INTRODUCTION
Gastric mucosa can be damaged by oxidative stress induced by ischemia-reperfusion (IR), due to free radicals and/or reactive oxygen species (ROS). The crucial role of oxygen free radicals in the pathogenesis of IR is supported by many lines of evidence demonstrating that superoxide dismutase (SOD), a highly specific scavenger of superoxide, prevents IR-induced gastrointestinal injury[1,2]. Experimental studies have also shown that IR-induced gastric mucosal damage produced by temporal clamping of the celiac artery in rats is accompanied by the formation of free radicals[3,4].
Treatments with antioxidants, such as melatonin, beta-carotene, allopurinol and SOD, are shown to protect the gastrointestinal mucosa against the IR-induced oxidative stress[5]. Additionally, some studies have shown that remote preconditioning with short ischemic episodes of the stomach and non-gastric tissue (heart, liver and limb) protects the gastric mucosa against acute gastric lesions caused by severe IR[6,7]. Moreover, reports from recent studies show that remote ischemic postconditioning (RIP) performed in the limbs reduces infarct size after acute myocardial infarction[8] and attenuates brain injury after focal ischemia[9]. More recently, work from our group has shown that RIP performed bilaterally in the lower limbs attenuates the intestinal injury induced by limb IR in rats[10]. However, whether RIP inhibits gastric injury after limb ischemia is unknown. The present study was therefore designed to investigate the mechanisms and gastroprotective effects of hindlimb RIP on rat models of gastric ischemic injury induced by limb IR.
MATERIALS AND METHODS
Animals
The present study was performed on 108 adult male Wistar rats (220-250 g) obtained from the Animal Experimental Center of the Gansu College of Traditional Chinese Medicine (Lanzhou, China). The animals were housed in individual cages and kept under standard conditions (12 h light-dark cycle and temperature between 22-24 °C) with ad libitum standard rat chow and water. All animals were allowed to acclimate for one week prior to the start of experimental procedures. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Lanzhou University.
Reagents
The reagents and detection kits used were as follows: SOD, malondialdehyde (MDA), xanthine oxidase (XOD), myeloperoxidase (MPO), lactate dehydrogenase (LDH) and creatine kinase (CK) detection kits were obtained from Nanjing Jiancheng Bioengineering Institute, China; tumor necrosis factor (TNF)-α and interleukin (IL)-10 enzyme-linked immunosorbent assay (ELISA) kits were obtained from Wuhan Boster Bioengineering Institute, China. All other chemicals were of analytical grade or equivalent.
Experimental protocol
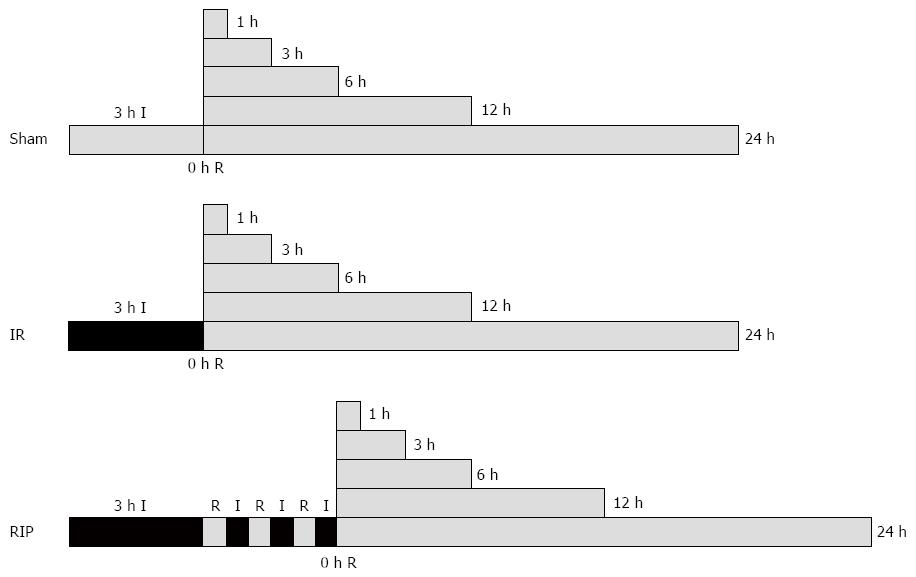
The animals were randomized into one of three groups (n = 36 each): IR group, animals were subjected to gastric IR injury induced for 3 h by placing an elastic rubber band under a pressure of 290-310 mmHg on the proximal part of both lower limbs[7,11] that was then released to allow reperfusion; RIP group, animals were additionally subjected to RIP, consisting of three cycles of 30 s femoral aortic declamping (reperfusion) and 30 s reocclusion at the onset of reperfusion; sham group, animals were not submitted to any IR (Figure 1). Global ischemia in the hindlimbs was verified by the absence of femoral arterio venular blood flow using laser Doppler flowmetry throughout the experiment. The animals were anesthetized by inhalation of 2%-3% isoflurane[12], and the anesthesia was only maintained during the experimental operation. Rats were kept supine for the duration of the experiment. The three groups underwent 0, 1, 3, 6, 12 or 24 h reperfusion. Following reperfusion, blood samples from the inferior vena cava were collected, and six rats were euthanized by venous bloodletting at each timepoint and the stomachs were immediately removed, cut along the lesser gastric curvature, rinsed in ice-cold saline and divided into three portions.
Figure 1 Surgery protocols.
Ischemia-reperfusion (IR, n = 36) was elicited by 3 h ischemia (I). For animals in the remote ischemic postconditioning group (RIP, n = 36), this was followed by three cycles of 30 s of reperfusion (R) followed by 30 s of I. There was no intervention in the sham group (n = 36). All groups were examined after 0, 1, 3, 6, 12 or 24 h of R.
Serum assays
Collected blood samples were left to clot at room temperature for 1 h before centrifugation (2500 rpm for 15 min at room temperature) to separate the serum, which was stored at -20 °C in microcentrifuge tubes until analysis. Serum LDH and CK were quantified using specific assay kits according to the manufacturer’s instructions. All the samples were analyzed in duplicate for LDH and CK in a 721(N) spectrophotometer (Shanghai, China) by the same analyst. Serum TNF-α and IL-10 concentrations were measured by ELISA according to the manufacturer’s instructions, and all samples were tested in duplicate in a SM-3 Auto ELISA Analyzer (Shanghai, China) by the same analyst.
Biochemical analysis
The tissue samples from the gastric mucosa were homogenized in a 0.9% saline solution and centrifuged at 2000-3000 rpm for 10 min at 4 °C. The supernatant was collected and MDA content and SOD, XOD and MPO activities were quantified using specific assay kits according to the manufacturer’s instructions. All assays were performed using the same spectrophotometer unless otherwise stated. Each sample measurement was performed in triplicate.
Measurement of gastric mucosal injury
Gastric mucosal samples were fixed in a 4% formaldehyde solution, paraffin-embedded and sectioned at a thickness of 4 μm. After deparaffinization and gradual hydration, sections were examined using hematoxylin and eosin staining. Gastric mucosal injury and repair were observed and photographed under a light microscope by an experienced pathologist who was unaware of the treatment. Based on a cumulative-length scale where an individual lesion was limited to the mucosal epithelium (including pinpoint erosions, ulcers, and hemorrhagic spots), the index was scored according to its length: (1) ≤ 1 mm; (2) > 1 mm and ≤ 2 mm; and (3) > 2 mm and ≤ 3 mm. For lesions > 1 mm in width, the score was doubled. The sum total of the scores of all lesions represented the gastric mucosal injury index as outlined by Zhang et al[13].
Statistical analysis
Data were entered into a database and analyzed using SPSS software (SPSS, Chiacgo, IL, United States) and are expressed as mean ± SD. Data were analyzed using a repeated measures analysis, and the means of all groups were compared using the least significant difference test for multiple comparisons. P < 0.05 was used to determine significance.
RESULTS
Serum LDH, CK, TNF-α and IL-10
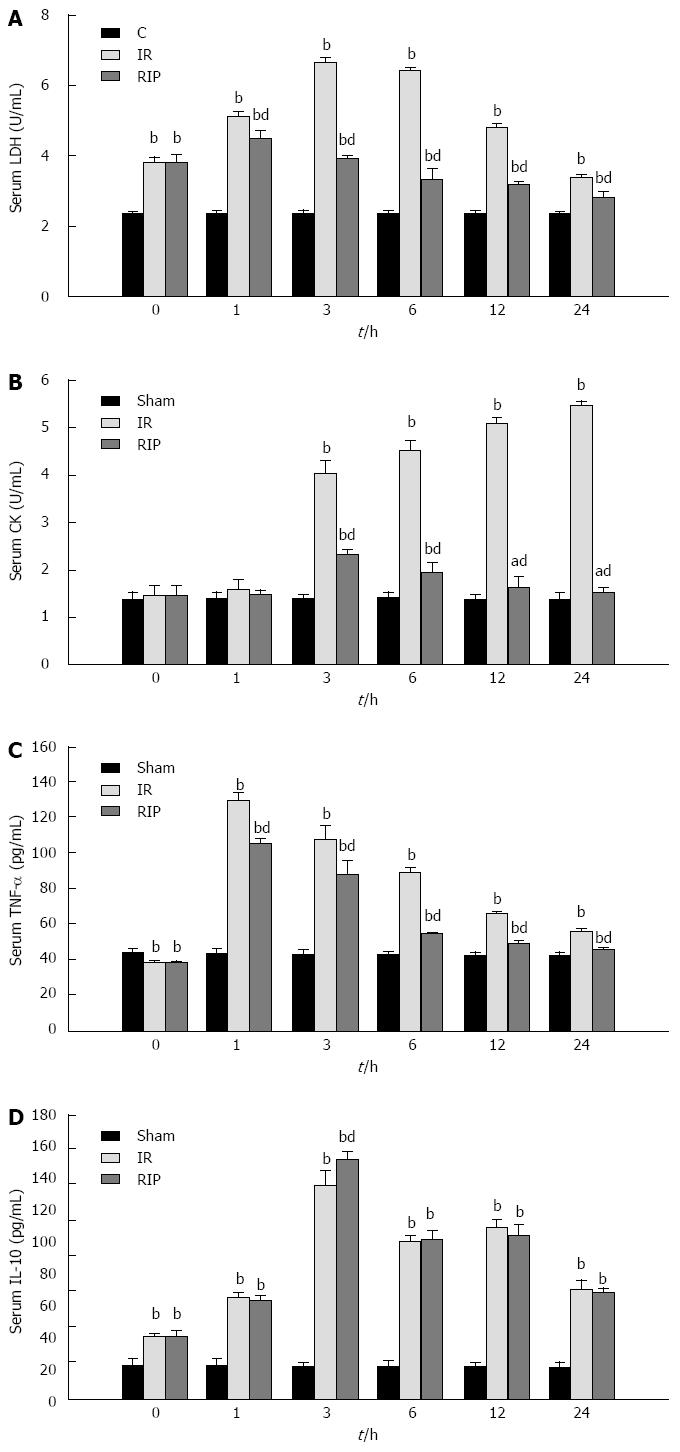
The activity of LDH and the concentration of IL-10 were significantly elevated in the IR and RIP groups immediately after ischemia was induced and at all reperfusion timepoints compared with the sham group (Figure 2A and D). The activity of CK and the concentration of TNF-α were also significantly elevated in the IR and RIP groups compared to the sham group during the reperfusion (Figure 2B and C). Compared to the IR group, RIP treatment significantly decreased the activities of LDH (6.46 ± 0.03 vs 3.31 ± 0.32; P < 0.01) and CK (4.54 ± 0.19 vs 1.94 ± 0.20; P < 0.01) at 6 h (Figure 2A and B) and significantly attenuated the concentration of TNF-α (88.50 ± 3.08 vs 53.82 ± 0.85; P < 0.01) at 6 h and throughout the 24 h reperfusion period (Figure 2C). However, RIP did not decrease IL-10 levels compared to the IR group, but increased the concentration at the 3 h timepoint (151.71 ± 4.06 vs 135.42 ± 8.62; P < 0.01) (Figure 2D).
Figure 2 Serum levels after ischemia reperfusion.
Serum levels of A: Lactate dehydrogenase (LDH); B: Creatine kinase (CK); C: tumor necrosis factor (TNF)-α; D: Interleukin (IL)-10 immediately after 3 h ischemia (0 h) and 1, 3, 6, 12 and 24 h reperfusion in sham, ischemia reperfusion (IR) and remote ischemic postconditioning (RIP) groups (n = 6 per group at each timepoint). aP < 0.05 vs sham group, bP < 0.01 vs sham group; dP < 0.01 vs IR group.
MDA content, SOD, XOD and MPO activities
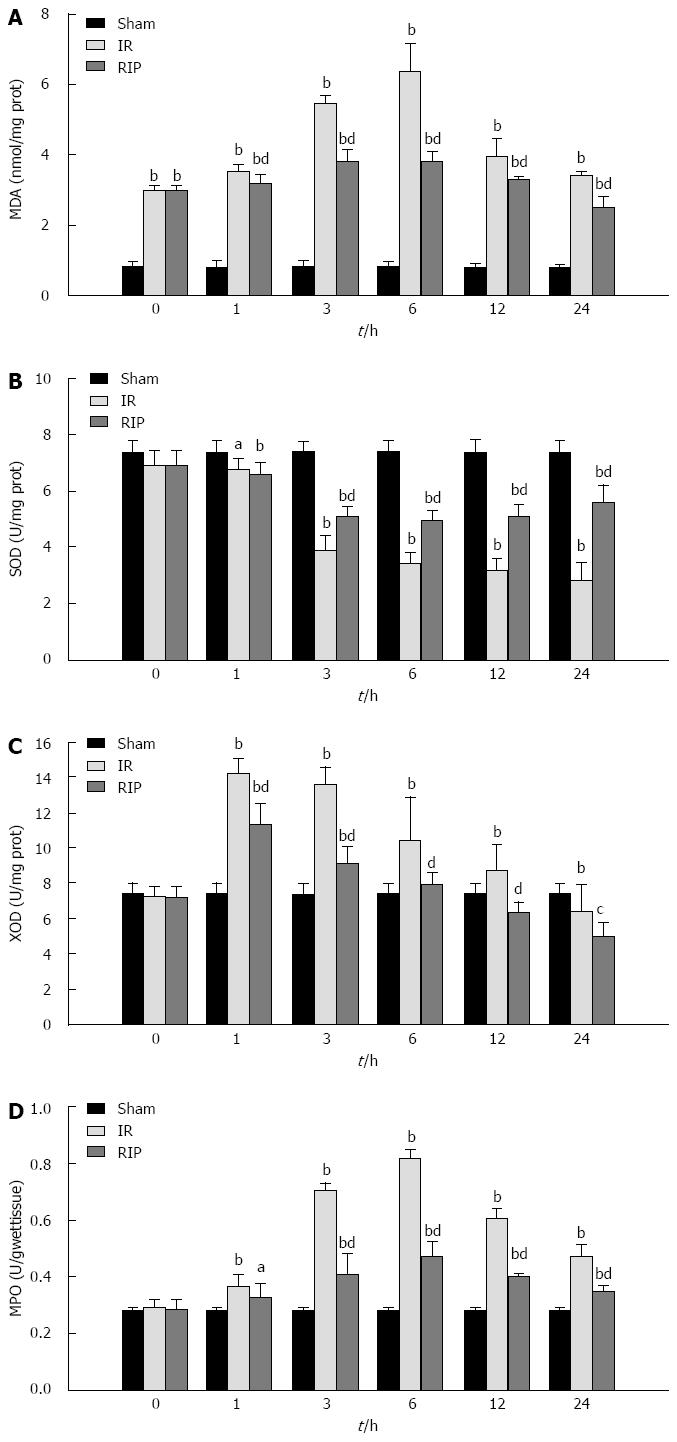
Ischemia and the subsequent reperfusion resulted in a significant elevation in MDA content in gastric tissue from both the IR and RIP groups compared to sham controls (Figure 3A), whereas a decrease in SOD activity was observed during reperfusion (Figure 3B). Treatment with RIP significantly attenuated the elevation in MDA compared with IR at 6 h (3.79 ± 0.29 vs 6.39 ± 0.81; P < 0.01) and throughout the 24 h reperfusion period. RIP also significantly attenuated the decrease in SOD activity compared to IR (6 h: 4.95 ± 0.32 vs 3.41 ± 0.38; P < 0.01).
Figure 3 Gastric oxidative stress and lipid peroxidation after ischemia reperfusion.
A: The gastric content of malondialdehyde (MDA); The activity levels of B: Superoxide dismutase (SOD); C: Xanthine oxidase (XOD); D: Myeloperoxidase (MPO), immediately after 3 h ischemia (0 h) and 1, 3, 6, 12 and 24 h reperfusion in sham, ischemia reperfusion (IR) and remote ischemic postconditioning (RIP) groups (n = 6 per group at each timepoint). aP < 0.05 vs sham group, bP < 0.01 vs sham group; cP < 0.05 vs IR group, dP < 0.01 vs IR group.
Reperfusion following the ischemic event resulted in increases in XOD and MPO activities in gastric tissues (Figure 3C and D). RIP treatment blocked the increase in XOD at 6 h (7.81 ± 0.75 vs 7.34 ± 0.63 in sham), and significantly attenuated the increase in MPO activity (0.82 ± 0.03 vs 0.47 ± 0.05 in IR; P < 0.01).
Effects of RIP on pathologic changes of gastric mucosa
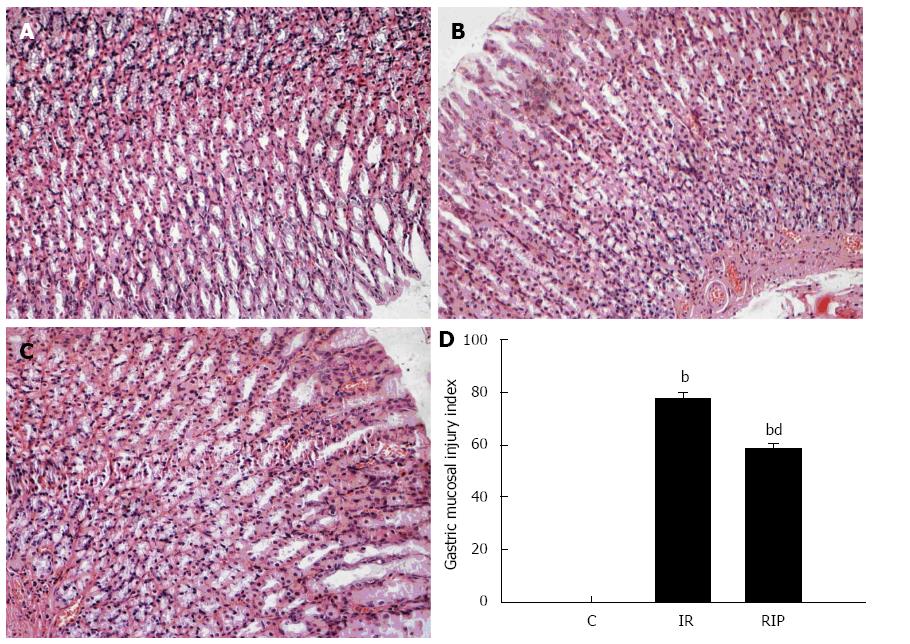
Ischemia reperfusion resulted in significant injury as evidenced by gastric mucosal edema, epithelial hemorrhage, hyperemia and erosion, and infiltration of inflammatory cells between the muscularis mucosa and the glands after 6 h (Figure 4B), which were ameliorated by RIP treatment (Figure 4C). Quantitative scoring of the lesions showed a significant reduction in the RIP group compared with the IR group (5.85 ± 0.22 vs 7.72 ± 0.43; P < 0.01) (Figure 4D).
Figure 4 Histologic evaluation of gastric tissue.
Hematoxylin and eosin staining of gastric sections obtained from A: Sham surgery; B: Ischemia reperfusion (IR); C: Remote ischemic postconditioning (RIP) after 6 h (magnification × 200); D: Quantitative scores for acute gastric lesions from sham, IR and RIP groups (mean ± SD; n = 6); bP < 0.01 vs sham group; dP < 0.01 vs IR group.
DISCUSSION
The local and remote consequences of acute limb IR injury (IRI) continue to be a serious clinical problem in trauma surgery. Protracted limb ischemic insult can produce ROS, cytokine release and cascade activation, which lead to skeletal muscle injury and organ specific and/or “systemic” effects[14]. Furthermore, the insult can produce consumption of endogenous antioxidants[10,15-17], resulting in remote organ injury to the heart, lungs, liver, kidneys and the gastrointestinal tract. Hypotension and sepsis are important predisposing factors in the etiology and pathogenesis of massive upper gastrointestinal hemorrhage following surgical operations[18], and a hemorrhagic shock of short duration is the most powerful factor causing gastric mucosal lesions[19]. These lesions are often referred to as stress ulcers resulting from ischemic-hypoxic gastric mucosa injury. Therefore, limb ischemia can result in IRI of the gastric mucosa, which is an important clinical condition with undesirable outcomes concerning patients’ morbidity and mortality.
In recent years, studies have revealed that ROS[20], microvascular dysfunction, polymorphonuclear (PMN) leukocyte infiltration[21], nitric oxide release[22] and gastric acid secretion[23] during reperfusion may play a role in the pathogenesis of gastric mucosal IRI. Several interventions (surgical and pharmacologic) are used to reduce such injury[20,24-26]. A potent surgical therapeutic technique called ischemic postconditioning (IPostC) is described as a modified schedule of reperfusion characterized by intermittent restoration of blood flow after a prolonged episode of ischemia[27]. Gyurkovics et al[28] demonstrated in a recent experimental study that IPostC provides effective functional protection to skeletal muscles from IRI and distant organ (lung and renal) injuries caused by bilateral lower limb IRI by attenuating the formation of ROS, PMN cell accumulation and decrease in inflammatory reaction mechanisms. In addition, a second remote ischemic condition technique known as RIP protects myocardium from IRI by reducing oxygen radical-induced injury and improving antioxidant action[29], and protects against focal cerebral ischemia by modulating nerve activity and protein synthesis[9]. Previous work showing that IPostC provides effective functional protection to lung and renal injuries caused by lower limb IRI[28] suggested that limb ischemia could be extrapolated to RIP. Indeed, the results of the current study show that RIP conducted in the hindlimb immediately after reperfusion markedly attenuates gastric ischemic injury.
The gastric mucosal injury induced by IR was mild immediately after ischemia, but severe after reperfusion, in agreement with a previous study indicating that reperfusion of acutely ischemic extremities produces structural and functional changes in the gastrointestinal tract[17]. It is well established that IR-induced gastric lesions are mediated, at least in part, by excessive formation of oxygen free radicals and/or ROS, resulting in suppression of gastric microcirculation, neutrophil infiltration, and release of proinflammatory cytokines[30]. These changes may elicit the progression of acute erosions observed immediately after the IR into deep gastric lesions appearing 12 and 24 h after reperfusion[31]. The results of the present study indicate that these changes occur much earlier, as reperfusion resulted in gastric mucosal lesions with edema, epithelial hemorrhage, hyperemia and erosion, and inflammatory cell infiltration observed just 6 h after reperfusion.
In this study, limb IRI produced an increase in gastric tissue levels of MDA, XOD and MPO, and a decrease in the level of SOD, which is in agreement with previous studies[21,31,32]. The observed elevation in serum LDH and CK levels may be attributed to cell membrane damage and/or ischemia-induced lactate accumulation[33], and to cell death induced by the acutely ischemic gut[34]. These changes were attenuated by RIP conducted immediately after reperfusion in the lower limb, which also protected against gastric ischemia. It is plausible that RIP protects the ischemic stomach through these antioxidative and cytoprotective effects, as SOD, MDA, XOD and MPO are crucial in the protection against gastric injuries.
TNF-α plays a major role in the generalized inflammatory process by activating neutrophils and upregulating the expression of adhesion molecules in endothelial cells and granulocytes[35]. IL-10 may be protective and can limit tissue damage caused by inflammation via inhibition of cytokine synthesis[36] and suppression of TNF-α production[37]. Results of the present study along with our previous work[10] have shown that RIP treatment, which attenuates small intestinal injury resulting from limb IRI, can reduce serum levels of TNF-α and increase levels of IL-10. Consistent with the theory that the release of the inflammatory mediators may induce end-organ injury at sites remote from the focus of IR, recent work also showed lower TNF-α levels and reduced distant organ (lung and renal) impairment with RIP treatment[28].
In summary, the present study demonstrates that treatment with RIP attenuates gastric IRI and helps to restore and maintain the structural integrity of gastric mucosa. This protective effect may be mediated by the reduced generation of ROS, and anti-inflammatory and antioxidant actions. Thus, intervention with RIP, which targets the first few minutes of reperfusion, may be a valuable clinical treatment for gastric IR.
COMMENTS
Background
Recent studies have reported that remote ischemic postconditioning (RIP) performed in the limbs reduces infarct size after acute myocardial infarction and attenuates brain injury after focal ischemia. RIP is ischemia conducted in a distant organ that protects another organ against an ischemic insult. RIP performed in the lower limbs also attenuates the intestinal injury induced by limb ischemia reperfusion (IR) in rats. However, it is not known whether RIP inhibits gastric injury after limb ischemia.
Research frontiers
Experimental evidence indicates that RIP protects against IR injury (IRI) to a similar degree in various organ systems. However, these have not been tested in great detail. Further research may validate the benefits and usefulness of clinical applications of RIP for treating ischemic injuries.
Innovations and breakthroughs
This study examined the protective effects of RIP in gastric ischemic injury in rats induced by hindlimb IR. This is the first study to report that RIP provides effective functional protection to gastric injury induced by limb IRI via anti-inflammatory and antioxidant actions.
Applications
By understanding the protective and pathologic mechanisms of RIP and gastric damage resulting from limb IR, this study presents a possible strategy for therapeutic intervention in the treatment of patients with gastric lesions induced by limb IR.
Terminology
RIP is ischemia conducted in a distant organ for protection of another organ against a prior ischemia. The mechanism by which RIP is protective and prevents cell injury may involve reduced oxygen radical formation and improved antioxidant action and anti-inflammatory effects.
Peer review
The authors evaluated the effects of RIP on gastric mucosal lesions induced by limb IR in rats. This is a very interesting study and demonstrates that treatment with RIP attenuates gastric IRI, and helps to restore and maintain the structural integrity of gastric mucosa. The paper describes biochemical and histologic documentation of the effects of RIP over a 24 h reperfusion time course.