Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8638
Revised: February 14, 2014
Accepted: April 2, 2014
Published online: July 14, 2014
Processing time: 185 Days and 19.7 Hours
AIM: To evaluate the application of bipolar coagulation (BIP) in hepatectomy by comparing the efficacy of BIP alone, cavitron ultrasonic surgical aspirator (CUSA) + BIP and conventional clamp crushing (CLAMP).
METHODS: Based on our database of patient records, a total of 380 consecutive patients who underwent hepatectomy at our hospital were retrospectively studied for the efficacy of BIP alone, CUSA + BIP and CLAMP. Of all the patients, 75 received saline-coupled BIP (Group A), 53 received CUSA + BIP (Group B), and 252 received CLAMP (Group C). The pre-, mid-, and postoperative clinical manifestations were compared, and the effects of those maneuvers were evaluated.
RESULTS: There was no obvious difference among the preoperative indexes between the different groups. The operative time was longer in Groups A and B than in Group C (P < 0.001 for both). The amount of bleeding and the rate of transfusion during the operation were significantly higher in Group C than in Groups A and B (P < 0.001 for all). The incidence of postoperative complications in Group C (46.43%) was higher than that in Groups A (30.67%, P = 0.015) and B (28.30%, P = 0.016). The patients’ liver function recovery and postoperative hospital stay were not significantly different. BIP could decrease intraoperative hemorrhage and postoperative complications compared to CLAMP.
CONCLUSION: Simple saline-coupled BIP should be considered a safe and reliable technique for liver resection to decrease intraoperative hemorrhage and postoperative complications.
Core tip: The aim of this clinical study is to recommend a simplified and feasible surgical technique for liver resection. In this study, we found that simple saline-coupled bipolar electrocautery (BIP) could reduce blood loss, blood transfusion and complications compared with clamp crushing. Therefore, saline-coupled BIP can accomplish hepatectomy excellently and would be a safe and reliable technique that is easily applied in liver resection.
- Citation: Guo JY, Li DW, Liao R, Huang P, Kong XB, Wang JM, Wang HL, Luo SQ, Yan X, Du CY. Outcomes of simple saline-coupled bipolar electrocautery for hepatic resection. World J Gastroenterol 2014; 20(26): 8638-8645
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8638
Hepatectomy and its related surgical complications remain major concerns for surgeons operating on the liver. Controlling hemorrhage, shortening the time of inflow occlusion, and performing anatomical limited resections are important strategies for safe and careful dissection of the liver parenchyma. For patients with hepatocellular carcinoma (HCC), massive hemorrhage and blood transfusions are the powerful determinants of early liver failure and immune repression, which cause adverse effects, often leading to early tumor recurrence, and seriously affecting long-term survival after resection[1,2].
Advancements in the theory and practice of liver surgery have led to the invention of different approaches, such as the cavitron ultrasonic surgical aspirator (CUSA), water jet scalpel, monopolar floating ball, ligasure, microwave and other procedures to transect the liver parenchyma favorably. CUSA combined with bipolar coagulation (BIP) and conventional clamp crushing (CLAMP) are favored by most surgeons at many medical centers[3-6]. Notably, it is critical to expose vasculature and decrease blood loss during hepatic resection. As a device for hemostasis, BIP has aroused increased interest because of its excellent hemostatic effect and low thermal damage to surrounding tissues. Because of these potential benefits, increasing numbers of surgeons are also applying BIP in hepatectomy as a preferred method. Although various BIP devices and techniques have been developed in some centers, the efficacy of BIP alone is actually unclear, hindering its wide adoption. This retrospective cohort analysis investigated the efficacy of BIP alone, CUSA + BIP and CLAMP.
Between April 2011 and May 2013, data from 380 consecutive patients who underwent liver resection at our hospital were collected, based on our database of patient records. Through CT, MRI or pathologic examinations of these patients, the following was detected: 245 malignant tumors (including 179 HCC), 47 benign tumors (such as hemangioma, hamartoma, and focal nodular hyperplasia), 70 cases of hepatolithiasis and 18 cases of other diseases (such as liver abscess, polycystic liver, and hepatic hydatid). Detailed characteristics of those patients are shown in Table 1. This study was approved by the ethics board of The First Affiliated Hospital of Chongqing Medical University, and written informed consent was obtained from all patients.
| Baseline characteristic | BIP (n = 75) | CUSA + BIP (n = 53) | CLAMP (n = 252) | P value | |
| Age1 (yr) | 54.1 (17-81) | 50.7 (16-85) | 53.7 (19-81) | 0.256 | |
| Sex | Male | 47 (62.67) | 33 (62.26) | 155 (61.51) | 0.981 |
| Cause of surgery | HCC | 38 (50.67) | 20 (37.74) | 121 (48.02) | 0.868 |
| Other malignancy | 14 (18.67) | 9 (16.98) | 43 (17.06) | ||
| Benign tumour | 8 (10.66) | 10 (18.87) | 29 (11.51) | ||
| Intrahepatic stone | 12 (16.00) | 11 (20.75) | 47 (18.65) | ||
| Others | 3 (4.00) | 3 (5.66) | 12 (4.76) | ||
| Child-Pugh | A | 56 (74.67) | 41 (77.36) | 210 (83.33) | 0.196 |
| B | 19 (25.33) | 12 (22.64) | 42 (16.67) | ||
| ICG-R15 | < 15 | 67 (89.33) | 46 (86.79) | 219 (86.90) | 0.849 |
| ≥ 15 | 8 (10.67) | 7 (13.21) | 33 (13.10) | ||
| Background liver | Normal | 31 (41.33) | 31 (58.49) | 130 (51.58) | 0.359 |
| Chronic hepatitis | 24 (32.00) | 12 (22.64) | 61 (24.21) | ||
| Cirrhosis | 20 (26.67) | 10 (18.87) | 61 (24.21) | ||
| Level of surgeon | Chief doctor | 31 (20.67) | 29 (27.36) | 127 (25.20) | 0.069 |
| Deputy director doctor | 56 (37.33) | 40 (37.74) | 226 (44.84) | ||
| Attending doctor | 63 (42.00) | 37 (34.90) | 151 (29.96) | ||
| Type of resection | Extended | 13 (17.33) | 8 (15.09) | 35 (13.89) | 0.347 |
| Hemihepatectomy | |||||
| Hemihepatectomy | 33 (44.00) | 20 (37.74) | 108 (42.85) | ||
| Lobectomy | 29 (38.67) | 21 (39.62) | 92 (36.51) | ||
| Segmentectomy | 0 | 4 (7.55) | 13 (5.16) | ||
| Wedge resection | 0 | 0 | 4 (1.59) | ||
| Tumor | Yes | 60 (80.00) | 39 (73.58) | 193 (76.59) | 0.689 |
| Tumor condition | Size, < 30 mm | 14 (23.33) | 7 (17.95) | 56 (29.02) | 0.300 |
| ≥ 30 mm | 46 (76.67) | 32 (82.05) | 137 (70.98) | ||
| Single | 36 (60.00) | 31 (79.49) | 115 (59.59) | 0.059 | |
| Multiple | 24 (40.00) | 8 (20.51) | 78 (40.41) | ||
| Superficial | 36 (60.00) | 28 (71.79) | 141 (73.06) | 0.151 | |
| Deep | 24 (40.00) | 11 (28.21) | 52 (26.94) | ||
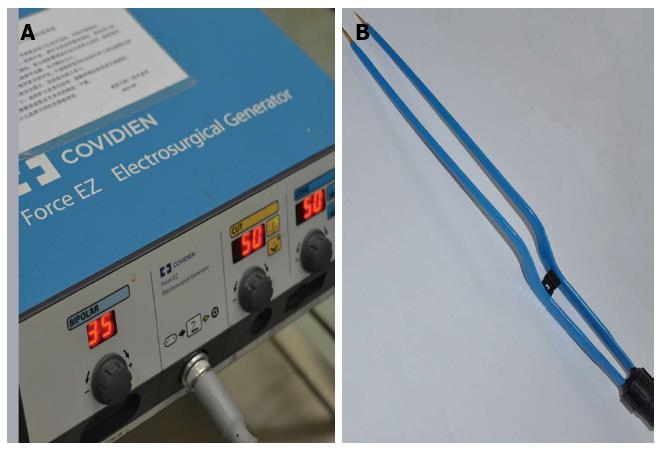
Patients were divided into 3 groups: Group A, patients treated by BIP, with BIP administered by Shuyou surgical instrument Inc, Zhejiang, China, and Force EZ Electrosurgical Generator; Covidien Inc, Boulder, Colo, United States; bipolar, 70W, Figure 1; Group B, patients treated by CUSA, with CUSA administered by Cavitron Ultrasonic Surgical Aspirator System 200; Valleylab Inc, Boulder, Colo, United States; amplitude, 70W; and Group C, patients receiving combined treatment of BIP and CLAMP. All patients received general anesthesia. For fair comparison and avoidance of the potential for patient selection bias with these various techniques by different surgeons, three experienced hepatic surgeons in our unit performed all operations. We chose one technique over the other due to equipment availability or change over time. CUSA and CLAMP were performed between April 2011 and April 2012, and BIP was performed from April 2012 to May 2013. There is no significant difference in the characteristics of the three groups of patients (P > 0.05), including the fibrotic or cirrhotic liver and tumor condition. Except for BIP, no other hemostatic device (i.e., argon beam coagulator) was used on any of these patients undergoing hepatic resection. The operation time (min), intraoperative bleeding (mL), the rate of blood transfusion and the amount of packed red blood cells transfusion (the amount of bleeding more than 25% of the blood volume or hemoglobin lower than 70 g/L was the indication of transfusion) were analyzed.
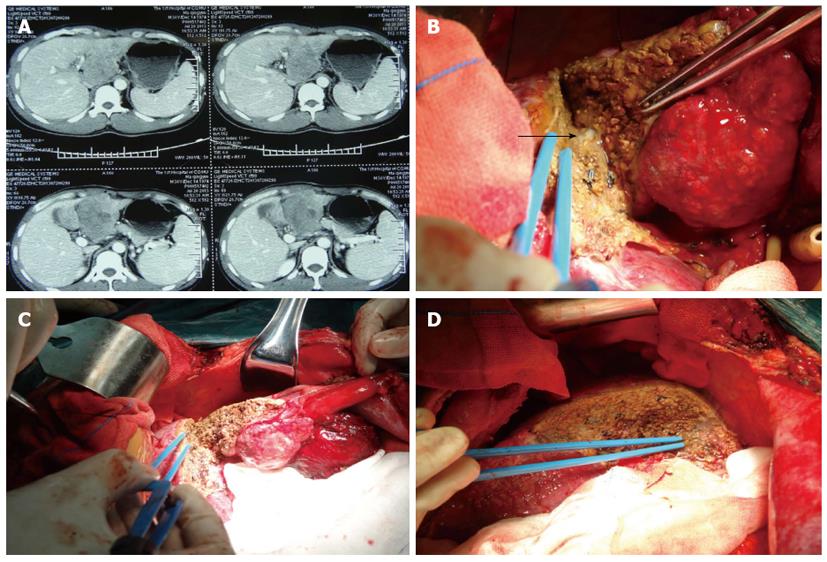
Hepatectomy was performed following standard procedures. The hemostatic forcep tip was moved vertically to the resectional line when transecting the parenchyma by CLAMP. The clamp held approximately 1 cm of the liver parenchyma each time. Hepatic parenchyma cells were disrupted and emulsified by vibration, and the bile ducts and vessels were preserved using CUSA; hemostasis was performed by BIP. The procedure of hepatic resection by saline-coupled BIP alone had water dripping from two sides of the bipolar electrocoagulator, and the power was set at 70 watt. BIP was combined with scissors and sucker for disruption, washing, hemostasis and suction. However, vessels of more than 3 mm in diameter were exposed to ligate, and vessels less than 3 mm in diameter were coagulated (Figure 2). Our results demonstrated that most of the hepatectomies did not require blocking the portal vein blood supply.
Postoperatively, all patients were admitted to the ICU ward for rehabilitation. Postoperative outcomes included recovery of liver function, length of postoperative hospitalization, and the rate of complications. To monitor liver function, albumin (ALB, g/L), total bilirubin (TB, μmol/L), alanine transaminase (ALT, μmol/L), and aspertate aminotransferase (AST, μmol/L) levels were measured on days 1 and 3 after resection. Complications for monitoring included: abdominal cavity infection, bile leakage, acute hepatic failure, respiratory failure, severe hemorrhage, reoperation within 30 d of surgery, hyperbilirubinemia, sepsis, intestinal obstruction, pulmonary embolism, hepatorenal syndrome, death within 30 d of surgery, pleural effusion, incision infection, incision dehiscence, pulmonary infection, delayed recovery of liver function, seroperitoneum, pulmonary atelectasis and deep vein thrombosis.
For statistical analysis, measurement values are expressed as mean with standard deviation or median with interquartile range. The differences in the measurement values were detected by ANOVA (F) or Kruskal-Wallis (H) rank test. The differences in enumeration data were compared by χ2 or Kruskal-Wallis (H) rank test. The difference between both groups was determined via Bonferroni correction. P < 0.05 was considered statistically significant. All data were analyzed using SPSS Version 17.0.
A total of 380 consecutive patients underwent liver resection. Preoperative data for patients in each group are presented in Table 1. The following was not significantly different between each group (P > 0.05 for all): age, gender, the cause of operation, liver function, indocyanine clearance (ICG-R15), liver disease, tumor condition (tumors located 2 cm or less from the liver surface were defined as superficial; those more than 2 cm from the surface were defined as deep), and extent of liver resection (resection range: right trilobectomy, extended left hepatactomy, right hepatactomy; right anterior lobe, right posterior lobe, left liver, central portion resection, S5+7, S5+6; Couinaud lobectomy; local hepatectomy; enucleation).
Intraoperative data are shown in Table 2. Median blood loss during surgery was 315 mL in Group A, 328 mL in Group B and 583 mL in Group C (P < 0.001). The percentage of patients whose hemorrhage volume was more than 1000 mL was 2.67% in Group A, 9.43% in Group B and 23.81% in Group C (P < 0.001). The blood transfusion rate was 16.00% for Group A, 13.21% for Group B and 46.43% for Group C (P < 0.001). The median amount of transfusion with packed red cells was 37 mL in Group A, 56 mL in Group B and 69 mL in Group C (P < 0.001). There was no significant difference between Group A and B (P > 0.05, Table 3).
| Outcome | BIP (n = 75) | CUSA+BIP (n = 53) | CLAMP (n = 252) | P value | |
| Blood loss1 | ≤ 1000 mL | 73 (97.33) | 48 (90.57) | 192 (76.19) | < 0.001 |
| > 1000 mL | 2 (2.67) | 5 (9.43) | 60 (23.81) | ||
| Intraoperative transfusion1 | 12 (16.00) | 7 (13.21) | 117 (46.43) | < 0.001 | |
| Blood loss (mL) median2 | 315 (50-1800) | 328 (50-2000) | 583 (50-5000) | < 0.001 | |
| Transfusion of RBC (mL) median | 37 (0-800) | 56 (0-1200) | 69 (0-2800) | < 0.001 | |
| Operative time (min) median | 315 (105-965) | 335 (80-730) | 265 (60-590) | < 0.001 | |
| BIP (A), CUSA + BIP (B), CLAMP (C) | (A vs B) | (B vs C) | (A vs C) | |
| P value | P value | P value | ||
| Blood loss, > 1000 mL | 0.973 | < 0.001 | < 0.001 | |
| Intraoperative transfusion | 0.662 | < 0.001 | < 0.001 | |
| Blood loss (mL), median | 0.670 | < 0.001 | < 0.001 | |
| Transfusion of RBC (mL), median | 0.731 | < 0.001 | < 0.001 | |
| Operative time (min), median | 0.409 | < 0.001 | < 0.001 | |
| Complications | Yes | 0.773 | 0.016 | 0.015 |
| Abdominal infection | 1.000 | 0.153 | 0.130 | |
| Bile leak | 0.635 | 0.260 | 0.108 | |
| Ascites | 0.760 | 0.149 | 0.014 | |
Postoperative complications of each group were compared and are presented in Table 4. The incidence of total complications in Group C was higher than that in Groups A and B (46.43% vs 30.67% vs 28.30%, P = 0.007). There were significant differences in single complication rates including abdominal infection (group A 2.67% vs Group B 1.89% vs Group C 8.73%, P = 0.035), bile leakage (Group A 1.33% vs Group B 1.89% vs Group C 7.14%, P = 0.039) and seroperitoneum (Group A 1.33% vs Group B 3.77% vs Group C 11.51%, P = 0.002). The results demonstrated that significant differences existed between Groups A and C for seroperitoneum (1.33% vs 11.51%, P = 0.014). Deaths within 30 d of surgery were not statistically significant among the groups. In Group A, one patient died from pulmonary embolism. No patient died in Group B. In Group C, one patient died from severe hemorrhage and multiple organ dysfunction (1.33% vs 0% vs 0.79%, P = 0.710). The incidence of other complications was not significantly different among the three groups (P > 0.05 for all).
| Complication | BIP | CUSA + BIP | CLAMP | P value |
| (n = 75) | (n = 53) | (n = 252) | ||
| Yes | 23 (30.67) | 15 (28.30) | 117 (46.43) | 0.007 |
| Abdominal infection | 2 (2.67) | 1 (1.89) | 22 (8.73) | 0.035 |
| Bile leak > 2 wk | 1 (1.33) | 1 (1.89) | 18 (7.14) | 0.039 |
| Acute liver failure | 1 (1.33) | 0 | 2 (0.79) | 0.710 |
| Return to operating room | 0 | 1 (1.89) | 3 (1.19) | 0.577 |
| Respiratory failure | 0 | 1 (1.89) | 2 (0.79) | 0.448 |
| Bleeding > 500 mL within 24 h | 1 (1.33) | 1 (1.89) | 7 (2.78) | 0.725 |
| Hyperbilirubinemia | 3 (4.00) | 5 (9.43) | 8 (3.17) | 0.181 |
| Sepsis | 0 | 0 | 2 (0.79) | 1.000 |
| Ileus | 0 | 0 | 3 (1.19) | 1.000 |
| Pulmonary embolism | 1 (1.33) | 0 | 1 (0.40) | 0.561 |
| Hepatorenal syndrome | 0 | 0 | 1 (0.40) | 1.000 |
| 30-d postoperative death | 1 (1.33) | 0 | 2 (0.79) | 0.710 |
| Deep venous thrombosis | 0 | 0 | 1 (0.40) | 1.000 |
| Pleural effusion | 4 (5.33) | 3 (5.66) | 24 (9.52) | 0.370 |
| Wound infection | 16 (21.33) | 12 (22.64) | 50 (19.84) | 0.884 |
| Wound dehiscence | 0 | 0 | 2 (0.79) | 1.000 |
| Pneumonia | 2 (2.67) | 2 (3.77) | 16 (6.35) | 0.359 |
| Hepatic insufficiency | 7 (9.33) | 2 (3.77) | 20 (7.94) | 0.432 |
| Ascites | 1 (1.33) | 2 (3.77) | 29 (11.51) | 0.002 |
| Atelectasis | 1 (1.33) | 1 (1.89) | 3 (1.19) | 0.928 |
Postoperative recovery conditions are shown in Table 5. No differences were noted in ALB, TB, ALT or AST levels on postoperative day 3 and preoperation (all P > 0.05). Elevated ALB levels were found in Group B when compared to Groups A and C (preoperative: 40.9 ± 5.3 vs 37.5 ± 6.1 vs 39.4 ± 5.8, P = 0.003; day 1: 29.4 ± 4.3 g/L vs 26.9 ± 5.6 g/L vs 26.2 ± 4.9 g/L, P < 0.001; day 3: 29.9 ± 4.8 g/L vs 27.2 ± 4.3 g/L vs 27.5 ± 4.3 g/L, P < 0.001). ALT levels were lower in Group C than in Groups A and B on postoperative day 1 (200 μmol/L vs 314 μmol/L vs 279 μmol/L, P < 0.001). There was no significant difference among the groups for other liver function recovery tests.
| Recovery evaluation | BIP | CUSA+BIP | CLAMP1 | P value | |
| (n = 75) | (n = 53) | (n = 251) | |||
| TB (μmol/L) | Pre- | 15.2 (4.3-360.7) | 15.5 (4.9-313.9) | 13.5 (3.5-358.9) | 0.425 |
| Day 1 | 26.5 (4.3-292.1) | 23.4 (4.3-292.3) | 25.9 (5.5-321.4) | 0.771 | |
| Day 3 | 22.1 (3.9-215.4) | 18.3 (6.6-422.0) | 21.9 (5.6-294.6) | 0.325 | |
| ΔTB2 | 6.4 (-189.9-160.3) | 2.9 (-135.3-261.9) | 6 (-137.7-156.9) | 0.585 | |
| ALT (μmol/L) | Pre- | 32 (11-337) | 48 (10-579) | 36 (7-984) | 0.187 |
| Day 1 | 314 (21-1616) | 279 (32-1760) | 200 (16-3449) | < 0.001 | |
| Day 3 | 136 (17-1717) | 135 (27-2246) | 130 (10-1114) | 0.272 | |
| ΔALT2 | 85 (-174-1691) | 84 (-449-2183) | 84 (-808-1065) | 0.388 | |
| AST (μmol/L) | Pre- | 35 (14-261) | 40 (6-531) | 33 (8-698) | 0.693 |
| Day 1 | 323 (43-1591) | 294 (20-3182) | 247 (14-3750) | 0.109 | |
| Day 3 | 88 (15-1404) | 59 (20-2388) | 72 (12-940) | 0.143 | |
| ΔAST2 | 38 (-133-1372) | 24 (-510-2350) | 31 (-617-853) | 0.168 | |
| ALB (g/L)3 | Pre- | 37.5 ± 6.1 | 40.9 ± 5.3 | 39.4 ± 5.8 | 0.003 |
| mean ± SD | Day 1 | 26.9 ± 5.6 | 29.4 ± 4.3 | 26.2 ± 4.9 | < 0.001 |
| Day 3 | 27.2 ± 4.3 | 29.9 ± 4.8 | 27.5 ± 4.3 | < 0.001 | |
| ΔALB2 | 10.3 ± 6.0 | 11.0 ± 6.2 | 12.0 ± 5.9 | 0.078 | |
| Hospital stay (d) mean ± SD | 16.6 ± 7.4 | 15.3 ± 9.2 | 16.5 ± 9.6 | 0.666 | |
These results demonstrated that the operation time in Group C was shorter than that in the other two groups (median time: 315 min in Group A, 335 min in Group B, 265 min in Group C, P < 0.001). There was no difference between Groups A and B (P = 0.409). There was no significant difference for hospital stays after surgery (Group A 16.6 ± 7.4 d vs Group B 15.3 ± 9.2 d, vs Group C 16.5 ± 9.6 d; P = 0.666).
Controlling bleeding and choosing the appropriate technique to transect the liver are the key points in hepatectomy[7-9]. For CLAMP, the whole surgical procedure is simple and fast during parenchyma transaction. However, the operation is relatively rough and can easily lead to severe liver damage. For CUSA + BIP, the surgeon can gradually grind and suction out parenchyma with clear anatomical structure under direct vision. Obviously, blood loss and postoperative complications were decreased using this method. Although Takayama et al[10] reported hepatectomy with CLAMP, which was associated with a significantly higher grade and appearance of the landmark hepatic vein on the cut surface than CUSA + BIP. Of note, the results of that study were based on the surgeon’s familiarity with CLAMP. Not every surgeon has such skilled operation levels. Therefore, some research centers could not fully agree with them[11,12]. Moreover, other methods to transect the liver parenchyma, such as water jet scalpel, habib procedures, monopolar floating ball and ligasure are not commonly used worldwide[13-16]. To our best knowledge, this is the first report about comparative analysis of the application of saline-coupled BIP alone in hepatectomy for the excellent hemostasis and satisfactory surgical results.
The majority of bleeding for liver resection comes from the process of transecting the liver parenchyma. The present study demonstrated that liver resection was relatively rough by CLAMP, especially for deep tissue. Because of long and large hemostatic forceps and its small operative field, the maneuver results in unnecessary vascular damage. For the rate of blood transfusion, BIP and CUSA + BIP were lower than CLAMP. A meta-analysis performed by Liu et al[17] revealed that perioperative blood transfusion was associated with adverse clinical outcomes for HCC. Moreover, blood loss of more than 2000 mL was also a poor prognostic factor for HCC[18]. Our results support that BIP and CUSA + BIP could improve quality of life and survival of HCC patients. Further comparison between BIP and CUSA + BIP showed that BIP could get the same effect for blood loss, blood transfusion, operational security and prognosis after simplified surgical procedures. Our results demonstrate that the incidence of postoperative complications, such as abdominal infection, bile leakage and seroperitoneum, were higher in the CLAMP group compared to the BIP and CUSA + BIP groups. The reason for that phenomenon was less blood transfusion and section integrity[19-21]. Delva et al[22] supported that the recovery of liver function was related to the Pringle maneuver length, cirrhosis, the quantity and quality of the remnant liver parenchyma and blood transfusion. Massive parenchyma was grasped and stitched with the clamp. Stable fibrillar structure features were formed, vessels and biliary ducts were closed ideally and less thermal damage was made by BIP. Postoperative complications were associated with operational technique, blood loss, blood transfusion and individual differences for surgery[23-26]. Therefore, BIP could be safer and decrease the risk of complications for hepatectomy.
Our results showed that, for an experienced hepatobiliary surgeon, the intrahepatic bile duct could be clearly freed by a single BIP without CUSA. Because the resectional extent of the ultrasonic section device was approximately 4 mm, it was easy to expose the tumor using CUSA when the surgeon could not ensure the distance between the section and tumor. There was a potential danger of spreading hepatitis when CUSA disrupted hepatic cells[27].
Compared to CLAMP, the cutting speed by BIP was lower, and the operative time was prolonged approximately 20%-30%. It is actually not surprising that the use of these instruments resulted in increased operative time because there is more attention paid to dissecting out blood vessels and coagulating or ligating them before dividing them. However, the operative time of BIP could be decreased if performed by highly skilled surgeons. BIP could not only separate the liver parenchyma but also reduce hemorrhage. Additionally, the operative time in the BIP group was an independent prognostic factor for postoperative complications.
In conclusion, simple saline-coupled BIP could reduce blood loss, blood transfusion and complications compared to CLAMP. Therefore, saline-coupled BIP would be a safe and reliable technique easily applied in liver resection.
The authors gratefully acknowledge the assistance of Bin Peng from Department of Epidemiology, Public Health College of Chongqing Medical University, who helped us with the statistics. The authors also thank Professor C Michael Dabney (National University, San Diego, United States) for the critical reading of the manuscript.
Liver resections are high risk procedures, the key points in which are controlling bleeding and choosing appropriate technique. Advancements in the theory and practice of liver surgery have led to the invention of multiple approaches to transect liver parenchyma. For these reasons, choosing a safe and reliable method of hepatectomy is important in decreasing intraoperative hemorrhaging and postoperative complications.
Cavitron ultrasonic surgical aspirator (CUSA) combined with bipolar coagulation (BIP) and conventional clamp crushing (CLAMP) are favored by most surgeons at many medical centers. As a device for hemostasis, more and more surgeons are applying BIP in hepatectomy as a preferred method. However, compared to CUSA and CLAMP, the efficacy of BIP alone is actually unclear.
The present study is the first report about comparative analysis of the application of saline-coupled BIP alone in hepatectomy for excellent hemostasis and satisfactory surgical results.
The study results suggest that saline-coupled BIP could reduce blood loss, blood transfusion and complications compared to CLAMP. Saline-coupled BIP can accomplish hepatectomy excellently and would be a safe and reliable technique easily applied in liver resection.
CUSA is a powerful ultrasonic aspirator that allows the surgeon to accomplish tissue resection with accurate control while fragmentation, suction, and irrigation occur simultaneously; BIP is a technique for surgical dissection and hemostasis that consists of two tips with current pass confined in a small amount of tissue.
This is an interesting manuscript describing outcomes of three different methods of parenchymal transection in hepatic surgery. As with any retrospective study, it has its inherent flaws but does show that liver transection can be safely performed by various methods.
| 1. | Barbas AS, Turley RS, Mallipeddi MK, Lidsky ME, Reddy SK, White RR, Clary BM. Examining reoperation and readmission after hepatic surgery. J Am Coll Surg. 2013;216:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Lapierre V, Aupérin A, Tiberghien P. Transfusion-induced immunomodulation following cancer surgery: fact or fiction? J Natl Cancer Inst. 1998;90:573-580. [PubMed] |
| 3. | Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814-22, discussion 822-3. [PubMed] |
| 4. | Rahbari NN, Elbers H, Koch M, Vogler P, Striebel F, Bruckner T, Mehrabi A, Schemmer P, Büchler MW, Weitz J. Randomized clinical trial of stapler versus clamp-crushing transection in elective liver resection. Br J Surg. 2014;101:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Patrlj L, Tuorto S, Fong Y. Combined blunt-clamp dissection and LigaSure ligation for hepatic parenchyma dissection: postcoagulation technique. J Am Coll Surg. 2010;210:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Riediger C, Mueller MW, Geismann F, Lehmann A, Schuster T, Michalski CW, Kuhn K, Friess H. Comparative analysis of different transection techniques in minor and major hepatic resections: a prospective cohort study. Int J Surg. 2013;11:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Palavecino M, Kishi Y, Chun YS, Brown DL, Gottumukkala VN, Lichtiger B, Curley SA, Abdalla EK, Vauthey JN. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery. 2010;147:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Lochan R, Ansari I, Coates R, Robinson SM, White SA. Methods of haemostasis during liver resection--a UK national survey. Dig Surg. 2013;30:375-382. [PubMed] |
| 10. | Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, Ijichi M, Hasegawa K. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922-928. [PubMed] |
| 11. | Campagnacci R, De Sanctis A, Baldarelli M, Di Emiddio M, Organetti L, Nisi M, Lezoche G, Guerrieri M. Hepatic resections by means of electrothermal bipolar vessel device (EBVS) LigaSure V: early experience. Surg Endosc. 2007;21:2280-2284. [PubMed] |
| 12. | Lee KF, Wong J, Ng W, Cheung YS, Lai P. Feasibility of liver resection without the use of the routine Pringle manoeuver: an analysis of 248 consecutive cases. HPB (Oxford). 2009;11:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Rau HG, Duessel AP, Wurzbacher S. The use of water-jet dissection in open and laparoscopic liver resection. HPB (Oxford). 2008;10:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Torzilli G, Donadon M, Montorsi M, Makuuchi M. Concerns about ultrasound-guided radiofrequency-assisted segmental liver resection. Ann Surg. 2010;251:1191-112; author reply 1191-112;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Torzilli G, Donadon M, Marconi M, Procopio F, Palmisano A, Del Fabbro D, Botea F, Spinelli A, Montorsi M. Monopolar floating ball versus bipolar forceps for hepatic resection: a prospective randomized clinical trial. J Gastrointest Surg. 2008;12:1961-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Ikeda M, Hasegawa K, Sano K, Imamura H, Beck Y, Sugawara Y, Kokudo N, Makuuchi M. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Ann Surg. 2009;250:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, Zhou Y, Zhou Y, Zhang Y. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e64261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 339] [Article Influence: 19.9] [Reference Citation Analysis (1)] |
| 19. | Benzoni E, Molaro R, Cedolini C, Favero A, Cojutti A, Lorenzin D, Intini S, Adani GL, Baccarani U, Bresadola F. Liver resection for HCC: analysis of causes and risk factors linked to postoperative complications. Hepatogastroenterology. 2007;54:186-189. [PubMed] |
| 20. | Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Orci LA, Toso C, Mentha G, Morel P, Majno PE. Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia-reperfusion injury and surgical stress response in patients undergoing liver resection. Br J Surg. 2013;100:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Delva E, Camus Y, Nordlinger B, Hannoun L, Parc R, Deriaz H, Lienhart A, Huguet C. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg. 1989;209:211-218. [PubMed] |
| 23. | Neal CP, Mann CD, Pointen E, McGregor A, Garcea G, Metcalfe MS, Berry DP, Dennison AR. Influence of hepatic parenchymal histology on outcome following right hepatic trisectionectomy. J Gastrointest Surg. 2012;16:2064-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Chok KS, Ng KK, Poon RT, Lo CM, Fan ST. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP, Prasad KR. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
P- Reviewer: Kapoor S S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Wang CH