Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.8846
Revised: February 13, 2014
Accepted: May 28, 2014
Published online: July 21, 2014
Processing time: 269 Days and 11.8 Hours
Disordered signalling between the brain and the gut are generally accepted to underlie the functional bowel disorder, irritable bowel syndrome (IBS). However, partly due to the lack of disease-defining biomarkers, understanding the aetiology of this complex and multifactorial disease remains elusive. This common gastrointestinal disorder is characterised by alterations in bowel habit such as diarrhoea and/or constipation, bloating and abdominal pain, and symptom exacerbation has been linked with periods of stress, both psychosocial and infection-related. Indeed, a high level of comorbidity exists between IBS and stress-related mood disorders such as anxiety and depression. Moreover, studies have observed alterations in autonomic output and neuro-endocrine signalling in IBS patients. Accumulating evidence indicates that a maladaptive stress response, probably mediated by the stress hormone, corticotropin-releasing factor contributes to the initiation, persistence and severity of symptom flares. Other risk factors for developing IBS include a positive family history, childhood trauma, dietary factors and prior gastrointestinal infection. An emerging role has been attributed to the importance of immune factors in the pathophysiology of IBS with evidence of altered cytokine profiles and increased levels of mucosal immune cells. These factors have also been shown to have direct effects on neural signalling. This review discusses how pathological changes in neural, immune and endocrine pathways, and communication between these systems, contribute to symptom flares in IBS.
Core tip: Irritable bowel syndrome (IBS) is a disorder characterised by symptoms such as diarrhoea and/or constipation, bloating and abdominal pain. However the underlying pathophysiology of this common disorder remains unclear. Nonetheless, a number of mechanisms have been proposed to contribute to the initiation, exacerbation and persistence of symptoms. Alterations in brain-gut communication, stress, previous infections, abnormal microbiota, altered cytokine profiles and increased intestinal permeability have all been proposed as contributors to IBS and indeed, we propose that complex interactions between neural, endocrine and immune factors underlie the heterogeneity of symptoms that is characteristic of IBS.
- Citation: Buckley MM, O’Mahony SM, O’Malley D. Convergence of neuro-endocrine-immune pathways in the pathophysiology of irritable bowel syndrome. World J Gastroenterol 2014; 20(27): 8846-8858
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/8846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.8846
Irritable bowel syndrome (IBS) is the most common functional digestive condition with a worldwide prevalence rate of 10%-20% in the general population[1,2]. As with other functional disorders it is often difficult to identify an unequivocal organic cause, at least with the diagnostic tools available. This disorder accounts for approximately 3% all general practice consultation and up to 40% of gastrointestinal (GI) referrals[3] leading to a large economic burden. At the level of the individual, IBS significantly impinges on the quality of life of a patient causing recurrent abdominal pain or discomfort coupled with disturbed bowel habits[4]. IBS is subtyped according to bowel habit pattern, therefore patients are classified as diarrhoea-(IBS-D) or constipation-predominant IBS (IBS-C) or an alternating subtype (IBS-A), which fluxes between the two states[5]. Some reports suggest that IBS-D and IBS-A are more prevalent[6] while others show an equal distribution between all three subtypes[7]. Although little mortality is associated with IBS a satisfactory treatment still does not exist, primarily due to the fact that the aetiology and pathophysiology of IBS are incompletely understood. Nonetheless, dysfunctional brain-gut axis signalling is hypothesised to be at the heart of symptoms of IBS[8] and this incorporates three major systems, neural, endocrine and immune signalling. In this review we discuss the contribution of each system to IBS symptoms and how convergence and interplay between factors from each system may provide a better understanding of the heterogeneity of IBS.
The two major persisting symptoms of IBS are visceral hypersensitivity and altered bowel habit[9] each of which are entwined within the nervous system. Functions of the GI tract are modulated by both intrinsic and extrinsic innervation[10]. Extrinsic innervation includes both branches of the autonomic nervous system, which are anatomically and functionally integrated within the brain-gut axis and are responsible for homeostatic regulation of GI function[11]. The parasympathetic nervous system stimulates smooth muscle and secretory actions while the sympathetic element inhibits motor and secretory activity of the GI tract. The parasympathetic afferent pathway runs primarily with the vagus and terminates in the nucleus solitary tact, which sends information regarding non-nociceptive information, including gastric accommodation and gastric-colic reflex, to corticolimbic structures[12]. The sympathetic afferent pathways mediates mainly nociceptive signals through spinal pathways primarily to the thalamus and then to the sensory cortex and pain matrix[13]. Information is also sent to specific brain regions such as the hippocampus, amygdala, prefrontal cortex[14] and the hypothalamus[15] for processing. These central regions which are capable of modulating gut function are also involved in emotional (e.g. mood, anxiety, pain) and cognitive behaviours (e.g. memory, decision making) and hence, in the development of coping strategies and general well-being[16]. A descending pain inhibitory pathway from the brainstem also exists in order to control the signals reaching the brain. The intrinsic or enteric nervous system works somewhat independently providing local reflexes, such as migrating motor complex and peristaltic reflexes, and yet also receives input from the central nervous system (CNS) via the autonomic nervous system.
A growing body of evidence suggests the existence of autonomic dysfunction in IBS[17-19] and some have shown correlations with symptoms[20]. Low vagal activity can lead to a reduction in bowel contractions, reduced motility, and constipation, while high vagal activity can result in increased contractions and diarrhoea[21]. The sympatho-vagal balance was found to be disturbed in IBS patients compared to healthy controls[22]. Furthermore, a study assessing female IBS patients with constipation and severe abdominal pain showed lower vagal activity than controls[23], which correlates with a study showing an increased parasympathetic tone in women with IBS-D compared to those with IBS-C[24]. Given the close association between the stress axis and the autonomic nervous system, increased sympathetic tone as seen with constipation may be due to the increase in corticotropin-releasing factor (CRF) expression[10], which is discussed in more detail in the next section. Indeed, the psychological disorders that often co-occur with IBS are also associated with altered autonomic balance[25].
Visceral hypersensitivity, as seen in a subset of IBS patients, is an exaggerated response to a stimulus such as colorectal distension, was first noted by Ritchie[26], 1973. Several neural theories have been proposed for this increased sensitivity, including sensitisation of primary afferent pathways, increased activity of endogenous pain facilitation and reduced engagement of endogenous pain inhibition[27]. IBS patients have significantly elevated levels of anxiety, interpersonal sensitivity, depression, hostility and somatization of effect, which can impact on pain perception[28]. Some studies indicate a difference in sensitivity to colon and rectal distension between diarrhoea and control subjects[29,30], while patients with constipation showed conflicting results[31,32]. However, a comparison between constipation and diarrhoea predominant IBS patients revealed no significant difference in pain threshold[33].
Preclinical studies of acute gut inflammation have shown that sensitization of primary afferent pathways can result in visceral hyperalgesia[34-36]. A subset of IBS patients develop symptoms following an acute GI infection[37]. Usually peripheral sensitization is temporary and response properties of primary afferents return to normal state after resolution of the inflammation[27]. Evidence from human mucosal biopsies suggests neuroplastic remodelling in the epithelium[38]. Such plastic changes can affect the response properties of primary afferents which include spinal and vagal afferents[39]. Changes in afferent nerve terminals could affect responsiveness to visceral stimuli and interfere with the release of neuropeptides from these terminals resulting in neurogenic inflammation[27].
In turn then there are multiple mechanisms by which the CNS can modulate afferent signals from the viscera, including increased activation of endogenous pain facilitation and reduced engagement of endogenous pain inhibition[27]. Of course, these modulatory systems are also influenced by stress and mood[27]. Neuroimaging studies consistently support a role for altered neural processing of visceral stimuli[40]. Indeed, some sophisticated studies now incorporate the contribution of emotional factors and cognitive influences such as expectation, attention and learning, to their analyses of functional connectivity between brain regions and actual CNS structural changes[40].
It was noted that a thinning of the anterior mid-cingulate and insular cortices was evident in IBS patients[41], these areas being associated with perception of the internal state. Moreover, regional structural changes including decreased grey matter in the medial and ventrolateral prefrontal cortex, thalamus and periaqueductal grey are seen in IBS patients as compared to healthy controls[42]. These may point towards an impaired ability to activate the descending pain inhibition system. This hypothesis is supported by the observation that the reduction in grey matter in the ventrolateral prefrontal cortex was only found in the patients presenting with a high level of pain[42]. Central areas involved in the processing of the affective component of pain such as the pregenual anterior cingulate cortex and the orbital frontal cortex showed an increase in grey matter in IBS patients, which was abolished once data was corrected for anxiety and depression in these patients[40]. These findings further confirm the involvement of emotional systems in the processing of visceral pain. Consistent with this, Chen et al[43] showed that white matter aberrations are seen in the anterior cingulate cortex and the insula. However, as it is still not known whether these changes are present before symptoms emerge, or are actually acquired due to altered visceral signalling, these results should be interpreted with caution.
A meta-analysis of functional magnetic resonance imaging studies in IBS patients reported differences in CNS response to colorectal distension[44]. The differences were seen in areas associated with visceral afferent signalling, attention and emotional arousal. The anterior cingulate cortex is one of the most commonly reported cortical areas that displays pain evoked activation during acute stimulation in patients[43]. Mertz et al[45] demonstrated that the anterior cingulate cortex, thalamus, the insula and the prefrontal cortex were more activated in IBS patients than controls and that the pattern of activation was dependent on previous experience. A greater activation of the thalamic, striatal and dorsolateral prefrontal cortex was seen in controls as compared to IBS patients during rectal distension indicating an abnormal descending modulation in IBS[46]. It has also been shown that female IBS patients have a greater engagement of the emotional arousal system during expectation of visceral pain than males[47]. These studies highlight the importance of the emotional status of patients in pain perception and that the female predominance may be in part due to the gender differences in the activation of circuits involved in stress and arousal[27]. Taken together these results indicate a role for both structural and functional abnormalities in the CNS in IBS pathophysiology.
Stress is a pervasive condition that effects everyone and is defined as a “stereotyped body response to any demand”[48]. However, the high co-morbidity of stress-associated mood disorders such as anxiety and depression and altered bowel function in IBS patients[49], suggests that these individuals are more sensitive to the effects of stress. Indeed, the relationship between severe and chronic stress and symptom intensity in IBS patients[50] is linked to chronic stress, with the onset and duration of symptoms increased[51]. As noted above, this may mediated via altered autonomic signalling[52], however the key signalling factor initiated by stress is an endocrine hormone, CRF.
CRF is the vital hormone in the body’s response to stress, activating the hypothalamic-pituitary-adrenal (HPA) axis in reaction to a variety of physical and psychological stressors. This results in enhanced levels of adrenocorticotropic hormone and cortisol in IBS patients as compared to healthy subjects[53,54]. CRF is secreted by the paraventricular nucleus (PVN) of the hypothalamus and its release is regulated by the amygdala, which is part of the limbic system[51].
CRF exerts its biological effects through activation of CRF1 and CRF2 receptors (CRFR1 and CRFR2), which are members of the seven transmembrane G-protein coupled receptor superfamily[55]. CRFR1 is prevalent in brain regions associated with affective, stress and nociceptive circuitries including the PVN, locus coeruleus and amygdala[56,57]. CRF neurons project from the PVN to the spinal cord, where they can alter the function of innervated organs[58].
Although much of the influence of CRF on GI function is mediated centrally, the presence of CRF ligands and its receptors in the colon[55,59,60] suggests that organ-specific activation of these receptors may also be important for stress-induced changes in bowel function. CRFR1 is expressed on enteric neurons and in the mucosal layer[59] and is likely to be the focal mechanism by which stress induces changes in GI function including delayed gastric emptying[61], accelerated colonic transit[62] and motility[63]. The importance of CRF to these effects has been demonstrated using the non-selective CRF receptor antagonist, α-helical CRF[64]. Furthermore, the use of CRFR1 -/- mice has revealed the importance of the CRFR1 subtype in IBS like-symptoms, as these knock-out mice exhibit decreased visceral sensitivity[65], as well as decreased anxiety and an impaired stress response[66]. In addition CRF-evoked defecation in rats[67] is inhibited by blocking CRF1 receptors[68]. These results translate to IBS patients, where peripheral administration of CRF1 receptor antagonists reduces abdominal pain and anxiety[64]. In contrast to CRFR1-mediated increases in GI contractile activity, stimulation of CRFR2 is likely to result in inhibition of GI motility[69,70] and contribute to stress-induced colonic permeability dysfunction[71].
Some of the key symptoms of IBS, such as colonic motility, alterations in bowel habit and abdominal pain associated with gut hypersensitivity may be a consequence of CRFR1 signalling[72]. Consistent with this is increased thalamic expression of CRFR1 following colonic distension in the maternally separated rat model of IBS[73]. Moreover, central administration of CRF increases pain behaviours in response to colonic distension in rats[74] demonstrating the bidirectional signalling between the CNS and the gut. Activation of the CRFR1 signalling pathways causes increases in colonic motor activity and visceral pain[75,76], and conversely, activation of central and peripheral CRFR2 receptors delays gastric emptying[70]. Furthermore, activation of either CRFR1 or CRFR2 causes increased colonic permeability and inflammation[77]. The pathophysiology of stress-induced exacerbation of IBS symptoms may be due to central hypersecretion of CRF, as it has been shown that inhibition of CRFR1[78] as well as central inhibition with CRF antagonists decreases the response to water avoidance stress[79]. Also, stress increases intestinal permeability, visceral hypersensitivity, causes alterations in gastrointestinal motility and leads to profound activation of mast cells, resulting in the release of many pro-inflammatory mediators[80-82] which will be discussed in more detail later. Williams et al[67] illustrated that acute restraint stress increased large intestine transit rates and stimulated defecation and this was associated with mucosal mast cell activation[83,84], which was mediated by CRF[83]. These studies imply that the brain-gut axis of IBS patients has a magnified response to CRF. Thus, targeting CRF signalling molecules has been proposed as a potential treatment for IBS[70]. However, thus far, clinical trials using a CRFR1 antagonist have been disappointing[85].
Mineralocorticoids and glucocorticoids are steroid hormones which mediate the actions of the adrenal hormone, cortisol in the initiation and termination of the stress response, respectively[86]. Cortisol, which is the natural ligand for corticosteroid receptors, is elevated in IBS patients both at baseline and in response to stress[53,54]. In rodent studies, application of corticosterone to the amygdala induces colonic hypersensitivity and anxiety[87,88] and alters colonic transit[89], actions that are mediated through both mineralocorticoid and glucocorticoid receptors[90]. These studies demonstrate that central signalling by corticosteroids are potential targets for treating bowel dysfunction in IBS.
A precipitating factor in symptom exacerbation is food ingestion, frequently in the form of abdominal pain and gas[91]. Although food intolerance has not been shown to cause IBS, ingestion of certain foods can result in abdominal pain, bloating, flatus and diarrhoea[92,93], especially carbohydrates, including gluten and lactose and fat-rich meals[92]. This appears to be more common in females and those who display increased anxiety levels again demonstrating the multi-factorial nature of IBS[93]. Prolonged and exaggerated colonic motor responses following a meal has been observed in IBS patients[94] and balloon distension in the jejunum demonstrated increased sensitivity in IBS patients following a meal[95]. Recent studies have reported the success of diets low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols[96-98]. Reduction of poorly absorbed short-chain carbohydrates such as lactose, fructose and sorbitol, fructo-oligosaccharides, galacto-oligosaccharides and incompletely absorbed sugar polyols such as sorbitol and mannitol relieves symptoms of IBS, such as bloating, distension, abdominal pain, excessive flatus[98] and osmotic diarrhoea[99]. Although release of gas by fermentation is normal, the sensitivity of IBS bowels to distension results in visceral pain. The pathophysiological changes resulting in these symptoms are not yet clear. However, an important physiological response to the arrival of food in the GI tract is the secretion of incretin hormones such as glucagon-like peptide 1 (GLP-1) which is secreted by L-cells. The biological activities of GLP-1 include stimulation of glucose-dependent insulin secretion and insulin biosynthesis, inhibition of glucagon secretion and gastric emptying, and the inhibition of food intake. One report has related GLP-1 to IBS pathophysiology by demonstrating that a GLP-1 mimetic alleviated some of the pathophysiological symptoms of IBS with anti-spasmodic and pain-relieving properties[100]. Although, the molecular mechanisms by which GLP-1 achieves this outcome are not completely understood, it is thought to act in a neurocrine fashion. Indeed, GLP-1 has been found to increase firing rates in afferent vagal nerves[101] and also decreasing neurally-evoked chloride secretion[102]. Interestingly, GLP-1 can also modulate GI secretion of cytokines and alter central CRF pathways that regulate stress-induced alterations in colonic transit[103]. GLP-1-expressing neurons are found in the enteric nervous system but also in brain regions such as the nucleus tractus solitarius and the ventrolateral medulla[104], revealing that the action of GLP-1 on gut function may be central or peripheral. GLP-1 activates the HPA axis through CRF neuronal stimulation, which may be important in the suppression of feeding behaviour[105]. Other GI hormones, such as motilin[106], which regulates the migrating motor complex in the fasting period and cholecystokinin[107] are elevated in IBS. In contrast, colonic expression of peptide YY[108] and circulating neuropeptide Y are lower in IBS patients[107]. Consideration must therefore be given to these and other GI factors in the pathogenesis of IBS.
Mounting evidence suggests that alterations in immune status such as elevations in mucosal mast cell numbers, pro-inflammatory cytokines and increased intestinal permeability are frequently noted in IBS patients[109]. Potential biomarkers of the disorder include alterations in cytokine profiles, mucosal and muscular infiltration of immune cells, changes in intestinal permeability and luminal microbiota which are discussed below.
Gross morphological evidence of inflammation is absent from IBS mucosal biopsies and other indicators of inflammation such as faecal levels of calprotectin and lactoferrin are not elevated[110,111]. Nonetheless, evidence is mounting on the important contribution of immune activation to the development of this syndrome. Indeed, one of clearest predictors of developing IBS is a prior history of bacterial or viral gastroenteritis[37,112], with one study showing a sevenfold increase in the risk of developing the functional bowel disorder following gastrointestinal infection[113]. Samples from individuals with post-infectious IBS show persistent increases in mucosal mononuclear immune cells[114], T-lymphocytes[115] and mast cells[116], which degranulate following stimulation releasing compounds such as histamine, tryptase and chymase. The extent of immune activation is an indicator of the severity of the infective gastroenteritis episode and the subsequent risk of developing IBS[114,117].
Expression of lymphocytes and mast cells are elevated in IBS mucosal samples[118,119], although not all studies detected increased numbers of mucosal mast cells[118,120,121]. Nonetheless, soluble mediators released from degranulated mast cells were found to induce excitation of rat sensory neurons[121]. This has implications for GI sensory and motor function, with one study demonstrating that the colon is more susceptible to effects of stress on enteric nerve function following a prior bout of inflammation[122].
Evidence of immune activation in IBS includes elevated levels of pro-inflammatory cytokines such as interleukin (IL)-6 and IL-8[53,123-125], although not all studies detected such increases[120,126]. Furthermore, in peripheral blood mononuclear cells isolated from IBS patients, abnormal secretion of pro-inflammatory cytokines in response to immune challenges was observed[123,125,127]. Studies reporting changes in mucosal levels of pro-inflammatory cytokines in IBS biopsies varied with some studies describing an upregulation[116,128] but several others describing down-regulation of these cytokines[54,129]. That said, anti-inflammatory cytokines such as IL-10 and transforming growth factor β appear to be decreased in IBS colonic and rectal biopsies[54,128,129]. Expression of chemokines, including IL-8, CXCL-9 and monocyte chemoattractant protein-1, which are important in mucosal defence, were also decreased in IBS biopsies[129].
The source of these immune messengers are likely to be from mast cells, the numbers of which are elevated in IBS[128,130,131] and can secrete IL-6 and IL-1β[132] in addition to histamine, tryptase, chymase and proteases. Indeed, Buhner et al[131] described how excitation of non-IBS submucosal neurons with IBS biopsy secretions was dependent on serotonin, tryptase and histamine. Furthermore, the proximity of activated mast cells to colonic nerves was found to correlate with visceral pain severity[130].
Cytokines have been shown to have neuromodulatory effects with IL-6[133], IL-1β[134] and tumour necrosis factor (TNF) α[135] stimulating submucosal secretomotor neurons. This may result in changes in gut function including contractility[136], absorption and/or secretion[133]. IL-6 and IL-1β also influence mucosal ion transport and epithelial permeability and enhance cholinergically-mediated neurotransmission[133,137,138]. Furthermore, IL-6 has a potential role in neurogenic secretory diarrhoea[125] as this cytokine can suppress the inhibitory and anti-secretory effects of norepinephrine by blocking its release from sympathetic fibres[139]. Others have provided evidence that IL-6 attenuates the pre-synaptic inhibition of noradrenalin release, thereby releasing the sympathetic brake[134], which further contributes to a pro-secretory state. Aside from altered GI motility, the other main debilitating symptom of IBS is visceral pain sensitivity. Given the demonstrated effects of cytokines on enteric neuron excitability[133-135] and proven roles in nociception and sensory pain pathways[140], activation of enteric neurons and subsequent evocation of visceral pain make cytokines attractive candidates for mediating the visceral pain-related features of IBS.
The permeability of the epithelial layer which acts as a barrier between the external environment of the gut lumen and the body’s internal milieu is an important consideration in immune activation in IBS. Indeed, some IBS patients have increased intestinal permeability[141], which may be due to proteasomal degradation of tight junction proteins[128,142]. Additionally, altered secretion of inflammatory cytokines may affect barrier function and permeability[129]. Breakdown of the mucosal barrier by IL-6 and other pro-inflammatory cytokines[137,143] may provide access for foreign proteins, thus initiating an immune response in the GI muscle layers resulting in changes in bowel function. In IBS, where circulating IL-6 levels are elevated and the HPA axis is hyper-activated[53], a coincident compromise of the mucosal barrier is observed. Thus, increased permeability of the mucosal barrier and the subsequent initiation of an immune response may contribute to the increase in sensitivity to visceral pain in IBS patients[144].
An additional factor contributing to brain-gut axis signalling in IBS currently gaining considerable attention is the importance of disrupting the luminal microbiota[145,146]. Indeed, microbiota dysbiosis, which may facilitate the adhesion of enteric pathogens in the human gut, has been reported in several IBS studies[147-150]. This virtual organ is integrated into the bi-directional communication in the brain-gut axis with studies demonstrating that microbiota dysbiosis exists in IBS patients and manipulation of the microbial environment with probiotics may lead to symptom improvement[151]. Probiotics have been shown to modulate the immune response in IBS, suppressing pro-inflammatory cytokines[152], maintaining intestinal barrier integrity[153], causing down-regulation of T cells and inhibition of nuclear factor kappa B[154]. Moreover, probiotics prevented adhesion of enteric pathogens to the wall of the GI tract[155]. However, more recent longer-term studies did not detect an improvement in symptoms[156,157]. Other members of the innate immune system that are altered in IBS include the pattern recognition receptors, toll-like receptors (TLRs), which recognise and respond to pathogens. Altered expression of TLR4, 5, 7 and 8 in mucosal biopsies from IBS patients further supports the importance of interactions between the luminal flora and the host in this disorder[158].
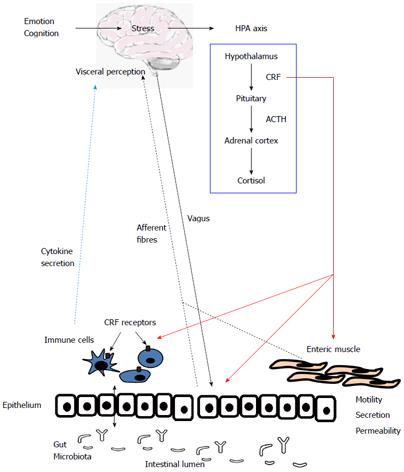
The pathophysiology of altered bowel function in IBS patients remains unclear, however a number of mechanisms have been proposed to contribute to the initiation, exacerbation and persistence of symptoms. Alterations in brain-gut communication[159], stress[70], previous infections[37], abnormal microbiota[160], altered cytokine profiles[53] and increased intestinal permeability[142] have all been discussed. However, we believe that complex interactions between neural, endocrine and immune factors underlie the heterogeneity of symptoms that is characteristic of IBS as diagrammed in Figure 1.
For example, a perceived threat or stressor, which frequently precedes symptom flares, evokes responses from both the immune and stress systems. In healthy individuals this is a crucial response for the adaptation and ultimate survival of an organism. However, in the case of PI-IBS, co-morbidity with anxiety or depression and the occurrence of stressful life events around the time of exposure to the enteric pathogen have been independent predictors of risk for the development of IBS[114,161,162], although not all studies, including the Walkerton cohort[112], detected this association. IBS patients are more likely to be stress-sensitive, as measured by the Holmes and Rahe stress scale, and exhibit elevated numbers of colonic mucosal mast cells[130]. Moreover, acute stress causes increases in the numbers of white blood cells, natural killer cells and CD8+ T-lymphocytes, decreases B cell numbers and stimulates secretion of pro-inflammatory cytokines[163,164], whereas secretion of glucocorticoids and an associated decrease in secretion of pro-inflammatory cytokines is noted in cases of chronic stress[165]. Patients with IBS often exhibit concurrent increases in markers of a hyperactive stress response and immune upregulation such as CRF-stimulated HPA axis hyper-responsivity which is related to the elevation in IL-6 levels[53]. CRF also stimulates the recruitment and activation of granulocytes[166] and mast cells[167] to the gut mucosa.
Immune cells express receptors for several different stress-related peptides including CRF[168]. Indeed, we have detected both CRFR1 and IL-6 receptors on T-helper cells[169]. CRF peptides have potent immunomodulatory actions[170], including degranulation of mast cells[171] and secretion of cytokines[53,172], although it is not yet clear whether these effects are pro-[173] or anti-inflammatory[174,175].
In terms of crosstalk between the stress system and the neural response, many of the psychological disorders frequently found to be co-morbid with IBS also have the capacity to disrupt autonomic balance[52] and indeed, anxiety and depression are associated with depressed parasympathetic activity in IBS patients. Enteric neurons, which directly regulate absorpto-secretory function and gut motility have been shown to express both CRF receptors and IL-6 receptors[169]. Indeed, cytokines such as IL-6 can directly induce excitation of enteric neurons in animal models of IBS[133,176]. IL-6[133], IL-1β[134] and TNFα[135] can cause activation of submucosal secretomotor neurons thereby acting as neuromodulatory factors that can directly influence such gut functions as motility, absorption, secretion and blood flow. IL-6 and IL-1β also have effects on mucosal ion transport and epithelial permeability, in addition to enhancing cholinergically-mediated neurotransmission[133,137,138]. Indeed, soluble mediators released from mast cells in IBS biopsies were found to have excitatory effects on rat sensory neurons[121].
Although the pathophysiology of IBS still requires further elucidation, recent progress in the field has demonstrated the importance of molecular factors such as the stress hormone, CRF and cytokine release and their influence on neural communication between the brain and gut. Further research will hopefully reveal the aberrant signalling between endocrine, immune and neural systems in IBS patients and pave the way towards effective new therapies for this common bowel disorder.
| 1. | Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 953] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 2. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] |
| 3. | Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46:78-82. [PubMed] |
| 4. | El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171-1185. [PubMed] |
| 5. | Su AM, Shih W, Presson AP, Chang L. Characterization of symptoms in irritable bowel syndrome with mixed bowel habit pattern. Neurogastroenterol Motil. 2014;26:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Andrews EB, Eaton SC, Hollis KA, Hopkins JS, Ameen V, Hamm LR, Cook SF, Tennis P, Mangel AW. Prevalence and demographics of irritable bowel syndrome: results from a large web-based survey. Aliment Pharmacol Ther. 2005;22:935-942. [PubMed] |
| 7. | Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 416] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Drossman DA, Chang L, Bellamy N, Gallo-Torres HE, Lembo A, Mearin F, Norton NJ, Whorwell P. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749-1759; quiz 1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol. 2012;47:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945-950. [PubMed] |
| 12. | Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919-933. [PubMed] |
| 13. | Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154 Suppl 1:S63-S70. [PubMed] |
| 14. | Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 588] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 15. | Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Gillanders S, Wild M, Deighan C, Gillanders D. Emotion regulation, affect, psychosocial functioning, and well-being in hemodialysis patients. Am J Kidney Dis. 2008;51:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Spaziani R, Bayati A, Redmond K, Bajaj H, Mazzadi S, Bienenstock J, Collins SM, Kamath MV. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterol Motil. 2008;20:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Spetalen S, Sandvik L, Blomhoff S, Jacobsen MB. Autonomic function at rest and in response to emotional and rectal stimuli in women with irritable bowel syndrome. Dig Dis Sci. 2008;53:1652-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | van Orshoven NP, Andriesse GI, van Schelven LJ, Smout AJ, Akkermans LM, Oey PL. Subtle involvement of the parasympathetic nervous system in patients with irritable bowel syndrome. Clin Auton Res. 2006;16:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Adeyemi EO, Desai KD, Towsey M, Ghista D. Characterization of autonomic dysfunction in patients with irritable bowel syndrome by means of heart rate variability studies. Am J Gastroenterol. 1999;94:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Pellissier S, Dantzer C, Canini F, Mathieu N, Bonaz B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. 2010;35:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Cain KC, Jarrett ME, Burr RL, Hertig VL, Heitkemper MM. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:110-118. [PubMed] |
| 24. | Heitkemper M, Jarrett M, Cain KC, Burr R, Levy RL, Feld A, Hertig V. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2001;46:1276-1284. [PubMed] |
| 25. | Jarrett ME, Burr RL, Cain KC, Hertig V, Weisman P, Heitkemper MM. Anxiety and depression are related to autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2003;48:386-394. [PubMed] |
| 26. | Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125-132. [PubMed] |
| 27. | Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 28. | Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci. 1980;25:404-413. [PubMed] |
| 29. | Prior A, Maxton DG, Whorwell PJ. Anorectal manometry in irritable bowel syndrome: differences between diarrhoea and constipation predominant subjects. Gut. 1990;31:458-462. [PubMed] |
| 30. | Simrén M, Abrahamsson H, Björnsson ES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut. 2001;48:20-27. [PubMed] |
| 31. | Harraf F, Schmulson M, Saba L, Niazi N, Fass R, Munakata J, Diehl D, Mertz H, Naliboff B, Mayer EA. Subtypes of constipation predominant irritable bowel syndrome based on rectal perception. Gut. 1998;43:388-394. [PubMed] |
| 32. | Slater BJ, Plusa SM, Smith AN, Varma JS. Rectal hypersensitivity in the irritable bowel syndrome. Int J Colorectal Dis. 1997;12:29-32. [PubMed] |
| 33. | Steens J, Van Der Schaar PJ, Penning C, Brussee J, Masclee AA. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterol Motil. 2002;14:241-247. [PubMed] |
| 34. | Chen J, Winston JH, Sarna SK. Neurological and cellular regulation of visceral hypersensitivity induced by chronic stress and colonic inflammation in rats. Neuroscience. 2013;248C:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, High KW, Kollarik M, Randich A, Undem B. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009;60:171-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. [PubMed] |
| 38. | Vergnolle N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol Motil. 2008;20 Suppl 1:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1045-G1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386-394. [PubMed] |
| 41. | Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 42. | Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48-57.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 43. | Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 45. | Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842-848. [PubMed] |
| 46. | Hall GB, Kamath MV, Collins S, Ganguli S, Spaziani R, Miranda KL, Bayati A, Bienenstock J. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil. 2010;22:276-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230-231. [PubMed] |
| 49. | Folks DG. The interface of psychiatry and irritable bowel syndrome. Curr Psychiatry Rep. 2004;6:210-215. [PubMed] |
| 50. | Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256-261. [PubMed] |
| 51. | Gué M, Tekamp A, Tabis N, Junien JL, Buéno L. Cholecystokinin blockade of emotional stress- and CRF-induced colonic motor alterations in rats: role of the amygdala. Brain Res. 1994;658:232-238. [PubMed] |
| 52. | Tougas G. The autonomic nervous system in functional bowel disorders. Gut. 2000;47 Suppl 4:iv78-i80; discussion iv87. [PubMed] |
| 53. | Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304-311. [PubMed] |
| 54. | Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol. 1998;12:1077-1085. [PubMed] |
| 56. | Sánchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365-377. [PubMed] |
| 57. | Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122-130. [PubMed] |
| 58. | Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165-186. [PubMed] |
| 59. | Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309-316. [PubMed] |
| 60. | Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799-1809. [PubMed] |
| 61. | Barquist E, Zinner M, Rivier J, Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992;262:G616-G620. [PubMed] |
| 62. | Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716-723. [PubMed] |
| 63. | Gue M, Junien JL, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964-970. [PubMed] |
| 64. | Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958-964. [PubMed] |
| 65. | Trimble N, Johnson AC, Foster A, Greenwood-van Meerveld B. Corticotropin-releasing factor receptor 1-deficient mice show decreased anxiety and colonic sensitivity. Neurogastroenterol Motil. 2007;19:754-760. [PubMed] |
| 66. | Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093-1102. [PubMed] |
| 67. | Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582-G586. [PubMed] |
| 68. | Lu L, Liu D, Ceng X, Ma L. Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur J Neurosci. 2000;12:4398-4404. [PubMed] |
| 69. | Martínez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221-234. [PubMed] |
| 70. | Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33-40. [PubMed] |
| 71. | Teitelbaum AA, Gareau MG, Jury J, Yang PC, Perdue MH. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am J Physiol Gastrointest Liver Physiol. 2008;295:G452-G459. [PubMed] |
| 72. | Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071-4088. [PubMed] |
| 73. | Tjong YW, Ip SP, Lao L, Wu J, Fong HH, Sung JJ, Berman B, Che CT. Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuro Endocrinol Lett. 2010;31:215-220. [PubMed] |
| 74. | Gué M, Del Rio-Lacheze C, Eutamene H, Théodorou V, Fioramonti J, Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271-279. [PubMed] |
| 75. | Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 76. | Taché Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend Med. 2005;2:146-154. [PubMed] |
| 77. | Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60 Suppl 7:33-46. [PubMed] |
| 78. | Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H, Saunders PR, Maillot C, Mayer EA, Taché Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res. 2003;985:32-42. [PubMed] |
| 79. | Taché Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol. 1999;13 Suppl A:18A-25A. [PubMed] |
| 80. | Söderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2001;280:G7-G13. [PubMed] |
| 81. | Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1037-G1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Konturek SJ, Brzozowski T, Konturek PC, Zwirska-Korczala K, Reiter RJ. Day/night differences in stress-induced gastric lesions in rats with an intact pineal gland or after pinealectomy. J Pineal Res. 2008;44:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol. 1996;271:G884-G892. [PubMed] |
| 84. | Castagliuolo I, Wershil BK, Karalis K, Pasha A, Nikulasson ST, Pothoulakis C. Colonic mucin release in response to immobilization stress is mast cell dependent. Am J Physiol. 1998;274:G1094-G1100. [PubMed] |
| 85. | Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012;11:462-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 86. | de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 3264] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 87. | Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav. 2000;69:379-382. [PubMed] |
| 88. | Myers B, Dittmeyer K, Greenwood-Van Meerveld B. Involvement of amygdaloid corticosterone in altered visceral and somatic sensation. Behav Brain Res. 2007;181:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Venkova K, Foreman RD, Greenwood-Van Meerveld B. Mineralocorticoid and glucocorticoid receptors in the amygdala regulate distinct responses to colorectal distension. Neuropharmacology. 2009;56:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res. 2010;214:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | Simrén M, Abrahamsson H, Björnsson ES. Lipid-induced colonic hypersensitivity in the irritable bowel syndrome: the role of bowel habit, sex, and psychologic factors. Clin Gastroenterol Hepatol. 2007;5:201-208. [PubMed] |
| 92. | Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS). Patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421. [PubMed] |
| 93. | Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108-115. [PubMed] |
| 94. | Rogers J, Henry MM, Misiewicz JJ. Increased segmental activity and intraluminal pressures in the sigmoid colon of patients with the irritable bowel syndrome. Gut. 1989;30:634-641. [PubMed] |
| 95. | Evans PR, Kellow JE. Physiological modulation of jejunal sensitivity in health and in irritable bowel syndrome. Am J Gastroenterol. 1998;93:2191-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 97. | Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis. 2009;3:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 98. | Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (2)] |
| 99. | Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 100. | Hellström PM, Näslund E, Edholm T, Schmidt PT, Kristensen J, Theodorsson E, Holst JJ, Efendic S. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil. 2008;20:649-659. [PubMed] |
| 101. | McKee DP, Quigley EM. Intestinal motility in irritable bowel syndrome: is IBS a motility disorder? Part 1. Definition of IBS and colonic motility. Dig Dis Sci. 1993;38:1761-1772. [PubMed] |
| 102. | Quigley EM. Antibiotics for irritable bowel syndrome: hitting the target, but what is it? Gastroenterology. 2011;141:391-393. [PubMed] |
| 103. | Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci. 2007;131:50-56. [PubMed] |
| 104. | Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, Brubaker PL. Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology. 2009;150:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 105. | Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445-4455. [PubMed] |
| 106. | Simrén M, Björnsson ES, Abrahamsson H. High interdigestive and postprandial motilin levels in patients with the irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Zhang H, Yan Y, Shi R, Lin Z, Wang M, Lin L. Correlation of gut hormones with irritable bowel syndrome. Digestion. 2008;78:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Simrén M, Stotzer PO, Sjövall H, Abrahamsson H, Björnsson ES. Abnormal levels of neuropeptide Y and peptide YY in the colon in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2003;15:55-62. [PubMed] |
| 109. | Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, Ardid D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 110. | Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 111. | Sidhu R, Drew K, McAlindon ME, Lobo AJ, Sanders DS. Elevated serum chromogranin A in irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD): a shared model for pathogenesis? Inflamm Bowel Dis. 2010;16:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445-450; quiz 660. [PubMed] |
| 113. | Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol. 2006;101:1894-1899; quiz 1942. [PubMed] |
| 114. | Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400-406. [PubMed] |
| 115. | Kai Y, Takahashi I, Ishikawa H, Hiroi T, Mizushima T, Matsuda C, Kishi D, Hamada H, Tamagawa H, Ito T. Colitis in mice lacking the common cytokine receptor gamma chain is mediated by IL-6-producing CD4+ T cells. Gastroenterology. 2005;128:922-934. [PubMed] |
| 116. | Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 117. | Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779-782. [PubMed] |
| 118. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [PubMed] |
| 119. | Goral V, Kucukoner M, Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepatogastroenterology. 2010;57:751-754. [PubMed] |
| 120. | Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, Presson AP, Yuan PQ, Cortina G, Gong H. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107:262-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 121. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. [PubMed] |
| 122. | Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996;111:1683-1699. [PubMed] |
| 123. | McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 124. | Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, Cooney J, Keeling PW. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570-2576. [PubMed] |
| 125. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [PubMed] |
| 126. | Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 127. | Elsenbruch S, Holtmann G, Oezcan D, Lysson A, Janssen O, Goebel MU, Schedlowski M. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? Am J Gastroenterol. 2004;99:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Coëffier M, Gloro R, Boukhettala N, Aziz M, Lecleire S, Vandaele N, Antonietti M, Savoye G, Bôle-Feysot C, Déchelotte P. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181-1188. [PubMed] |
| 129. | Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467-1476. [PubMed] |
| 130. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] |
| 131. | Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425-1434. [PubMed] |
| 132. | Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135-142. [PubMed] |
| 133. | O’Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2011;300:G241-G252. [PubMed] |
| 134. | Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH, Wood JD. IL-1beta and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J Clin Invest. 1999;103:1309-1316. [PubMed] |
| 135. | Rehn M, Hübschle T, Diener M. TNF-alpha hyperpolarizes membrane potential and potentiates the response to nicotinic receptor stimulation in cultured rat myenteric neurones. Acta Physiol Scand. 2004;181:13-22. [PubMed] |
| 136. | Zhang L, Hu L, Chen M, Yu B. Exogenous interleukin-6 facilitated the contraction of the colon in a depression rat model. Dig Dis Sci. 2013;58:2187-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 137. | Natale L, Piepoli AL, De Salvia MA, De Salvatore G, Mitolo CI, Marzullo A, Portincasa P, Moschetta A, Palasciano G, Mitolo-Chieppa D. Interleukins 1 beta and 6 induce functional alteration of rat colonic motility: an in vitro study. Eur J Clin Invest. 2003;33:704-712. [PubMed] |
| 138. | Kindt S, Vanden Berghe P, Boesmans W, Roosen L, Tack J. Prolonged IL-1beta exposure alters neurotransmitter and electrically induced Ca(2+) responses in the myenteric plexus. Neurogastroenterol Motil. 2010;22:321-e85. [PubMed] |
| 139. | Rühl A, Hurst S, Collins SM. Synergism between interleukins 1 beta and 6 on noradrenergic nerves in rat myenteric plexus. Gastroenterology. 1994;107:993-1001. [PubMed] |
| 140. | De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96:1096-1103, table of contents. [PubMed] |
| 141. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [PubMed] |
| 142. | Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196-201. [PubMed] |
| 143. | Hiribarren A, Heyman M, L’Helgouac’h A, Desjeux JF. Effect of cytokines on the epithelial function of the human colon carcinoma cell line HT29 cl 19A. Gut. 1993;34:616-620. [PubMed] |
| 144. | Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41-46. [PubMed] |
| 145. | Cremonini F, Talley NJ. Treatments targeting putative mechanisms in irritable bowel syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:82-88. [PubMed] |
| 146. | Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050-16055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2500] [Cited by in RCA: 2815] [Article Influence: 187.7] [Reference Citation Analysis (1)] |
| 147. | Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213-222. [PubMed] |
| 148. | Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 502] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 149. | Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. [PubMed] |
| 150. | Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 646] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 151. | Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 152. | O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [PubMed] |
| 153. | Prakash S, Rodes L, Coussa-Charley M, Tomaro-Duchesneau C. Gut microbiota: next frontier in understanding human health and development of biotherapeutics. Biologics. 2011;5:71-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 154. | O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 155. | Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 156. | Begtrup LM, de Muckadell OB, Kjeldsen J, Christensen RD, Jarbøl DE. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 157. | Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 158. | Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13:147-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 268] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 159. | Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861-869. [PubMed] |
| 160. | Barbara G, Stanghellini V, Cremon C, De Giorgio R, Gargano L, Cogliandro R, Pallotti F, Corinaldesi R. Probiotics and irritable bowel syndrome: rationale and clinical evidence for their use. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S214-S217. [PubMed] |
| 161. | Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651-1659. [PubMed] |
| 162. | Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150-153. [PubMed] |
| 163. | Pawlak CR, Jacobs R, Mikeska E, Ochsmann S, Lombardi MS, Kavelaars A, Heijnen CJ, Schmidt RE, Schedlowski M. Patients with systemic lupus erythematosus differ from healthy controls in their immunological response to acute psychological stress. Brain Behav Immun. 1999;13:287-302. [PubMed] |
| 164. | Ackerman KD, Martino M, Heyman R, Moyna NM, Rabin BS. Immunologic response to acute psychological stress in MS patients and controls. J Neuroimmunol. 1996;68:85-94. [PubMed] |
| 165. | Leonard BE. Changes in the immune system in depression and dementia: causal or co-incidental effects? Int J Dev Neurosci. 2001;19:305-312. [PubMed] |
| 166. | Zheng PY, Feng BS, Oluwole C, Struiksma S, Chen X, Li P, Tang SG, Yang PC. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 167. | Söderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099-1108. [PubMed] |
| 168. | Song C, Earley B, Leonard BE. Behavioral, neurochemical, and immunological responses to CRF administration. Is CRF a mediator of stress? Ann N Y Acad Sci. 1995;771:55-72. [PubMed] |
| 169. | O’Malley D, Cryan JF, Dinan TG. Crosstalk between interleukin-6 and corticotropin-releasing factor modulate submucosal plexus activity and colonic secretion. Brain Behav Immun. 2013;30:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 170. | Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1-27. [PubMed] |
| 171. | Wu Y, Hu J, Zhang R, Zhou C, Xu Y, Guan X, Li S. Enhanced intracellular calcium induced by urocortin is involved in degranulation of rat lung mast cells. Cell Physiol Biochem. 2008;21:173-182. [PubMed] |
| 172. | Ando T, Rivier J, Yanaihara H, Arimura A. Peripheral corticotropin-releasing factor mediates the elevation of plasma IL-6 by immobilization stress in rats. Am J Physiol. 1998;275:R1461-R1467. [PubMed] |
| 173. | Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. 2004;89:5352-5361. [PubMed] |
| 174. | Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN. Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett. 2005;579:4259-4264. [PubMed] |
| 175. | Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol. 2007;210:774-783. [PubMed] |
| 176. | O’Malley D, Dinan TG, Cryan JF. Altered expression and secretion of colonic interleukin-6 in a stress-sensitive animal model of brain-gut axis dysfunction. J Neuroimmunol. 2011;235:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
P- Reviewers: Chen L, Hoensch HP, Gonzalez-Reimers E, Pozo MJ, Zhu YL S- Editor: Ma YJ L- Editor: A E- Editor: Ma S