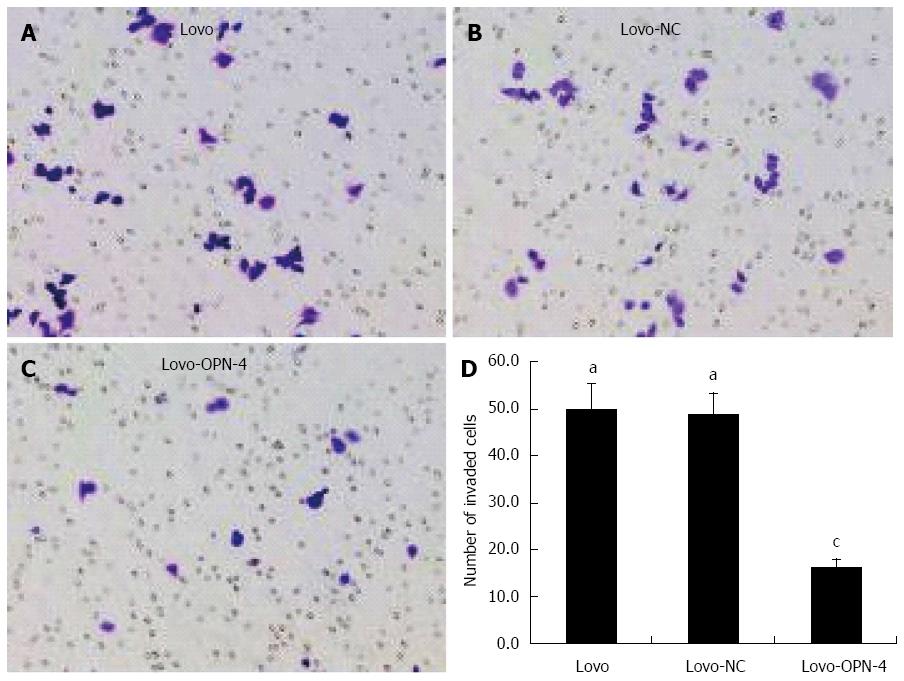
Next, Lovo cell adhesion and invasion of Matrigel-coated membranes following OPN knockdown were examined. The results show that the A595 value of adhered Lovo-OPN-4 cells (0.215 ± 0.036) was decreased significantly compared to the values for Lovo (0.490 ± 0.037) and Lovo-NC cells (0.462 ± 0.043) (P < 0.05) (Figure 5). The number of invasive Lovo-OPN-4 cells (16.1 ± 1.9) was also significantly decreased compared to the number of Lovo (49.9 ± 5.4) and Lovo-NC cells (48.8 ± 4.5) (P < 0.05) (Figure 6). There were no significant differences in adhesion and invasion values between Lovo and Lovo-NC cells.
To elucidate the possible mechanism of reduced proliferation, adhesion, and invasion by OPN suppression, the expression levels of four important tumor markers, namely VEGF, MMP-2, MMP-9, and μPA, were measured in Lovo cells using ELISA assays. The expression level of VEGF in culture supernatants from Lovo-OPN-4 cells (1687.85 ± 167.84 ng/L) was significantly lower than that in Lovo and Lovo-NC supernatants (2348.54 ± 143.80 and 2284.39 ± 138.62 ng/L, respectively) (P < 0.05). Similarly, expression of MMP-2 was lower in Lovo-OPN-4 cells (2966.07 ± 177.36) compared to Lovo and Lovo-NC cells (4084.74 ± 349.54 and 4011.41 ± 424.48 μg/L, respectively) (P < 0.05). Levels of MMP-9 were also significantly reduced in Lovo-OPN-4 cells (3782.89 ± 300.64) compared to Lovo and Lovo-NC cells (5062.90 ± 303.02 and 4986.38 ± 300.75 μg/L, respectively) (P < 0.05). Finally, μPA levels were also significantly reduced in Lovo-OPN-4 cells (1152.69 ± 120.79 μg/L vs 1380.90 ± 147.25 μg/L and 1449.80 ± 189.92 μg/L, respectively; P < 0.05). There were no significant differences between Lovo and Lovo-NC cells in any of the enzyme levels examined.
OPN is a secreted glycoprotein involved in malignant transformation. The arginine-glycine-aspartic acid (RGD)-rich sequence was first reported by Tang et al[13] in 1979, and officially named by Oldberg et al[14] in 1986. OPN can be cleaved by thrombin into an N-terminal RGD-containing domain that binds to cell surface integrin receptors, and a C-terminal domain that binds cell adhesion molecule CD44, which can induce unique intracellular signal pathways[15]. Compared to normal human intestinal epithelial cell lines, human colon cancer cell lines (HT-29, SW480, HCT-8) show high OPN expression[16]. Furthermore, Irby et al[17] demonstrated that increased endogenous OPN expression (via stable transfection) as well as exogenous OPN (added to culture medium) enhanced the motility and invasive capacity of these cell lines in vitro. Previous work from our group has shown that the proliferation, invasiveness, and mobility of oral squamous carcinoma KB cells were much higher than those of FaDu cells, which express lower amounts of OPN[18].
Tumor metastasis involves many processes, including angiogenesis, adhesion, degradation, movement, and reattachment, which involve a variety of adhesion molecules, proteolytic enzymes and cytokines[19,20]. Regarding angiogenesis, studies have shown that OPN expression is positively correlated with tumor microvessel density, and the OPN-derived peptide SVVYGLR potentiates tube formation in three-dimensional collagen gels[21,22]. This enhancement of angiogenesis may be through PI3K/AKT- and ERK-mediated pathways with VEGF acting as a positive feedback signal, as the inhibition of angiogenesis was stronger with an OPN-antibody than with a VEGF-antibody, or PI3K or ERK inhibitor[23]. Furthermore, Tang et al[13] showed that the proliferation, migration, and tube formation of human umbilical vein endothelial cells was reduced following OPN knockdown in gastric cancer cells. Additional studies have also shown that the expression of some tumor-associated genes that play an important role in metastatic processes, such as VEGF, MMP-2, MMP-9, and μPA, are closely associated with OPN[24-26], which is likely one of numerous prognostic factors related to colon cancer metastasis[27].
In this study, an OPN-siRNA-containing plasmid vector was used to knockdown expression in cancer cells to determine if OPN could be a molecular target for colon cancer therapy. Our results showed that four recombinant plasmids were successfully constructed, as assessed by enzyme digestion and sequencing. Quantification of mRNA and protein revealed that stable transfection of Lovo cells with p-OPN-siRNA-4 significantly and drastically reduced OPN levels. Furthermore, Lovo cells expressing this siRNA construct showed significantly suppressed proliferation, adhesion, and invasion. This may have been due to the low expression of CD44 and overexpression of E-cadherin that result from OPN knockdown[28,29]. The secretion of several tumor angiogenesis markers is associated with OPN in models of breast cancer and melanoma[30,31]. Importantly, the expressions of several of these markers, MMP2, MMP-9, uPA, and VEGF, were found to be significantly reduced in our experiments with OPN knockdown. Liu et al[26] have reported that OPN induces expression of MMP-2 and -9 via NF-κB-mediated signaling pathways in prostate cancer. In addition, the expression of MMP-2 and MMP-9 is correlated with angiogenesis and metastasis of colorectal cancer[32]. Thus, OPN downregulation could inhibit not only the MMPs, but also VEGF and μPA expression in colon cancer cells, which might lead to decreased invasion and angiogenesis capacity of colon cancer cells[24,33-36].
In conclusion, this study demonstrates that knockdown of OPN expression inhibits the proliferation, invasion, and adhesion of human colon cancer cells. Furthermore, the angiogenic role of OPN was confirmed, as verified by reduced expression of VEGF, MMP-2, MMP-9, and uPA in stably siRNA transfected cells. Therefore, OPN may be a potential molecular target for siRNA-targeted gene therapy of colon cancer.
Osteopontin (OPN) is a secreted multifunctional glyco-phosphoprotein that plays an important role in the tumorigenesis, tumor invasion, and metastasis of many cancer types. Recently, much interest has been focused on the biological role of OPN in the pathogenesis of angiogenesis, which may facilitate tumor progression. The mechanisms by which OPN promotes growth and angiogenesis of colon cancer remain unclear.
This study utilized selective inhibition of OPN expression using expression vector-mediated RNA interference (RNAi). This technique is highly specific and efficient, easy to control and manipulate, versatile, and time saving.
This study demonstrates that the knockdown of OPN expression inhibits human colon cancer cell proliferation, adhesion, and invasion. In addition, it demonstrated an anti-angiogenic effect of OPN downregulation, as verified by the reduced expression of the angiogenic factors vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, MMP-9, and urokinase-type plasminogen activator.
The results of the present study suggest that OPN may be a potential molecular and anti-angiogenic target for future gene therapy of colon cancer.
RNAi is a post-transcriptional process triggered by the introduction of double-stranded RNA, which leads to gene silencing in a sequence-specific manner. RNAi is rapidly becoming an important method for analyzing gene functions in eukaryotes and holds promise for the development of therapeutic gene silencing.
The results of this study provide an important contribution to the evaluation of the role of OPN in colon cancer pathogenesis. Furthermore, the results implicate OPN as a potential gene therapy target.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25606] [Article Influence: 1707.1] [Reference Citation Analysis (11)] |
| 2. | Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179-184. [PubMed] |
| 4. | Hopps L. Road to confidence. Nurs Times. 1988;84:38-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Huang J, Pan C, Hu H, Zheng S, Ding L. Osteopontin-enhanced hepatic metastasis of colorectal cancer cells. PLoS One. 2012;7:e47901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Phillips RJ, Helbig KJ, Van der Hoek KH, Seth D, Beard MR. Osteopontin increases hepatocellular carcinoma cell growth in a CD44 dependant manner. World J Gastroenterol. 2012;18:3389-3399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Sun L, Pan J, Peng L, Fang L, Zhao X, Sun L, Yang Z, Ran Y. Combination of haptoglobin and osteopontin could predict colorectal cancer hepatic metastasis. Ann Surg Oncol. 2012;19:2411-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Macrì A, Versaci A, Lupo G, Trimarchi G, Tomasello C, Loddo S, Sfuncia G, Caminiti R, Teti D, Famulari C. Role of osteopontin in breast cancer patients. Tumori. 2009;95:48-52. [PubMed] |
| 9. | Zhang X, Tsukamoto T, Mizoshita T, Ban H, Suzuki H, Toyoda T, Tatematsu M. Expression of osteopontin and CDX2: indications of phenotypes and prognosis in advanced gastric cancer. Oncol Rep. 2009;21:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Boldrini L, Donati V, Dell’Omodarme M, Prati MC, Faviana P, Camacci T, Lucchi M, Mussi A, Santoro M, Basolo F. Prognostic significance of osteopontin expression in early-stage non-small-cell lung cancer. Br J Cancer. 2005;93:453-457. [PubMed] |
| 11. | Hass HG, Nehls O, Jobst J, Frilling A, Vogel U, Kaiser S. Identification of osteopontin as the most consistently over-expressed gene in intrahepatic cholangiocarcinoma: detection by oligonucleotide microarray and real-time PCR analysis. World J Gastroenterol. 2008;14:2501-2510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Likui W, Hong W, Shuwen Z, Yuangang Y, Yan W. The potential of osteopontin as a therapeutic target for human colorectal cancer. J Gastrointest Surg. 2011;15:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Tang H, Wang J, Bai F, Hong L, Liang J, Gao J, Zhai H, Lan M, Zhang F, Wu K. Inhibition of osteopontin would suppress angiogenesis in gastric cancer. Biochem Cell Biol. 2007;85:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 212] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Oldberg A, Franzén A, Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci USA. 1986;83:8819-8823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 799] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Likui W, Hong W, Shuwen Z. Clinical significance of the upregulated osteopontin mRNA expression in human colorectal cancer. J Gastrointest Surg. 2010;14:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Irby RB, McCarthy SM, Yeatman TJ. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis. 2004;21:515-523. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Shi L, Han F, Zhang JT, Wu XL. The experimental study of the relationship of osteopontin and biological characteristics of oral carcinoma cell lines. Kouqing Yixue. 2012;32:333-335, 346. |
| 19. | Liotta LA, Mandler R, Murano G, Katz DA, Gordon RK, Chiang PK, Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci USA. 1986;83:3302-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 364] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Ishihara Y, Nishikawa T, Iijima H, Matsunaga K. Expression of matrix metalloproteinase, tissue inhibitors of metalloproteinase and adhesion molecules in silicotic mice with lung tumor metastasis. Toxicol Lett. 2003;142:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Du XL, Jiang T, Sheng XG, Gao R, Li QS. Inhibition of osteopontin suppresses in vitro and in vivo angiogenesis in endometrial cancer. Gynecol Oncol. 2009;115:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Hamada Y, Nokihara K, Okazaki M, Fujitani W, Matsumoto T, Matsuo M, Umakoshi Y, Takahashi J, Matsuura N. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem Biophys Res Commun. 2003;310:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Dai J, Peng L, Fan K, Wang H, Wei R, Ji G, Cai J, Lu B, Li B, Zhang D. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412-3422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Chen RX, Xia YH, Xue TC, Zhang H, Ye SL. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol Rep. 2011;25:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ, Tsai CH, Hsu HC, Liu SH, Tang CH. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Physiol. 2009;221:98-108. [PubMed] |
| 26. | Liu H, Chen A, Guo F, Yuan L. A short-hairpin RNA targeting osteopontin downregulates MMP-2 and MMP-9 expressions in prostate cancer PC-3 cells. Cancer Lett. 2010;295:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as colon cancer tumor progression marker. C R Biol. 2003;326:1041-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Robertson BW, Chellaiah MA. Osteopontin induces beta-catenin signaling through activation of Akt in prostate cancer cells. Exp Cell Res. 2010;316:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Smit DJ, Gardiner BB, Sturm RA. Osteonectin downregulates E-cadherin, induces osteopontin and focal adhesion kinase activity stimulating an invasive melanoma phenotype. Int J Cancer. 2007;121:2653-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Chakraborty G, Jain S, Kale S, Raja R, Kumar S, Mishra R, Kundu GC. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol Med Rep. 2008;1:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Rangaswami H, Kundu GC. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol Rep. 2007;18:909-915. [PubMed] |
| 32. | Waas ET, Wobbes T, Lomme RM, DeGroot J, Ruers T, Hendriks T. Matrix metalloproteinase 2 and 9 activity in patients with colorectal cancer liver metastasis. Br J Surg. 2003;90:1556-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Li XD, Chen J, Ruan CC, Zhu DL, Gao PJ. Vascular endothelial growth factor-induced osteopontin expression mediates vascular inflammation and neointima formation via Flt-1 in adventitial fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2250-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci. 2003;94:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Chen RX, Xia YH, Xue TC, Ye SL. Osteopontin promotes hepatocellular carcinoma invasion by up-regulating MMP-2 and uPA expression. Mol Biol Rep. 2011;38:3671-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Tilli TM, Mello KD, Ferreira LB, Matos AR, Accioly MT, Faria PA, Bellahcène A, Castronovo V, Gimba ER. Both osteopontin-c and osteopontin-b splicing isoforms exert pro-tumorigenic roles in prostate cancer cells. Prostate. 2012;72:1688-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
P- Reviewer: de Medina FS, Ghobadloo SM, Macrì A, Sadik R, Yao YM S- Editor: Qi Y L- Editor: Webster JR E- Editor: Ma S