Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9621
Revised: January 29, 2014
Accepted: April 1, 2014
Published online: July 28, 2014
Processing time: 193 Days and 21.9 Hours
Endoscopic biopsy is essential to the proper diagnosis and treatment of gastric cancer. Unfortunately, the results of endoscopic biopsy are not always the same as what is expected based on gross endoscopic findings. The results of endoscopic biopsy can be negative for malignancy in Borrmann type IV advanced gastric cancer (AGCa) or gastric lymphoma. However, in the case of type II AGCa, repeated biopsies negative for malignancy have not been reported. A 49-year-old male patient underwent esophagogastroduodenoscopy three times due to large gastric ulcer suspected to be Borrmann type II cancer. However, three repeat endoscopic biopsies with multiple specimens showed necrosis and superficial regenerative epithelium without malignant findings. The patient underwent laparoscopic distal gastrectomy with D2 lymph node dissection. The surgical specimen revealed that the mucosal layer was completely replaced with regenerative epithelium without cancer cells.
Core tip: We present a case of Borrmann type II gastric cancer undiagnosed by three repeat endoscopic biopsies with multiple specimens due to necrosis and superficial regenerative epithelium. When endoscopic and ultrasonography findings highly suggest malignancy, operation or surgical biopsy should be considered.
- Citation: Hur J, Chang JH, Kim BK, Ko HY, Lee JH, Kim SJ, Song MA, Kim TH, Kim CW, Han SW. Undiagnosed Borrmann type II gastric cancer due to necrosis and regenerative epithelium. World J Gastroenterol 2014; 20(28): 9621-9625
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9621.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9621
Endoscopic examination and biopsy are the cornerstones of the detection of gastric cancer[1]. Endoscopic biopsy has high sensitivity, with specificity[2]. The accuracy of biopsy can be increased by multiple-specimen collection and repeated performance[3]. In rare cases, endoscopic biopsies cannot reveal malignancy in patients with gastric cancer. Certain instances of Borrmann type IV advanced gastric cancer (AGCa) and gastric lymphoma are not revealed by endoscopic biopsy[4-6]. However, in the case of type II AGCa, repeated biopsies negative for malignancy have not been reported. Herein, we report a case of Borrmann type II gastric cancer undiagnosed by three repeat endoscopic biopsies with multiple specimens and discuss the underlying reasons.
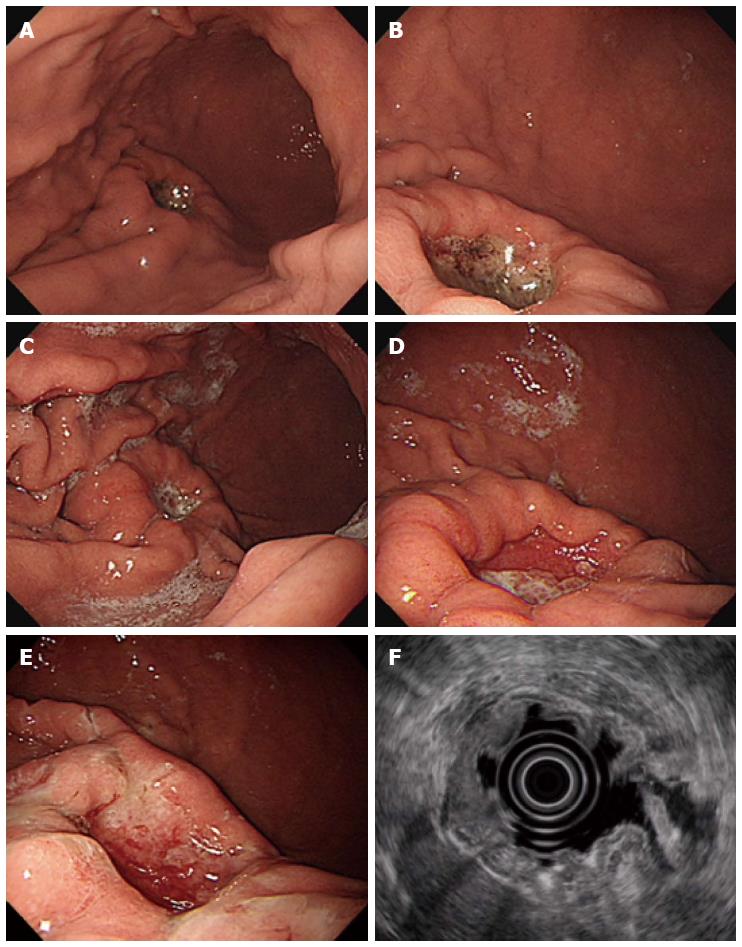
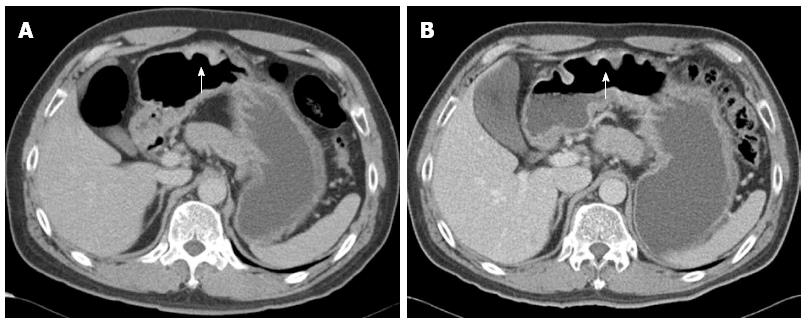
A 49-year-old male patient underwent upper gastrointestinal series at a primary clinic because he had mild epigastric discomfort. This examination showed abnormal findings that were suspicious of a gastric submucosal tumor. The patient was referred to our medical center for further evaluation. He had a past history of hypertension and benign prostatic hyperplasia. His father had a history of gastric cancer. The patient underwent esophagogastroduodenoscopy (EGD), during which a 2 cm × 2 cm-sized, deep round ulcer, with an elevated thick and fused mucosal fold at the greater curvature side of the lower body, was found (Figure 1A and B). This large ulcer was suspected to be Borrmann type II gastric cancer or, less likely, a benign gastric ulcer. Eight biopsy specimens were obtained at the margin of the ulcer and base. Abdominal computed tomography (CT) was performed, which showed a 3 cm ulcerofungating lesion at the lower body, without enlarged lymph nodes (Figure 2A). The result of endoscopic biopsy was necrosis and acute inflammation, suggesting benign ulceration without a finding of malignancy. The patient was treated with a proton pump inhibitor for three weeks and then underwent EGD again to rule out gastric malignancy. On EGD, the gastric ulcer was still noted, without discernible change (Figure 1C). Some regenerating tissue was found at the ulcer base (Figure 1D). We performed endoscopic biopsy again, collecting ten biopsy specimens at the margin of the ulcer to exclude malignancy. However, the result of biopsy was also ulceration with regenerating epithelium. Despite the negative biopsy findings, we recommended gastric surgery due to the strong potential for gastric cancer. The patient refused surgery. We had no choice but to recommend endoscopic ultrasonography (EUS) and abdominal CT three months later, with treatment with a proton pump inhibitor during the intervening period.
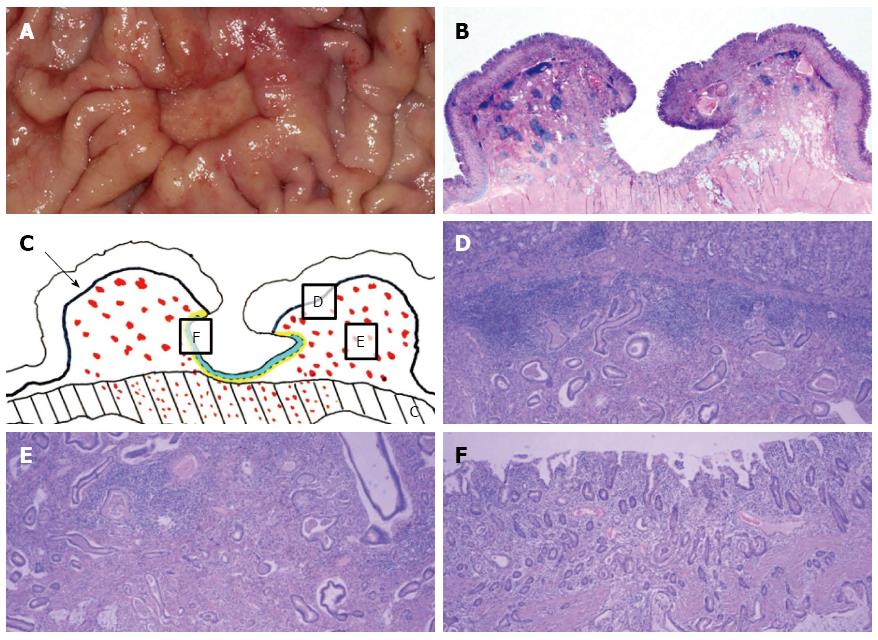
After three months, EGD/EUS and abdominal CT were performed. EGD still showed a large ulcer that had not grossly changed since the last EGD. The surrounding gastric mucosa was slightly eroded (Figure 1E). On EUS, the mucosal, submucosal and muscular layers of the stomach were involved in the ulcerative lesion, and the serosa was focally abutted (Figure 1F). Endoscopic biopsy was performed again, and five biopsy specimens were obtained. The pathologic result was ulceration with regenerative epithelium and without malignancy. Abdominal CT followed, demonstrating a 3 cm ulcerofungating lesion in the gastric body, without definitive change since the last exam, including no enlargement in the lymph nodes (Figure 2B). We strongly recommended surgery, including surgical biopsy, because malignancy was highly suspected on EUS. Finally, the patient agreed to surgery. The tumor markers CEA and CA 19-9 were present at 1.2 ng/mL and 8.16 U/mL, respectively. During the operation, a frozen biopsy specimen from the ulcer revealed malignancy. The patient underwent laparoscopic distal gastrectomy with D2 lymph node dissection. The gross specimen showed a round ulcerofungating lesion among the gastric folds with focal fold conversions (Figure 3A). Microscopic examination showed moderately differentiated tubular adenocarcinoma cells. The tumor focally penetrated the serosa, and lymphatic invasion was observed. Vascular, perineural invasion and lymph node metastasis were absent. The large ulcer was covered with superficial regenerative epithelium. Bunches of cancer cells were scattered below the regenerative mucosa (Figure 3B). The mucosa surrounding the ulcer was normal epithelium without cancer cells. The reconstruction of a cross-sectional specimen was performed (Figure 3C). Multiple cancer cells were noted in the submucosa (Figure 3D and E), muscle and serosa. However, there were no cancer cells in the mucosal layer in the ulcer, including at the margins (Figure 3F). The mucosal layer was completely replaced with regenerative epithelium without cancer cells. After surgery, the patient recovered well and was discharged. Thereafter, he received adjuvant chemotherapy and was regularly followed up at an outpatient clinic.
All gastric ulcers should be evaluated for malignancy. A study reported that less than 3% of gastric ulcers are malignant[7]. The gross characteristics suggesting malignancy are as follows: effaced, interrupted, fused or nodular mucosal folds approaching the margin of the crater[8]. In the case of suspicious malignancy, multiple biopsies should be performed, and short-term follow-up endoscopy should be considered if the pathologic result is a negative finding. Although endoscopic biopsy has high sensitivity and high specificity[2], false-negative results can occur under several circumstances. First, the results depend on the site of biopsy. In ulcerative gastric cancer, the ulcer’s margin is considered to be a suitable place for biopsy because the ulcer base is often covered with necrotic tissue[9]. In the present case, we performed multiple biopsies at the ulcer’s margin, but these biopsies yielded a negative result due to necrosis and superficial regenerative epithelium. Second, the number and amount of biopsy specimens are important. Biopsies should be performed a sufficient number of times and in sufficient amounts. It has been reported that at least 3 or 4 biopsies from gastric cancer should be performed for a pathologic diagnosis[3]. In the present case, we performed biopsies to collect more than five specimens of ample amounts, despite the negative result. Third, the type of gastric cancer can affect the biopsy results. There have been reports concerning the difficult diagnosis of type IV AGCa and gastric lymphoma[4-6]. In type IV AGCa and in gastric lymphoma, the cancer cells can spread below the mucosal layer, so there are cases of biopsies that are negative for malignancy. However, Borrmann type II AGCa not diagnosed by repeat endoscopic biopsy with multiple specimens has not been reported. In the present case, the first biopsy result was ulceration with necrotic and inflammatory cells, and the following two biopsies indicated ulceration with regenerative epithelium.
EUS and CT can be used in the diagnosis and staging of gastric cancer. These studies occasionally show crucial findings indicating gastric cancer. Universally, EUS is the most accurate test for the diagnosis of the depth of a lesion. EUS can also be utilized for regional lymph node staging and has high sensitivity for perigastric lymph nodes. However, EUS has certain shortcomings. Lesions located in the upper third of the stomach and with a depressed morphology can be overestimated or underestimated[10,11]. Thickening of the gastric wall due to perifocal inflammatory change or the absence of the serosal layer in certain areas of the stomach induces overestimation on EUS. In contrast, microscopic infiltration can be underestimated[10]. In the present case, EUS was helpful for the diagnosis of gastric cancer, showing invasion of the third muscular layer with focal serosal irregularity. These findings strongly supported a malignant ulcer, despite pathologic inconsistency. CT is widely available and noninvasive. In evaluating lymph nodes, the accuracy of CT is slightly lower compared with the accuracy of EUS. However, CT is good for the evaluation of widely metastatic disease. When peritoneal metastasis and hematogenous metastasis are smaller than 5 mm, they may not visible on CT[12]. In the present case, the CT findings did not give us a confirmative result.
In the case of suspected gastric malignancy, endoscopic biopsy should be performed carefully, at multiple sites and abundantly. If the biopsy results do not match gross endoscopic findings, repeat biopsy is needed. However, necrosis or regenerative epithelium can hinder proper diagnosis, as in our case. Therefore, when endoscopic and EUS findings highly suggest malignancy, operation or surgical biopsy should be considered.
Borrmann type II gastric cancer undiagnosed by three repeat endoscopic biopsies.
Borrmann type II gastric cancer.
Benign gastric ulcer.
Tumor markers were normal.
Esophagogastroduodenoscopy showed a 2 cm x 2 cm-sized, deep round ulcer, with an elevated thick and fused mucosal fold at the greater curvature side of the lower body. Computed tomography (CT) showed a 3 cm ulcerofungating lesion at the lower body, without enlarged lymph nodes. Endoscopic ultrasonography showed the mucosal, submucosal and muscular layer of the stomach were involved in the ulcerative lesion, and the serosa was focally abutted.
Surgical specimen showed moderately differentiated tubular adenocarcinoma cells. The tumor focally penetrated the serosa, and lymphatic invasion was observed. Vascular, perineural invasion and lymph node metastasis were absent. The large ulcer was covered with superficial regenerative epithelium.
The patient underwent laparoscopic distal gastrectomy with D2 lymph node dissection.
Necrosis or regenerative epithelium can hinder proper diagnosis of ulcerative gastric cancer.
Interesting and well written case report. No great new news as the approach to unresolving ulcer is operation. Nice pictures including CT and the pathology that is very informative.
| 1. | Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers (Basel). 2013;5:48-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Tatsuta M, Iishi H, Okuda S, Oshima A, Taniguchi H. Prospective evaluation of diagnostic accuracy of gastrofiberscopic biopsy in diagnosis of gastric cancer. Cancer. 1989;63:1415-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Choi Y, Choi HS, Jeon WK, Kim BI, Park DI, Cho YK, Kim HJ, Park JH, Sohn CI. Optimal number of endoscopic biopsies in diagnosis of advanced gastric and colorectal cancer. J Korean Med Sci. 2012;27:36-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Ahn JB, Ha TK, Lee HR, Kwon SJ. An Insufficient Preoperative Diagnosis of Borrmann Type 4 Gastric Cancer in Spite of EMR. J Gastric Cancer. 2011;11:59-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kim GH, Choi BG, Lee JN, Park SH, Lee BE, Ryu DY, Song GA, Park do Y. [2 cases of gastric mucosa-associated lymphoid tissue lymphoma presenting as a submucosal tumor-like lesion]. Korean J Gastroenterol. 2010;56:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Song W, Chen CY, Xu JB, Ye JN, Wang L, Chen CQ, Zhang XH, Cai SR, Zhan WH, He YL. Pathological diagnosis is maybe non-essential for special gastric cancer: case reports and review. World J Gastroenterol. 2013;19:3904-3910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 446] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 8. | Liu SH. Gastric Ulcer and Ulcerative Gastric Cancer. Korean Soc Gastrointest Endosc. 2011;42:27-30. |
| 9. | Hatfield AR, Slavin G, Segal AW, Levi AJ. Importance of the site of endoscopic gastric biopsy in ulcerating lesions of the stomach. Gut. 1975;16:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Park JM, Ahn CW, Yi X, Hur H, Lee KM, Cho YK, Han SU. Efficacy of endoscopic ultrasonography for prediction of tumor depth in gastric cancer. J Gastric Cancer. 2011;11:109-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Tsendsuren T, Jun SM, Mian XH. Usefulness of endoscopic ultrasonography in preoperative TNM staging of gastric cancer. World J Gastroenterol. 2006;12:43-47. [PubMed] |
| 12. | Moschetta M, Ianora AAS, Cazzato F, Scardapane A, Angelelli G. The Role of Computed Tomography in the Imaging of Gastric Carcinoma. Management of Gastric Cancer. Croatia: InTech 2011; . [DOI] [Full Text] |
P- Reviewer: Carter D, Chavalitdhamrong D S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH