Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.10062
Revised: March 12, 2014
Accepted: April 30, 2014
Published online: August 7, 2014
Processing time: 283 Days and 14.8 Hours
AIM: To investigate the role of nuclear translocation of calcyclin binding protein, also called Siah-1 interacting protein (CacyBP/SIP), in gastric carcinogenesis.
METHODS: The expression of CacyBP/SIP protein in gastric cancer cell lines was detected by Western blot. Immunofluorescence experiments were performed on gastric cancer cell lines that had been either unstimulated or stimulated with gastrin. To confirm the immunofluorescence findings, the relative abundance of CacyBP/SIP in nuclear and cytoplasmic compartments was assessed by Western blot. The effect of nuclear translocation of CacyBP/SIP on cell proliferation was examined using MTT assay. The colony formation assay was used to measure clonogenic cell survival. The effect of CacyBP/SIP nuclear translocation on cell cycle progression was investigated. Two CacyBP/SIP-specific siRNA vectors were designed and constructed to inhibit CacyBP/SIP expression in order to reduce the nuclear translocation of CacyBP/SIP, and the expression of CacyBP/SIP in stably transfected cells was determined by Western blot. The effect of inhibiting CacyBP/SIP nuclear translocation on cell proliferation was then assessed.
RESULTS: CacyBP/SIP protein was present in most of gastric cancer cell lines. In unstimulated cells, CacyBP/SIP was distributed throughout the cytoplasm; while in stimulated cells, CacyBP/SIP was found mainly in the perinuclear region. CacyBP/SIP nuclear translocation generated a growth-stimulatory effect on cells. The number of colonies in the CacyBP/SIP nuclear translocation group was significantly higher than that in the control group. The percentage of stimulated cells in G1 phase was significantly lower than that of control cells (69.70% ± 0.46% and 65.80% ± 0.60%, control cells and gastrin-treated SGC7901 cells, P = 0.008; 72.99% ± 0.46% and 69.36% ± 0.51%, control cells and gastrin-treated MKN45 cells, P = 0.022). CacyBP/SIPsi1 effectively down-regulated the expression of CacyBP/SIP, and cells stably transfected by CacyBP/SIPsi1 were then chosen for further cellular assays. In CacyBP/SIPsi1 stably transfected cells, CacyBP/SIP was shown to be distributed throughout the cytoplasm, irregardless of whether they were stimulated or not. After CacyBP/SIP nuclear translocation was reduced, there had no major effect on cell proliferation, as shown by MTT assay. There had no enhanced anchorage-dependent growth upon stimulation, as indicated by colony formation in flat plates. No changes appeared in the percentage of cells in G0-G1 phase in either cell line (71.09% ± 0.16% and 70.86% ± 0.25%, control cells and gastrin-treated SGC7901-CacyBP/SIPsi1 cells, P = 0.101; 74.17% ± 1.04% and 73.07% ± 1.00%, control cells and gastrin-treated MKN45-CacyBP/SIPsi1 cells, P = 0.225).
CONCLUSION: CacyBP/SIP nuclear translocation promotes the proliferation and cell cycle progression of gastric cancer cells.
Core tip: Calcyclin binding protein, also called Siah-1 interacting protein (CacyBP/SIP), is a component of the ubiquitination pathway. It can translocate to the cell nucleus. In colon cancer cells, it was found that gastrin, epidermal growth factor, prostaglandin E2 and hypoxia can induce CacyBP/SIP nuclear translocation. Our previous study found that CacyBP/SIP-positive staining was observed in the cytoplasm and nuclei of some cancer cells in gastric cancer tissues. Thus, there is considerable interest in determining whether CacyBP/SIP can translocate into the nucleus or not in gastric cancer cells, and whether nuclear translocation of CacyBP/SIP mediates the proliferation of gastric cancer cells.
- Citation: Zhai HH, Meng J, Wang JB, Liu ZX, Li YF, Feng SS. CacyBP/SIP nuclear translocation induced by gastrin promotes gastric cancer cell proliferation. World J Gastroenterol 2014; 20(29): 10062-10070
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/10062.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.10062
Calcyclin binding protein (CacyBP) was first found in the cytosolic fraction of Ehrlich ascites tumor cells, where it interacted with S100A6 (calcyclin) in the physiological range of Ca2+ concentration[1]. Further investigations showed that Siah-1 interacting protein (SIP) was a human ortholog of CacyBP[2]. Hence, calcyclin binding protein is formally named “CacyBP/SIP”. In neurons and neuroblastoma NB-2a cells, CacyBP/SIP translocated to the cell nucleus and was phosphorylated in response to changes in intracellular Ca2+ concentration induced by KCl or BAPTA/AM[3]. This phenomenon was also observed in retinoic acid-induced neuronal differentiation of neuroblastoma SH-SY5Y cells[4]. In colon cancer cells, it was showed that CacyBP/SIP can translocate to the nucleus upon stimulation by KCl or gastrin, which leads to an increase in [Ca2+][5]. However, the function of CacyBP/SIP nuclear translocation is unclear.
Our laboratory was the first to show that CacyBP/SIP is involved in the multidrug resistance of gastric cancer cells[6,7]. By using monoclonal antibodies against CacyBP/SIP produced in our laboratory[8], we found that CacyBP/SIP showed no or minimal expression in the stomach, but strong expression in cancerous gastric tissues. The positive staining was located mostly in the cytoplasm and nuclear envelope[9].
Thus, there is considerable interest in determining whether nuclear translocation of CacyBP/SIP mediates the proliferation of gastric cancer cells. We set out to address these questions in the present study, using gastrin to induce CacyBP/SIP nuclear translocation and observing the proliferation of gastric cancer cells. Our results indicate that nuclear translocation of CacyBP/SIP induced by gastrin promotes gastric cell proliferation.
Cells were cultured in RPMI 1640 (HyClone, Logan, UT) supplemented with 10% FBS (Sijiqing, China), penicillin (100 units/mL) and streptomycin (100 μg/mL). We used the following human gastric cancer cell lines, which were preserved at our institute: AGS (gastric adenocarcinoma), BGC823, MKN45, SGC7901 (poorly differentiated adenocarcinoma), KATOIII (signet ring cell carcinoma), and MKN28 (well-differentiated adenocarcinoma). Stably transfected SGC7901-CacyBP/SIPsi and MKN45-CacyBP/SIPsi cells were cultured in RPMI 1640 medium, 10% FBS and 200 μg/mL G418 (Invitrogen, Carlsbad, CA). Gastrin (Sigma, St. Louis, MO) was dissolved in RPMI 1640 and used to treat cells.
Immunofluorescence experiments were performed on gastric cancer cell lines that had been either unstimulated or stimulated with gastrin. Cells were plated onto poly-L-lysine coated coverslips and fixed with 4% paraformaldehyde. Slides were blocked with 4% sheep serum and stained with an anti-CacyBP MAb (1:10), followed by incubation with a secondary FITC-conjugated anti-mouse antibody (1:50; Santa Cruz Biotech., United States). Sections were mounted on glass slides with 20% glycerol and analyzed under a confocal laser microscope (Bio-Rad Laboratories, United States). For control experiments, cells were incubated with pre-immune serum.
Cells were seeded at 1 × 104 cells per well in 200 μL of complete culture medium in 96-well microtiter plates at 37 °C in a humidified chamber. Twenty-four hours after seeding, cells were exposed to gastrin at various concentrations (10-6, 10-7, 10-8, 10-9 and 10-10 mol/L) in serum-free culture medium for 24 and 48 h, then incubated with MTT (0.5 mg/mL) for 4 h at 37 °C. After removal of MTT, 150 μL of DMSO was added to the cells, and the absorbance values were determined at 492 nm on the enzyme-linked immunosorbent assay reader (DASIT, Milan, Italy).
The colony formation assay was used to measure clonogenic cell survival. Cells were seeded in 6-well dishes at 200 cells/well with or without 10-8 mol/L gastrin. The number of foci > 100 μm was determined after 10-14 d.
Cells were plated at a density of 50000 cells/well in 2 mL of complete RPMI1640 in 6-well plates, and allowed to grow for 24 h until 60%-70% confluence. To achieve synchronization, cells were starved in serum-free medium for 24 h. Upon return to regular growth medium, cells were treated or untreated by 10-8 mol/L gastrin. After 48 h of culture, cells were fixed overnight in 70% ethanol at 4 °C, and then re-suspended in a buffer containing propidium iodide (PI). After 30 min of incubation, cells were subjected to DNA content analysis by flow cytometry (Coulter EPICS XL) using CellQuest software. This cell cycle analysis was performed in three independent experiments.
Cells were lysed in 300 μL of freshly prepared extraction buffer [1% SDS, 1 mmol/L Na3VO4, 0.1 mol/L Tris (pH 7.4)]. To distinguish cytosolic from nuclear CacyBP/SIP, cell fractions were extracted using the NE-PER™ nuclear and cytoplasmic extraction kit (Pierce Biotechnology, Rockford, IL). Proteins were resolved at 40 μg/lane on 12% SDS-polyacrylamide gels and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) for 20-50 min at 20 V. Membranes were incubated at 4 °C overnight with one of the following primary antibodies: polyclonal antibody PARP (1:1000) (Cell Signaling Technology, Boston); and monoclonal antibodies against CacyBP/SIP (1:1000) and β-actin (1:2000) (Sigma Chemical, St. Louis, MO). Membranes were then incubated with anti-mouse IgG or anti-rabbit IgG (Amersham Biosciences, Piscataway, NJ) and were detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology Inc., Rockford, IL). For each Western blotting result, at least three independent experiments were conducted, and representative images were shown in the Results.
Two short interfering RNA (siRNA) specifically targeting the CacyBP/SIP RNA was used to reduce CacyBP/SIP expression. The siRNAs were generated with a pSilencer siRNA Construction Kit (Ambion, Austin, TX). Cell transfection was performed with Lipofectamine2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. For stable transfection, G418 (200 μg/mL) was added to cells after 24 h of transfection. Mixed clones were screened and expanded for an additional 6 wk. The target siRNA sequences were derived from the CacyBP/SIP coding region and did not reveal any overlapping regions between target sequences and other human genes. For siRNA control we used pSilencer negative control, whose sequence was not found in the mouse, human, or rat genome databases. Cells were harvested for Western blotting and cell cycle analysis.
Bands from Western blots were quantified with Quantity One software (BioRad). Relative protein levels were calculated by comparing absolute protein levels to the amount of β-actin. Numerical data are presented as mean ± SD. Calculation of the difference between means was performed with ANOVA and then a post-hoc test. All statistical analyses were performed using SPSS software (version 11.0; Chicago, IL, United States). Differences with P < 0.05 were considered statistically significant.
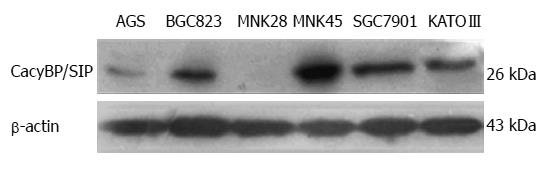
CacyBP/SIP protein was present in the AGS, BGC823, SGC7901, MKN45 and KATOIII gastric cancer cell lines but not in the MKN28 gastric cancer cell line (Figure 1). Meanwhile, an immunoreactive protein band representing CacyBP/SIP was detected in SGC7901 and MKN45 cell lines at a much higher intensity than in other gastric cancer cell lines. Thus, these two cell lines were chosen for further studies.
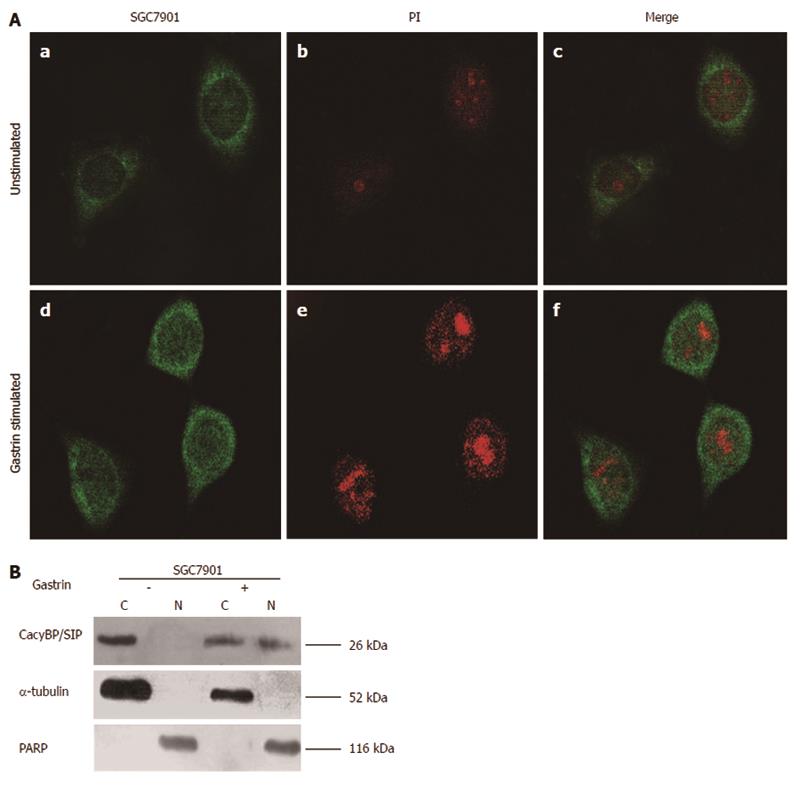
Our previous results showed that CacyBP/SIP could be detected in the nuclei of gastric cancer cells from tissue specimens. CacyBP/SIP has been reported to translocate into the nucleus after an increase in intracellular [Ca2+]i levels. Gastrin is a carcinogen of gastric cancer, and it may also induce the mobilization of intracellular Ca2+. Based on this knowledge, we speculated that gastrin might induce CacyBP/SIP nuclear translocation, so we assessed the localization of CacyBP/SIP before and after stimulation by gastrin. We observed that in unstimulated cells, CacyBP/SIP was distributed throughout the cytoplasm (Figure 2A), while in stimulated cells, CacyBP/SIP was found mainly in the perinuclear region (Figure 2A). To confirm the immunofluorescence findings, we assessed the relative abundance of CacyBP/SIP in nuclear and cytoplasmic compartments by Western blot. CacyBP/SIP was detected in both the cytoplasm and nuclei after stimulation by gastrin (10-8 mol/L), but only in the cytoplasm without gastrin stimulation (Figure 2B).
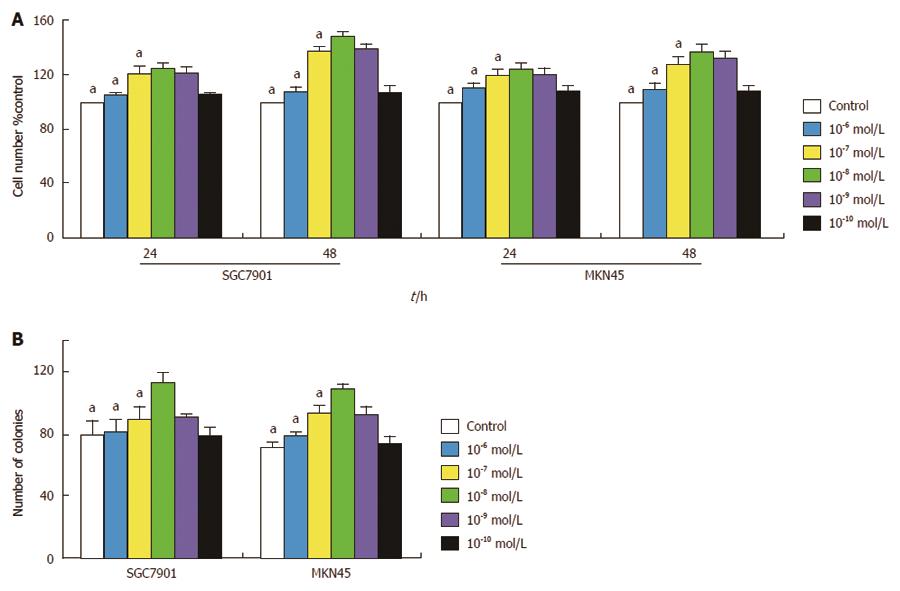
The effect of gastrin on cell proliferation was first examined. SGC7901 and MKN45 cells were treated with various concentrations of gastrin in serum-free culture medium for different time periods, and then assessed by MTT assay. As shown in Figure 3A, treatment with gastrin at any concentration (10-6, 10-7, 10-8, 10-9, and 10-10 mol/L) generated a growth-stimulatory effect on cells, which appeared to be significant at 10-8 mol/L of gastrin. We also compared the effect of gastrin (10-8 mol/L) on cell growth rates under anchorage-dependent cell growth conditions. After 10-14 d of culture in flat dishes, the number of colonies in the gastrin-treated group was significantly higher than that in the untreated group (P < 0.05; Figure 3B).
The effect of gastrin on cell cycle phase distribution was investigated in SGC7901 and MKN45 cells exposed to gastrin (10-8 mol/L) or not for 2 d. After 2 d of culture, 69.70% ± 0.46% of untreated and 65.80% ± 0.60% of gastrin-treated SGC7901 cells were found in the G1 peak, while there were 72.99% ± 0.46% of untreated and 69.36% ± 0.51% of gastrin-treated MKN45 cells in the G1 peak. In both cell lines, the analysis showed that the G1 phase of treated cells was shorter than that of the untreated cells (P < 0.05; Table 1).
| Cell line | Control | Gastrin-treated | P value |
| SGC7901 | 69.70% ± 0.46% | 65.80% ± 0.60% | 0.008 |
| MKN45 | 72.99% ± 0.46% | 69.36% ± 0.51% | 0.022 |
| SGC7901-CacyBP/SIPsi | 71.09% ± 0.16% | 70.86% ± 0.25% | 0.101 |
| MKN45-CacyBP/SIPsi | 74.17% ± 1.04% | 73.07% ± 1.00% | 0.225 |
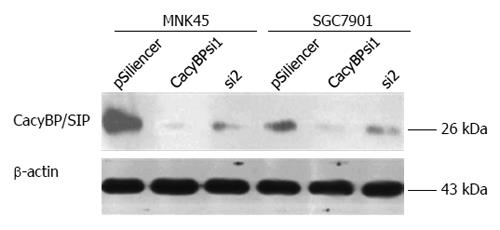
To verify that the proliferation of gastric cancer cells promoted by gastrin was induced by CacyBP/SIP nuclear translocation, we inhibited CacyBP/SIP expression to reduce the nuclear translocation of CacyBP/SIP and then observed the effect of gastrin on cell proliferation. Two CacyBP/SIP-specific siRNA vectors, named CacyBP/SIPsi1 and CacyBP/SIPsi2, were designed and constructed. After cell transfection and selection with G418 for 6 wk, the expression of CacyBP/SIP in stably transfected cells was determined by Western blot. CacyBP/SIPsi1 effectively down-regulated the expression of CacyBP/SIP in SGC7901 and MKN45 cells, while the effect of CacyBP/SIPsi2 on CacyBP/SIP expression was minimal (Figure 4). SGC7901 and MKN45 cells stably transfected with CacyBP/SIPsi1 were then chosen for further cellular assay.
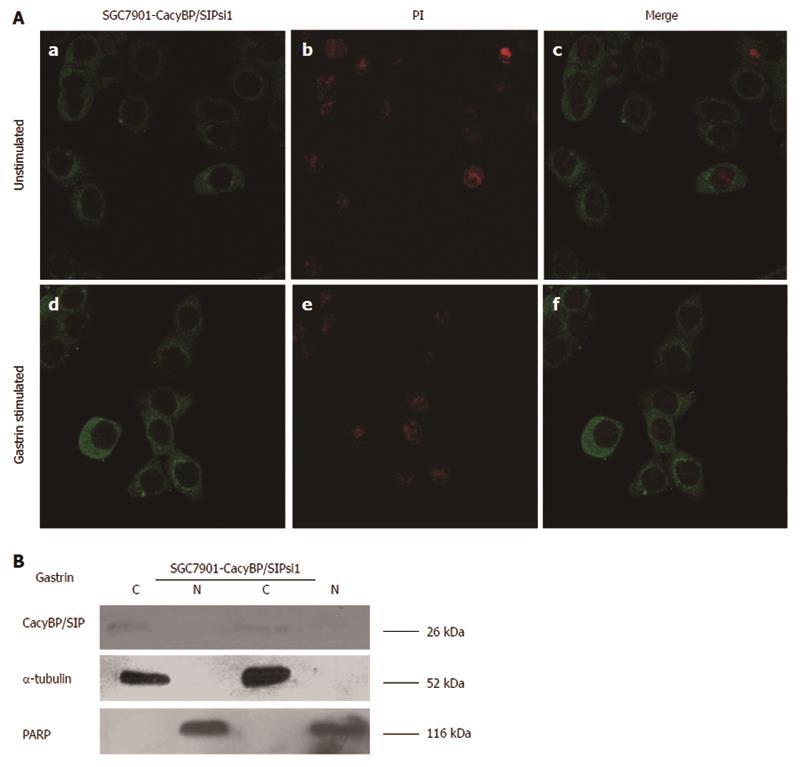
In SGC7901-CacyBP/SIPsi1 and MKN45-CacyBP/SIPsi1 cells, CacyBP/SIP was shown by immunofluorescence to be distributed throughout the cytoplasm irregardless of whether they were stimulated with gastrin (10-8 mol/L) or not (Figure 5A); this was also confirmed by Western blot (Figure 5B). This finding showed that the amount of translocated CacyBP/SIP induced by gastrin was effectively reduced after CacyBP/SIP expression was suppressed.
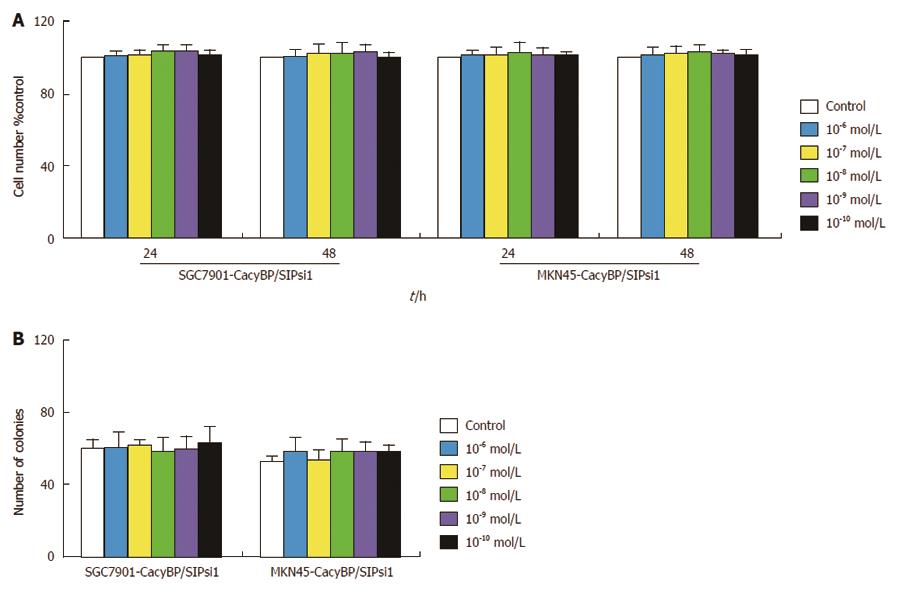
After CacyBP/SIP nuclear translocation was reduced, gastrin treatment had no major effect on cell proliferation, as shown by MTT assay (Figure 6A). In agreement with the results of the MTT assay, SGC7901-CacyBP/SIPsi1 and MKN45-CacyBP/SIPsi1 cells exhibited no enhanced anchorage-dependent growth upon stimulation with gastrin, as indicated by colony formation in flat plates (Figure 6B). After 2 d of treatment, 71.09% ± 0.16% of untreated and 70.86% ± 0.25% of gastrin-treated SGC7901-CacyBP/SIPsi1 cells were found in the G1 peak, while there were 74.17% ± 1.04% of untreated and 73.07% ± 1.00% of gastrin-treated MKN45-CacyBP/SIPsi1 cells in the G1 peak. Cell cycle analyses showed that no change appeared in the percentage of cells in G0-G1 phase in either cell line, whether untreated or treated with gastrin (P > 0.05; Table 1).
It was found that CacyBP/SIP can translocate into the nucleus, but its function is unknown. In this study, we examined the role of CacyBP/SIP nuclear translocation in gastric cancer cells. Our results suggest that nuclear translocation of CacyBP/SIP can promote the growth of gastric cancer cells.
Our previous study found that CacyBP/SIP could be translocated into the nucleus in colon cancer cells after treatment with gastrin[5], epidermal growth factor[10], prostaglandin E2[11] and hypoxia[12]. We also found that CacyBP/SIP expressed in the nuclei of cells in gastric cancer tissue, and the present study was aimed to verify this result in individual cells[9]. Using Western blot, it was found that CacyBP/SIP protein was present most of the gastric cancer cell lines. So we assessed the localization of CacyBP/SIP in gastric cancer cells before and after stimulation. CacyBP/SIP has been reported to translocate into the nucleus after an increase in intracellular [Ca2+]i levels[3,5]. Gastrin is a carcinogen of gastric cancer, and it may also induce the release of intracellular Ca2+[13,14]. Based on this knowledge, we speculated that gastrin might induce CacyBP/SIP nuclear translocation. We observed that CacyBP/SIP was detected in both the cytoplasm and nuclei after stimulation by gastrin, but only in the cytoplasm of cells without gastrin stimulation.
It is unknown whether CacyBP/SIP nuclear translocation plays a crucial role in the growth of gastric cancer cells. According to our study, CacyBP/SIP nuclear translocation promoted the proliferation of gastric cancer cells, based on the results of MTT and colony-forming assays. Inhibition of CacyBP/SIP nuclear translocation through inhibition of its expression abolished the proliferation of gastric cancer cells. Although gastrin had a proliferative effect on CacyBP/SIP siRNA-transfected cells, these cells showed no statistically significant difference in proliferation compared to control cells growing in the absence of gastrin. Taken together, these data suggest that CacyBP/SIP nuclear translocation promotes the growth of gastric cancer cells.
Inconsistent with our finding, some studies suggested that CacyBP/SIP might suppress cell growth and invasion in gastric cancer cells[15]. It was also found in renal cell carcinoma that CacyBP/SIP suppressed proliferation and tumorigenesis of renal cancer[16]. However, there were different results in breast and pancreatic cancers. CacyBP/SIP expression was significantly higher in pancreatic cancer tissue than in adjacent tissues and was associated with distal metastasis[17]. It was also found that CacyBP/SIP expression was greater in more advanced breast cancer, including metastasis[18]. These studies implicate CacyBP/SIP in tumorigenesis, although whether it promotes or suppresses cancer may depend on the cell type.
In our study, cell cycle analysis showed an increase in the number of cells in S phase at the expense of those in G1 phase. In eukaryotic cells, the transition from G1 to S is regulated mainly by the G1-specific kinases, including P27kip1. Matsuzawa firstly found function of CacyBP/SIP for thymocyte development and G1 checkpoint. It was found that SIP-/- embryonic fibroblasts showed a growth-rate increase resulting from defects in G1 arrest[19]. Recently, it was proven that CacyBP/SIP could regulate the glucose limitation-induced p27 degradation[20]. We supposed that CacyBP/SIP nuclear translocation affected the degradation of P27. This hypothesis may be proved by the finding that CacyBP/SIP could promote proliferation and G1/S transition of human pancreatic cancer cells[21].
In conclusion, the present study provides evidence that CacyBP/SIP nuclear translocation promotes the proliferation and cell cycle progression of gastric cancer cells. However, identification of the mechanism of CacyBP/SIP nuclear translocation in gastric cancer still requires further study.
Calcyclin binding protein, also called Siah-1 interacting protein (CacyBP/SIP), as a target protein of the S100 family, could translocate to the cell nucleus and was phosphorylated in response to changes in intracellular Ca2+ concentration in neurons and neuroblastoma NB-2a cells. This phenomenon was also observed in retinoic acid-induced neuronal differentiation of neuroblastoma SH-SY5Y cells. However, the function of CacyBP/SIP nuclear translocation is unclear. In colon cancer cells, it was found that CacyBP/SIP can translocate to the nucleus upon stimulation by KCl or gastrin, which leads to an increase in [Ca2+]. Further study found that epidermal growth factor, prostaglandin E2 and hypoxia also could induce CacyBP/SIP nuclear translocation. However, the role of CacyBP/SIP nuclear translocation is unknown. Their previous study found that CacyBP/SIP-positive staining was observed simultaneously in the cytoplasm and nuclei of some cancer cells. Thus, there is considerable interest in determining whether CacyBP/SIP can translocate into the nucleus or not in gastric cancer cells, and whether nucleus translocation of CacyBP/SIP mediates the proliferation of gastric cancer cells. The authors set out to address these questions in the present study, using gastrin to induce CacyBP/SIP nuclear translocation and observing the proliferation of gastric cancer cells.
Using Western blot, it was found that CacyBP/SIP protein was present in most of the gastric cancer cell lines. It was observed that CacyBP/SIP can translocate into the nucleus after stimulation by gastrin using immunofluorescence and Western blot. Further study showed that CacyBP/SIP nuclear translocation promoted the proliferation of gastric cancer cells, based on the results of MTT and colony-forming assays. Inhibition of CacyBP/SIP nuclear translocation through inhibition of its expression abolished the proliferation of gastric cancer cells. Cell cycle analysis showed an increase in the number of cells in S phase at the expense of those in G1 phase.
To explore whether CacyBP/SIP nuclear translocation promotes or suppresses growth of gastric cancer cells, Western blot was used to detect the expression of CacyBP/SIP in several gastric cancer cells; immunofluorescence and Western blot were used to observe the location of CacyBP/SIP in gastric cells stimulated by gastrin; MTT assay, colony-forming assay and cell cycle analysis were conducted to examine the effect of CacyBP/SIIP nuclear translocation on proliferation of gastric cancer cells. The study found that CacyBP/SIP nuclear translocation can promote the growth of gastric cancer cells through shortening G1 phase.
The study provides evidence that CacyBP/SIP nuclear translocation promotes the proliferation and cell cycle progression of gastric cancer cells. CacyBP/SIP may be a potential therapy target.
In this report, the authors present data demonstrating that CacyBP/SIP nuclear translocation can promote the proliferation of gastric cancer cells. It may be through affecting G1/S transition. These results may provide a therapeutic target against gastric tumorigenesis. The data are presented nicely and the findings merit publication.
| 1. | Filipek A, Kuźnicki J. Molecular cloning and expression of a mouse brain cDNA encoding a novel protein target of calcyclin. J Neurochem. 1998;70:1793-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 488] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Filipek A, Jastrzebska B, Nowotny M, Kwiatkowska K, Hetman M, Surmacz L, Wyroba E, Kuznicki J. Ca2+-dependent translocation of the calcyclin-binding protein in neurons and neuroblastoma NB-2a cells. J Biol Chem. 2002;277:21103-21109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Wu J, Tan X, Peng X, Yuan J, Qiang B. Translocation and phosphorylation of calcyclin binding protein during retinoic acid-induced neuronal differentiation of neuroblastoma SH-SY5Y cells. J Biochem Mol Biol. 2003;36:354-358. [PubMed] |
| 5. | Zhai HH, Chen X, Lu YY, Wang X, Fan DN. Expression and nucleus translcation of calcyclin binding protein in colon cancer cells. Shijie Huaren Xiaohua Zazhi. 2008;16:3953-3957. [DOI] [Full Text] |
| 6. | Liang J, Luo G, Ning X, Shi Y, Zhai H, Sun S, Jin H, Liu Z, Zhang F, Lu Y. Differential expression of calcium-related genes in gastric cancer cells transfected with cellular prion protein. Biochem Cell Biol. 2007;85:375-383. [PubMed] |
| 7. | Shi Y, Hu W, Yin F, Sun L, Liu C, Lan M, Fan D. Regulation of drug sensitivity of gastric cancer cells by human calcyclin-binding protein (CacyBP). Gastric Cancer. 2004;7:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Zhai H, Shi Y, Yu J, Hong L, Tang H, Wang J, Hu S, Bai F, Fan D. Establishment and characterization of calcyclin binding protein (CacyBP) monoclonal antibody. Hybridoma (Larchmt). 2006;25:91-94. [PubMed] |
| 9. | Zhai H, Shi Y, Jin H, Li Y, Lu Y, Chen X, Wang J, Ding L, Wang X, Fan D. Expression of calcyclin-binding protein/Siah-1 interacting protein in normal and malignant human tissues: an immunohistochemical survey. J Histochem Cytochem. 2008;56:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Xie FL, Zhai HH. Effects of epidermal growth factor on the nuclear translocation of CacyBP/SIP in colon cancer cells transfected by over expressed lentivirus vector of CacyBP/SIP. Shandong Yiyao. 2012;52. [DOI] [Full Text] |
| 11. | Xie FL, Zhai HH. Study on the effect of Prostaglandin E2 on nuclear translocation of CacyBP/SIP. Chongqing Yiyao. 2012;41:3020-3022. [DOI] [Full Text] |
| 12. | Xie FL, Qiu CQ, Zhao YY, Yang B, Feng SS, Zhai HH. Effect of CoCl2 on CacyBP/SIP nuclear translocation of colon carcinoma cell lines. Jichu Linchuang Yixue. 2012;32. |
| 13. | Seva C, Scemama JL, Pradayrol L, Sarfati PD, Vaysse N. Coupling of pancreatic gastrin/cholecystokinin-B (G/CCKB) receptors to phospholipase C and protein kinase C in AR4-2J tumoral cells. Regul Pept. 1994;52:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Bertrand V, Bastié MJ, Vaysse N, Pradayrol L. Inhibition of gastrin-induced proliferation of AR4-2J cells by calcium channel antagonists. Int J Cancer. 1994;56:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ning X, Sun S, Hong L, Liang J, Liu L, Han S, Liu Z, Shi Y, Li Y, Gong W. Calcyclin-binding protein inhibits proliferation, tumorigenicity, and invasion of gastric cancer. Mol Cancer Res. 2007;5:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Sun S, Ning X, Liu J, Liu L, Chen Y, Han S, Zhang Y, Liang J, Wu K, Fan D. Overexpressed CacyBP/SIP leads to the suppression of growth in renal cell carcinoma. Biochem Biophys Res Commun. 2007;356:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Chen X, Han G, Zhai H, Zhang F, Wang J, Li X, Huang S, Wang X, Fan D. Expression and clinical significance of CacyBP/SIP in pancreatic cancer. Pancreatology. 2008;8:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Wang N, Ma Q, Wang Y, Ma G, Zhai H. CacyBP/SIP expression is involved in the clinical progression of breast cancer. World J Surg. 2010;34:2545-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Fukushima T, Zapata JM, Singha NC, Thomas M, Kress CL, Krajewska M, Krajewski S, Ronai Z, Reed JC, Matsuzawa S. Critical function for SIP, a ubiquitin E3 ligase component of the beta-catenin degradation pathway, for thymocyte development and G1 checkpoint. Immunity. 2006;24:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Nagano Y, Fukushima T, Okemoto K, Tanaka K, Bowtell DD, Ronai Z, Reed JC, Matsuzawa S. Siah1/SIP regulates p27(kip1) stability and cell migration under metabolic stress. Cell Cycle. 2011;10:2592-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Chen X, Mo P, Li X, Zheng P, Zhao L, Xue Z, Ren G, Han G, Wang X, Fan D. CacyBP/SIP protein promotes proliferation and G1/S transition of human pancreatic cancer cells. Mol Carcinog. 2011;50:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
P- Reviewer: Wang NJ S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S