Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9570
Revised: February 25, 2014
Accepted: April 30, 2014
Published online: July 28, 2014
Processing time: 208 Days and 2.2 Hours
AIM: To evaluate the safety and clinical application of high-intensity focused ultrasound (HIFU) therapy for unresectable pancreatic cancer (PC).
METHODS: Thirty PC patients (16 cases in stage III and 14 cases in stage IV) with visualized pancreatic tumors were admitted for HIFU therapy as an optional local therapy in addition to systemic chemotherapy or chemoradiotherapy. Informed consent was obtained. This study began at the end of 2008 and was approved by the ethics committee of our hospital [Institutional Review Board (IRB): 890]. The HIFU device used was the FEP-BY02 (Yuande Bio-Medical Engineering, Beijing, China).
RESULTS: The mean tumor size after HIFU therapy changed to 30.9 ± 1.7 mm from 31.7 ± 1.7 mm at pre-therapy. There were no significant changes in tumor size, mean number of treatment sessions (2.7 ± 0.1 mm), or mean total treatment time (2.4 ± 0.1 h). The rate of symptom relief effect was 66.7%. The effectiveness of primary lesion treatment was as follows: complete response, 0; partial response, 4; stable disease, 22; progressive disease, 4. Treatment after HIFU therapy included 2 operations, 24 chemotherapy treatments, and 4 best supportive care treatments. Adverse events occurred in 10% of cases, namely pseudocyst formation in 2 cases and mild pancreatitis development in 1. However, no severe adverse events occurred in this study.
CONCLUSION: We suggest that HIFU therapy is safe and has the potential to be a new method of combination therapy for PC.
Core tip: The results of chemotherapy and chemoradiotherapy for unresectable pancreatic cancer (PC) have not been satisfactory. Therefore, new advances in therapy are expected. High-intensity focused ultrasound (HIFU), which uses focused ultrasound energy for therapeutic methods such as tissue ablation from an external body, is a new ultrasound technology. HIFU therapy is a new technology therapy for PC. The HIFU device used in this study was the FEP-BY02 (Yuande Bio-Medical Engineering, Beijing, China), which is designed to reduce adverse events. According to the current safety trial, HIFU therapy is non-invasive and promising for clinical effectiveness. HIFU therapy has the potential to be a new method of combination therapy for PC.
- Citation: Sofuni A, Moriyasu F, Sano T, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Tanaka R, Umeda J, Tonozuka R, Honjo M, Mukai S, Fujita M, Itoi T. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J Gastroenterol 2014; 20(28): 9570-9577
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9570.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9570
Pancreatic cancer (PC) is known to have one of the poorest prognoses among malignant tumors. The yearly mortality of PC is on the increase, and is currently at more than 216000 cases[1]. Most cases of PC are only discovered (with recent advances in diagnostic imaging) at an unresectable condition, when the prognosis is poor and the 5-year survival rate is less than 1%[1].
Most PC patients who undergo common treatments such as surgery, chemotherapy, and radio-chemotherapy receive only a small benefit because of the histological and anatomical characteristics of the tumors. Treatments are expected to both increase antitumor efficacy[2-4] and improve clinical benefit response (CBR)[5]. However, the effect of chemotherapy is not sufficient for unresectable PC. Therefore, new advances in treatment are needed.
Recently, the advancement of ultrasound technology has been expanded to not only diagnostic imaging and needle biopsy for percutaneous procedures, but also directive treatment of tumors. Moreover, ultrasound technology has been developed making use of focused-ultrasound energy for therapeutic intention such as tissue ablation.
High-intensity focused ultrasound (HIFU) is a new non-invasive therapy technique for PC. HIFU has the ability to ablate the deep tissue inside the body from an external source using a HIFU system, and can induce cell destruction and tissue necrosis. Results[6] from a China report in 251 patients with advanced PC suggested that HIFU therapy could provide relief from pain and reduce the size of tumors without causing complications, thus prolonging patient survival. Moreover, some reports[7-17] from China suggested that HIFU is a useful palliative treatment for unresectable PC.
The aim of our study is to evaluate the safety and clinical application of HIFU therapy for unresectable PC.
This is a prospective single center research study using continuous registration of subjects by non-random sampling.
The trial was performed at the Department of Gastroenterology and Hepatology at Tokyo Medical University in Japan from December 2008 until December 2011 under the cancer research project group of Tokyo Medical University.
The sample size calculations by a two-tailed test indicated that approximately 30 patients would be required for evaluation of an adverse event. The settings were α = 0.05 and β = 0.20 when π0 = 0.10 and π = 0.30 (the predictive value of the complication was set at 10%). If it were more than 30%, the research would be judged to be too dangerous and halted.
Thirty patients with unresectable PC were included in this study (19 men and 11 women), which ages ranging from 44 to 85 years old (mean 64.3 ± 1.5). Patient characteristics are shown in Table 1. According to the UICC guidelines, of the 30 patients, 16 were in stage III and 14 were in stage IV. The tumor was located in the head of the pancreas in 13 cases, uncus in 4 cases, body in 9 cases, tail of the pancreas in 1 case, and residual pancreas after surgery in 3 cases. Performance status was mostly 0 to 1, as indicated in Table 1. The occasionally overlapping therapies before HIFU included 3 operations, 28 chemotherapy treatments, 4 radiation treatments, 5 interventional radiology treatments, and 1 best supportive care (BSC) treatment.
| Number of cases | 30 |
| Age (yr) | 64.3 ± 1.5 SD |
| Sex (M/F) | 9/11 |
| Performance status (0/1/2/3/4) | 18/8/ 3/1/0 |
| Location (head/uncus/body/tail/others) | 13/4/9/1/ 3 |
| Mean tumor size (mm) | 31.67 ± 1.7 SD |
| Pre-therapy (operation/chemotherapy/radiation/IVR/BSC) | 3/28/4/5/1 |
In patients with unresectable progressive PC including recurrence PC after surgery, where standard therapy was not effective or suitable, where pain control was defective, or a combination of these.
The inclusion criteria were as follows: (1) age ≥ 20 years old; (2) status of former therapy is indeterminate; (3) if previously treated with radiation therapy, the patient must wait for at least 4 wk; (4) for other therapies, a waiting period of 4 wk may be required in order to avoid affecting the study; (5) the functions of the main internal organs (heart, liver, lung, and kidney) were stable; (6) WBC ≥ 2000/μL, PLT ≥ 5 × 104/μL; (7) a survival period of at least 4 weeks was anticipated; (8) visualization of the lesion is possible by ultrasound (US); and (9) an adequate acoustic window for treatment is available and the depth of the tumor is within 2 to 10 cm from the skin surface.
All patients provided written informed consent (IC) before admission into the trial. The Institutional Review Board in our institution reviewed and approved the trial.
The exclusion criteria were as follows: (1) a known or possible malignant tumor in other internal organs; (2) a known or potential pregnancy; (3) active inflammation or infection is present; (4) obstructive jaundice is present (although acceptable after drainage); (5) serious cardiopathy or encephalopathy is present; (6) coagulopathy is present or expected, such as in cases of antiplatelet therapy or anticoagulant therapy; (7) a physician assessed a patient as being an improper subject for this research; (8) a tumor embolism was present in the inferior vena cava; and (9) a calcified lesion was present in a major artery (unless it could be avoided).
A patient who met the above criteria for this research was contacted by the diagnostic imaging committee, which then evaluated the imaging data and confirmed the patient’s suitability. When a favorable decision was made, the patient was given a registration number and entered the study.
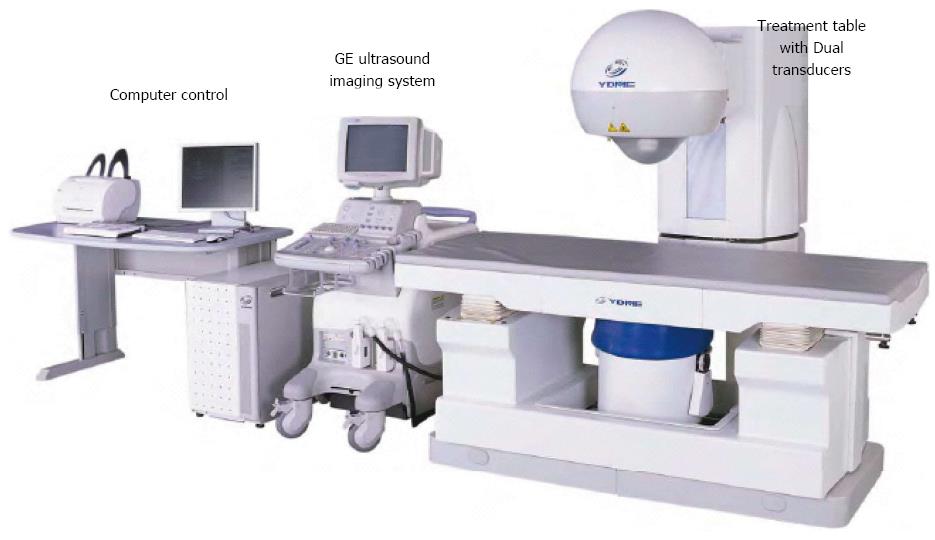
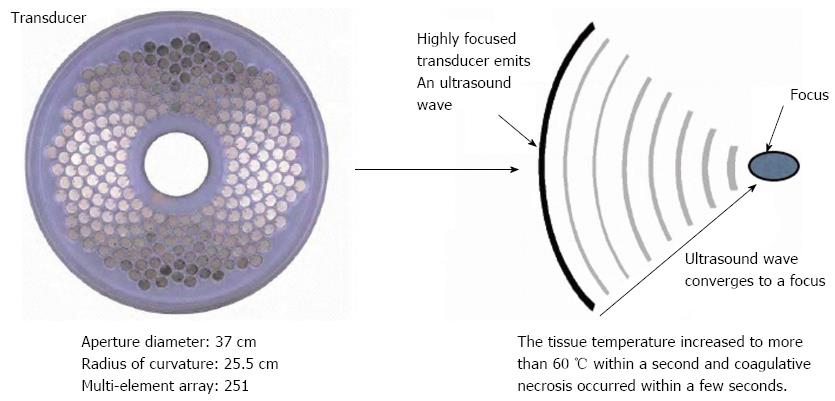
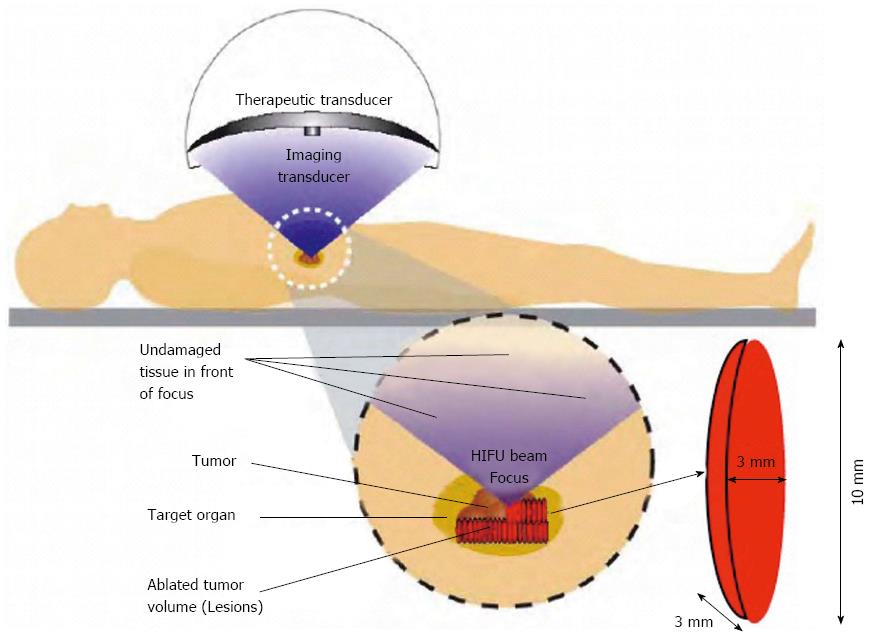
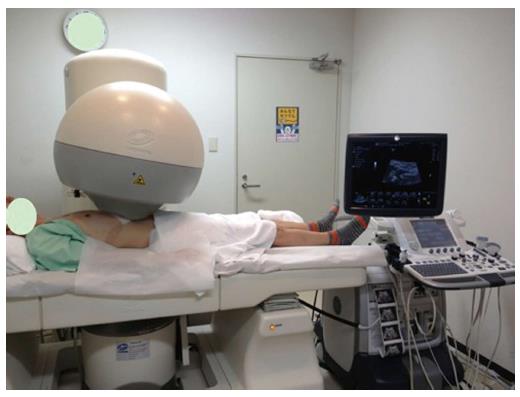
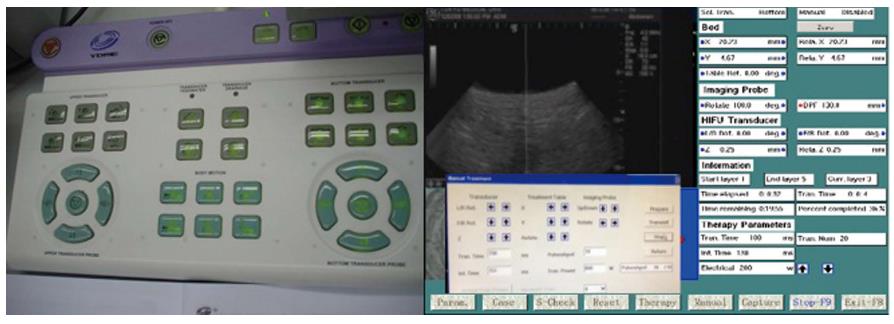
The HIFU therapeutic system used was the Model FEP-BY02 HIFU system (Yuande Bio-Medical Engineering, Beijing, China) (Figure 1). The FEP-BY Series HIFU therapeutic system has the capacity to convey HIFU from an external source deep into tissue, with a large convergence angle. The aperture of the ultrasound array is 37 cm, the radius of curvature is 25.5 cm, and there is an array of 251 elements (1.1 MHz frequency). The focus is oval in shape, with a short axis of approximately 3 mm and a long axis of 10 mm (Figures 2 and 3). There was no anesthesia or sedation required during the entire treatment procedure (Figure 4).
The position and size of the tumor and its relationship to the contiguous organ were determined by imaging ultrasonography prior to therapy. The intestinal cavity of the patients was cleansed by fasting. During therapy, the patient lied supine on the treatment table. The system has 2 transducers, with the upper transducer being used for PC. The B-mode ultrasound was used to define the target area, therapeutic range, therapeutic layers, and power. The therapy was planned and performed using a dot accumulation type (i.e., the line is formed by dots, the plane by lines, and the space by planes). After a treatment area was focused, an echo probe was scanned in accordance with the size of the tumor from front to back, from right to left, and from top to bottom; the focal zone was set up in advance. The detailed parameters as follows: (1) the distance between dots was 0.2-0.3 cm; (2) the focal area was extended 1 cm out of the tumor boarder; and (3) the distance between planes was 0.5-1.0 cm. The therapeutic probe was then moved according to the planned procedure. The number of treatments per individual patient was dependent upon tumor size.
The input electric power ranged from 0.5 to 2 kW and the effective treatment depth ranged from 2 to 15 cm. The practice focused sphere was 3 mm × 3 mm × 8 mm, whereas the effective focused sphere was 6 mm × 6 mm × 10 mm. The primary parameters for treatment and their ranges included the following: (1) input electric power (500-1350 W); (2) unit transmit time (t1) (0.1-0.2 s; (3) intermission time (t2) (0.2-0.4 s) and t2/t1 ratio (2/1); (4) total number of shots at each treatment point (1-10 times); and (5) treated layer distance (5-8 mm) and spot distance (2-5 mm). The parameters were adjusted based on the location and depth of the tumor, the density of tumor tissue, and the attenuation of the ultrasound. Therapy dose was affected by tissue attenuation. The attenuation (α) was calculated with the thickness of the abdominal wall (t) and the distance with skin and target focus (DSF). The energy of the focus was calculated with the attenuation (α), electric power (W), pulse (P), transmit time (t1), intermission time (t2), and the distance of the treatment focus; it was set at a dose of 1000 J. The shift of focus was manually moved, and the whole procedure of the treatment was automatically controlled by the computer (Figures 5 and 6).
We established an efficacy and safety evaluation decision committee and a diagnostic imaging committee to ensure a fair evaluation decision and uniformity.
The anticipated adverse events from the HIFU therapy were skin burn, pancreatitis, formation of a pancreatic pseudocyst, leakage of pancreatic and bile duct juice, intestinal perforation, gastrointestinal bleeding, tumor bleeding, hematoma formation, obstruction of the celiac and superior mesenteric arteries, and tumor dissemination. A severe adverse event would be which could lead to death, urgent open surgery, or hospitalization of one month and more. The predictive value of adverse events was set at 10%. If it reached more than 30%, the research would be stopped due to it being judged too dangerous.
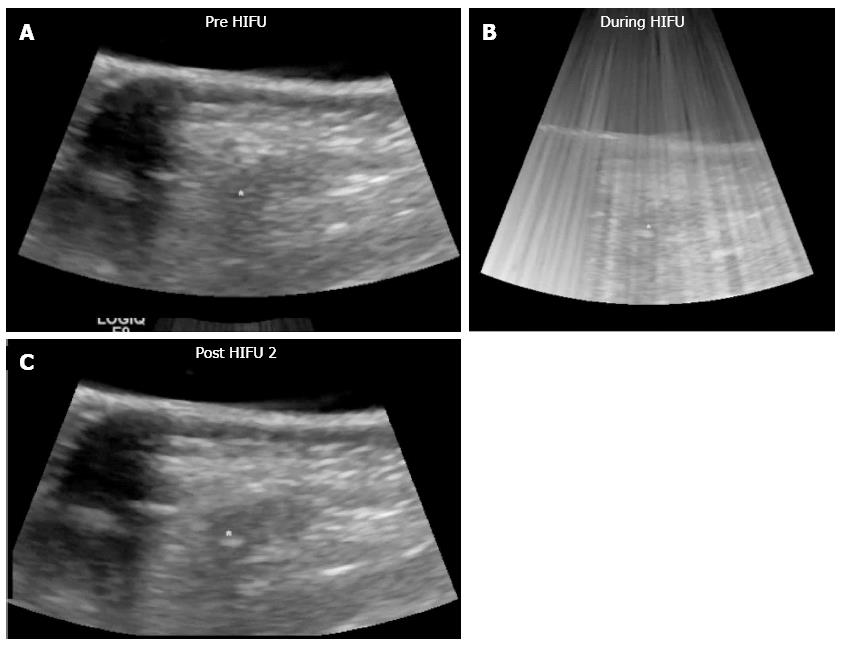
The therapeutic effect of imaging after HIFU therapy was evaluated by the change of B-mode ultrasonography (US) and computed tomography (CT) imaging; specifically the echogenicity in B-mode US and the vascularity change using contrast agent in US and CT. Complete tumor ablation was defined as when the entire tumor changed in high echo in B-mode US and/or the reduction of vascularity change in contrast-enhanced ultrasonography (CE-US) and/or CE-CT.
Evaluation of efficacy was based on WHO criteria; response to treatment was classified as complete response, partial response (PR), stable disease (SD), or progressive disease.
Evaluation of pain was based on the Numeric Rating Scale (NRS), which is the most widespread method. The NRS divides pain into 11 steps, with the degree of pain being given a number; “0” meaning “no pain” and “10” meaning “the strongest pain experienced so far”.
The primary study endpoints were to evaluate the safety of HIFU therapy for unresectable PC and to establish a HIFU therapeutic method for PC. We evaluated the following points: (1) therapeutic effects, namely changes in tumor marker (serum CA19-9 level; normal values < 37 U/mL) and imaging, including B-mode US, CE-US, CT, magnetic resonance imaging, and positron emission tomography (PET); (2) CBR, namely changes in clinical symptoms including NRS pain score (a greater than 30% improvement in pain relief was considered effective), appetite, fatigue, sleep, and weight, based on the patient’s declaration (“up” or “down”); and (3) adverse events. After the procedures, the results (e.g., symptoms, blood tests, and adverse events) on the following days were recorded. The efficacy of HIFU therapy was evaluated initially and every 3 mo after HIFU therapy. All patients were hospitalized for HIFU therapy and observation.
All pancreatic tumors were visualized by the HIFU ultrasound monitor system, and the tumor locations could be fixed. They were treated while under visualization. Clinical data is shown in Table 2. The mean total therapeutic time was short (2.4 h).
| Mean number of procedures (range) | 2.7 ± 0.1 SD |
| Mean total therapeutic time (h) per case (range) | 2.4 ± 0.1 SD |
| Rate of complete tumor ablation | 24/30 (80) |
| Rate of anesthesia/sedation administered | 0/30 (0) |
| Rate of pain killer administered | 1/30 (3.3) |
The therapeutic effect data is shown in Table 3. With regards to treatment effectiveness of the primary lesion, 4 patients achieved PR and 2 patients could receive surgery after HIFU therapy.
| Rate of pain relief effect1 (%) | 16/21 (66.7) |
| Rate of clinical benefit2 (%) | 22/30 (73.3) |
| Rate of usefulness of evaluation after HIFU | 22/30 (73.3) |
| using CE-US and/or CT/PET3 (%) | |
| Rate of half reduction in CA19-9 level (%) | 3/14 (21) |
| Mean tumor size (mm) | 30.9 ± 1.7 SD |
| Efficacy of primary lesion (CR/PR/SD/PD) | 0/4/22/4 |
| Therapy after HIFU (operation/chemotherapy/radiation/BSC) | 2/24/0/4 |
| Adverse events4 (%) | 3/30 (10) |
The CBR takes into consideration pain, appetite, fatigue, sleep, weight, and other factors. The pain relief effect rate was 66.7% and the rate of symptom relief effect was 73.3% (Table 3).
Adverse events were pseudocyst formation in 2 patients and development of mild pancreatitis in 1 patient (which occurred after 2 wk, with the patient having had a past history of pancreatitis after radiation therapy). One case of pseudocyst was treated conservatively by medication and resolved. Another case of pseudocyst was 20 mm in size and at first merely observed for a time. However, the pseudocyst was increasing in size and the patient complained of symptoms of pressure after 3 mo. Therefore, endoscopic drainage and stenting was performed. A mild pancreatitis case was treated by medication and recovered after about 2 wk. No severe adverse events occurred in this study.
HIFU is a new technique of noninvasive therapy for PC. The principle of HIFU therapy is to ablate the deep tissues inside the body from the outside using a HIFU system. HIFU can induce cell destruction, tissue necrosis, and eventually fibrosis. HIFU therapy is different from conventional hyperthermia therapy[18-20]. The results of previous animal experiment reports showed that temporary temperature was from 70 °C to 100 °C at the focal area inside the body[7,18]. According to some studies[7,17-20], HIFU could damage tumors or control the growth of tumor tissue. The effects of HIFU[7] are considered as mainly thermal and mechanical. Mechanical effects are associated with cavitation, microstreaming, and radiation forces. HIFU can biologically cause tissue heating, coagulation necrosis, and eventually fibrosis.
PC has one of the worst prognoses among malignant tumors. The difficulty in therapy is due to its fast progression and the complexity of its anatomical characteristics. The median survival time of unresectable PC is about 6 mo. In addition, the therapeutic results for unresectable PC patients who undergo common therapies such as chemotherapy and radio-chemotherapy are still not satisfactory. Moreover, such patients frequently endure severe cancer pain. HIFU therapy may be useful as an optional therapy for patients with progressive symptoms that are not candidates for surgery, and may become one of the palliative therapies for unresectable PC.
Results from a Chinese study consisting of 251 patients with unresectable PC suggested that HIFU therapy could elicit an anti-tumor effect without causing complications, and thus prolong survival[6]. Moreover, 84% of patients with pain due to advanced cancer obtained significant relief of their pain after HIFU therapy. According to several reports from China[7,8], HIFU therapy is suggested to be useful as one of the palliative therapies for unresectable PC.
Although HIFU therapy may be non-invasive and effective, this therapeutic method is a novel technique and requires further investigation. The safety of HIFU therapy for unresectable PC needs to be established; randomized controlled trials should be conducted to determine whether HIFU therapy for unresectable PC results in local tumor response or clinically beneficial outcome by improving pain relief, functional status, quality of life, or survival in the future.
The most important aspect of HIFU therapy is its clinical safety. Previous animal studies suggested that HIFU therapy was safe in treating abdominal animal tumors[7,17]. There are significant differences between animal and human bodies. Moreover, the pancreas is a highly sensitive organ which can incur serious inflammatory change from heat effects when using HIFU. The anticipated adverse events from the HIFU procedure include skin burn, development of pancreatitis, formation of a pancreatic pseudocyst, leakage of pancreatic and bile duct juice, intestinal perforation, gastrointestinal bleeding, tumor bleeding, hematoma formation, obstruction of the celiac and superior mesenteric arteries, and tumor dissemination. In our safety trial, there were 2 cases of pancreatic pseudocysts and 1 case of mild pancreatitis after 2 wk of HIFU therapy. The pancreatitis also occurred after previous radiation therapy; the patient was probably sensitive to the stimulus and the HIFU procedure may have become a trigger. The adverse events observed during this study are similar to those described in a previous report from China. No serious adverse events, such as intestinal perforation or arterial obstruction, were observed. Vigilance for these serious adverse events is needed during the therapy. Our results suggest that HIFU therapy for unresectable PC is also safe.
The required measures to prevent the aforementioned complications are described as follows. Obtaining accurate and appropriate positioning before therapy is necessary. Although the pancreas moves only slightly with each breath, it is important to observe the range of the breath movement effect in the therapeutic lesion, and establish the treatment range and tumor edge in order to prevent damage to contiguous tissues. Intestinal cleansing preparation is required before therapy, as gas and residue in the intestine will interfere with ultrasound view and positioning. Constipation needs to be prevented in order to minimize intestinal gas in the therapeutic area. Ultrasound waves could be scattered by gas, resulting in a reduction of the therapeutic effect. Scattered ultrasound waves may also damage or injure the adjacent intestines. The therapeutic target should start at the bottom and edge of the pancreatic tumor, and then move toward the upper and central areas. The appropriate target spot, area, and distance should be recognized by moving the treatment probe in 3 mm increments in the target area.
Treatment parameters chosen for this study yielded adequate tumor recession without increasing the risk for detrimental biological effects. The therapeutic effectiveness for the current study was almost comparable to the previous studies in China, although there was the discrepancy in the rate of half reduction of CA19-9 level. The tumor size was slightly reduced after HIFU therapy, but there was no significant difference. However, an interesting point is that two cases resulted in surgical operation after HIFU therapy in this study. It is expected that such cases will increase in the future. It is necessary to increase the rate of complete tumor ablation for more effective therapy. Therefore, not only our technique, but also further development of the treatment instruments is needed. As for the evaluation of therapeutic effect, it is unclear what examination is the best. Treatment effectiveness for the current study should be judged comprehensively by the relief of pain, CBR, CE-US, CT, and PET results from our study. The current study shows that HIFU therapy is safe and has the potential to be a new method of combination therapy for PC.
In conclusion, the clinical application of HIFU therapy for unresectable PC is safe, and may become one of the preferred combination therapies for PC in the future.
The authors are indebted to the Department of International Medical Communications of Tokyo Medical University for the editorial review of the English manuscript.
The results of chemotherapy and chemo-radiotherapy for unresectable pancreatic cancer (PC) have not been satisfactory. Therefore, new advances in therapy are expected.
The research frontiers are new technology therapy using ultrasound for unresectable PC.
Ultrasound technology has been developed which uses focused ultrasound energy for therapeutic intention, such as tissue ablation from an external body. The therapy to which the technique applied is High-Intensity Focused Ultrasound (HIFU) therapy. HIFU therapy is a new technology therapy for PC. The HIFU device used in this study was the FEP-BY02 (Yuande Bio-Medical Engineering, Beijing, China), which is designed to reduce adverse events. According to the current safety trial, HIFU therapy is noninvasive and promising for clinical effectiveness. HIFU therapy has the potential to be a new method of combination therapy for PC.
HIFU therapy for PC is safe and noninvasive. Moreover, no anesthesia or sedation is needed. The clinical effectiveness is promising. HIFU therapy has the potential to be a new method of combination therapy for PC.
New advances in therapy for unresectable PC are expected. HIFU therapy, which uses focused ultrasound energy for therapeutic intention such as tissue ablation, is promising. The current study suggests that HIFU therapy is safe and has the potential to be a new method of combination therapy for PC.
The manuscript describes clinical trial results from pancreatic cancer treatment with HIFU trial. This is a timely and important topic, as HIFU applications continue to emerge, and their safety, efficacy, and applicability are being evaluated in a clinical setting. The manuscript is well-written and should be published. The authors designed a case series of patients with unresectable pancreatic cancer treated by HIFU therapy to evaluate its safety and clinical output. They found no severe adverse events and concluded that HIFU therapy is safe and has the potential to be a new method of combination therapy for PC. The study design is good and the manuscript is written in a clear style.
| 1. | World Health Organization. The world health report. Geneva: WHO 2013; . |
| 2. | Rothman H, Cantrell JE, Lokich J, Difino S, Harvey J, Ahlgren J, Fryer J. Continuous infusion 5-fluorouracil plus weekly cisplatin for pancreatic carcinoma. A Mid-Atlantic Oncology Program study. Cancer. 1991;68:264-268. [PubMed] |
| 3. | Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751-755. [PubMed] |
| 4. | Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33 Suppl 1:S18-S22. [PubMed] |
| 5. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 6. | He SX, Wang GM. The noninvasive treatment of 251 cases of advanced pancreatic cancer with focused ultrasound surgery. Proceedings from the 2nd International Symposium on Therapeutic Ultrasound. Seattle, WA: University of Washington 2002; 51-56. |
| 7. | He SX, Xiong LL, Yao SS, Yu JS, Lan J, Yu QH. The preclinical research of high intensity focused ultrasound. Beijing Yikedaxue Xue Bao. 1999;31:573-576. |
| 8. | Xiong LL, He CJ, Yao SS, Zeng JQ, Zhang GX, Huang K, He SX. The preliminary clinical results of the treatment for advanced pancreatic carcinoma by high intensity focused ultrasound [in Chinese]. Zhonghua Putong Waike Zazhi. 2001;16:345-347. |
| 9. | Xie D, Chen D, Teng H, Lin X, Chen J. The preliminary clinical study of high intensity focused ultrasound combination with gemcitabine in treatment for advanced pancreatic carcinoma. Lingnan Xiandai Yiyao Zazhi. 2001;1:226-228. |
| 10. | Wang X, Sun JZ. Preliminary study of high intensity focused ultrasound in treating patients with advanced pancreatic carcinoma. Zhonghua Putong Waike Zazhi. 2002;17:654-655. |
| 11. | Yuan C, Yang L, Yao C. Observation of high intensity focused ultrasound treating 40 cases of pancreatic cancer. Linchang Gandanbing Zazhi. 2003;19:145-146. |
| 12. | Wang RS, Mu QX, Liu LX, Shu YQ. A clinical study of thermotherapy of HIFU in combination with chemotherapy on treating advanced pancreatic cancer [in Chinese]. Nanjing Yike Daxue Xuebao. 2003;23:460-463. |
| 13. | Xie DR, Chen D, Teng H. A multicenter nonrandomized clinical study of high intensity focused ultrasound in treating patients with local advanced pancreatic carcinoma. Zhongguo Zhongliu Linchang. 2003;30:630-634. |
| 14. | Xu YQ, Wang GM, Gu YZ, Zhang HF. The acesodyne effect of high intensity focused ultrasound on the treatment of advanced pancreatic carcinoma [in Chinese]. Zhonghua Yixue Zazhi. 2003;10:322-323. |
| 15. | Gu Y, Wang G, Xia H, Xu YQ, Pan YH, Zhang HF, Wang WP. Application of high intensity focused ultrasound in treating 45 cases of carcinoma of the pancreas. Fudan Xuebao Yixueban. 2005;31:135-141. |
| 16. | Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, Li KQ, Jin CB, Xie FL, Su HB. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034-1040. [PubMed] |
| 17. | Dubinsky TJ, Cuevas C, Dighe MK, Kolokythas O, Hwang JH. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol. 2008;190:191-199. [PubMed] |
| 18. | Sofuni A, Moriyasu F, Sano T, Yamada K, Itokawa F, Tsuchiya T, Tsuji S, Kurihara T, Ishii K, Itoi T. The current potential of high-intensity focused ultrasound for pancreatic carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Hynynen K, Lulu BA. Hyperthermia in cancer treatment. Invest Radiol. 1990;25:824-834. [PubMed] |
| 20. | Hwang JH, Wang YN, Warren C, Upton MP, Starr F, Zhou Y, Mitchell SB. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
P- Reviewer: Kawakubo K, Li ZS, Seip R S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S