Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8583
Revised: February 22, 2014
Accepted: March 12, 2014
Published online: July 14, 2014
Processing time: 250 Days and 18 Hours
AIM: To investigate the prognostic significance of preoperative fibrinogen levels in colon cancer patients.
METHODS: A total of 255 colon cancer patients treated at the Affiliated Tumor Hospital of Xinjiang Medical University from June 1st 2005 to June 1st 2008 were enrolled in the study. All patients received radical surgery as their primary treatment method. Preoperative fibrinogen was detected by the Clauss method, and all patients were followed up after surgery. Preoperative fibrinogen measurements were correlated with a number of clinicopathological parameters using the Student t test and analysis of variance. Survival analyses were performed by the Kaplan-Meier method and Cox regression modeling to measure 5-year disease-free survival (DFS) and overall survival (OS).
RESULTS: The mean preoperative fibrinogen concentration of all colon cancer patients was 3.17 ± 0.88 g/L. Statistically significant differences were found between preoperative fibrinogen levels and the clinicopathological parameters of age, smoking status, tumor size, tumor location, tumor-node-metastasis (TNM) stage, modified Glasgow prognostic scores (mGPS), white blood cell (WBC) count, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and carcinoembryonic antigen (CEA) levels. Univariate survival analysis showed that TNM stage, tumor cell differentiation grade, vascular invasion, mGPS score, preoperative fibrinogen, WBC, NLR, PLR and CEA all correlated with both OS and DFS. Alpha-fetoprotein (AFP) and body mass index correlated only with OS. Kaplan-Meier analysis revealed that both OS and DFS of the total cohort, as well as of the stage II and III patients, were higher in the hypofibrinogen group compared to the hyperfibrinogen group (all P < 0.05). In contrast, there was no significant difference between OS and DFS in stage I patients with low or high fibrinogen levels. Cox regression analysis indicated preoperative fibrinogen levels, TNM stage, mGPS score, CEA, and AFP levels correlated with both OS and DFS.
CONCLUSION: Preoperative fibrinogen levels can serve as an independent prognostic marker to evaluate patient response to colon cancer treatment.
Core tip: Inflammatory cytokines have been implicated in the development of several malignancies, including colon cancer. Specifically, fibrinogen has been correlated with some clinicopathological factors of cancer. However, the prognostic value of fibrinogen measurements remains unclear, particularly in colon cancer patients. In this study, preoperative fibrinogen levels were correlated with a poor prognosis in a cohort of colon cancer patients. These data suggest that preoperative fibrinogen levels can serve as an independent prognostic factor for colon cancer patients following radical surgery.
- Citation: Sun ZQ, Han XN, Wang HJ, Tang Y, Zhao ZL, Qu YL, Xu RW, Liu YY, Yu XB. Prognostic significance of preoperative fibrinogen in patients with colon cancer. World J Gastroenterol 2014; 20(26): 8583-8591
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8583.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8583
Recently, the incidence of gastrointestinal cancer has increased as standards of living have improved. Colon cancer is one of the most common malignancies of the digestive track in humans. Early detection is strongly correlated with an improved prognosis, thus it is important to develop simple, non-invasive methods to accurately detect colon cancer and assess patient prognosis. Recent evidence indicates that fibrinogen, a major protein in the blood clotting process, is linked to a number of malignancies[1-4]. Elevated fibrinogen levels have been correlated with tumor progression, invasiveness, and metastasis[5-7]. However, the role of fibrinogen in colon cancer remains unclear.
To assess the prognostic significance of fibrinogen for colon cancer patients, preoperative fibrinogen levels, clinicopathological parameters, and follow-up data were analyzed from a cohort of 255 colon cancer patients undergoing radical surgery. The results indicate that preoperative fibrinogen levels correlate with a number of cancer clinicopathological parameters, and are negatively correlated with 5-year survival rates. Therefore, fibrinogen measurements could provide a useful prognostic tool for colon cancer patients.
Clinicopathological parameters and follow-up data were assessed from 255 colon cancer patients (135 male, 120 female) who received radical surgery in the Affiliated Tumor Hospital of Xinjiang Medical University between June 1st 2005 and June 1st 2008. Mean patient age was 59.47 ± 12.63 years. Prior to participation, a diagnosis of colon cancer was confirmed by histopathology for all patients. Tumor-node-metastasis (TNM) stage was determined according to the American Joint Committee on Cancer/International Union Against Cancer TNM staging system of colorectal cancer (2010, 7th edition). The American Society of Anesthesiologists score of the patients was 1 in 198 cases and 2 in 57 cases. No patient received preoperative chemotherapy or immunotherapy. The presence of complex metastases, such as uncertain lumps, micrometastases (particularly in the liver), and abdominal/pelvic lymph node metastases, were diagnosed using enhanced computed tomography, magnetic resonance imaging, positron emission tomography-computed tomography, and puncture biopsies.
The following criteria were used to exclude patients: confirmed metastasis (39 cases), use of procoagulant or anticoagulant drugs within 8 wk of the enrollment (11 cases), hepatitis infection (23 cases), kidney disease (9 cases), myocardial infarction (14 cases), hypertension (47 cases), or other infectious or hematologic diseases which could influence fibrinogen levels (22 cases). Postoperative adjuvant chemotherapy was administered to stage II patients with high-risk factors (including poor differentiation, large lumps, T4 stage, less than 1 cm of tumor resection margin, fewer than 12 lymph nodes for postoperative biopsy) and stage III patients.
Blood samples were obtained by phlebotomy; 5-7 d prior to surgery, 2 mL of blood was collected from each patient. Preoperative fibrinogen levels were measured by the Clauss standard method with bovine thrombin (100 NIH U/mL).
After surgery, patients underwent follow-up assessments once every 3 mo for the first 2 years, and then once every 6 mo until 5 years after surgery. Follow-ups were either by outpatient or inpatient review, or by telephone. Thirty patients did not participate in follow-up analyses because they did not maintain any communication with physicians after surgery. In addition, 3 patients developed dysthymia and were unable to cooperate with the study procedures, one patient committed suicide due to the burden of the cancer, and 2 patients did non participate in follow-up assessments for unknown reasons. Therefore, the total follow-up rate in the study was 88.2%.
The study design and procedures described below were approved by the Ethics Committee of the Affiliated Tumor Hospital of Xinjiang Medical University (No. W-201305). All patients provided informed written consent prior to their participation.
All data is presented as mean ± SD. Univariate analyses of preoperative fibrinogen and each clinicopathological parameter were performed using the Student t-test and analysis of variance. Survival rates were determined using the Kaplan-Meier method and the log-rank test to perform comparisons between the hypofibrinogen (< 2.61 g/L) and hyperfibrinogen (≥ 2.61 g/L) groups. Multivariate survival analysis was performed using the Cox regression model to determine relative risk (RR) and 95% confidence interval (CI). All statistical analyses were carried out using SPSS for Windows, version 18 (SPSS Inc., Chicago, IL, United States). Statistical significance was defined by P≤ 0.05.
The mean level of preoperative fibrinogen levels of all 255 colon cancer patients was 3.17 ± 0.88 g/L. Statistical analyses were performed to assess any relationships between fibrinogen levels and clinicopathological factors (Table 1). Preoperative fibrinogen levels were associated with age, smoking status, tumor size, tumor location, TNM stage, modified Glasgow prognostic (mGPS) score, white blood cell (WBC) count, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and carcinoembryonic antigen (CEA) levels (P < 0.05 for all parameters). Meanwhile, preoperative fibrinogen was not related to the patient’s nationality, body mass index (BMI), tumor grade, gross tumor type, tumor invasion into the nervous or lymphatic system, or serum alpha-fetoprotein (AFP) levels.
| Parameters | n | Fibrinogen (g/L, mean ± SD) | P value |
| Age (yr) | |||
| < 60 | 119 | 3.29 ± 1.01 | 0.047 |
| ≥ 60 | 136 | 3.07 ± 0.73 | |
| Nationality | |||
| Han | 216 | 3.16 ± 0.87 | 0.892 |
| Uygur | 30 | 3.24 ± 0.92 | |
| Others | 9 | 3.12 ± 0.94 | |
| BMI (kg/m2) | |||
| < 18.5 | 8 | 3.54 ± 0.82 | 0.638 |
| 18.5-23.99 | 115 | 3.15 ± 0.94 | |
| 24-27.99 | 102 | 3.14 ± 0.83 | |
| ≥ 28 | 30 | 3.23 ± 0.79 | |
| Smoking | |||
| Never | 173 | 3.06 ± 0.82 | 0.018 |
| Light | 18 | 3.43 ± 0.74 | |
| Heavy | 64 | 3.39 ± 0.99 | |
| Grade (tumor differentiation) | |||
| Low | 203 | 3.12 ± 0.86 | 0.080 |
| High | 52 | 3.36 ± 0.92 | |
| TNM | |||
| I | 29 | 2.70 ± 0.58 | 0.002 |
| II | 139 | 3.24 ± 0.95 | |
| III | 87 | 3.21 ± 0.78 | |
| Tumor size (cm) | |||
| < 4 | 51 | 2.81 ± 0.72 | 0.000 |
| 4-6 | 114 | 3.01 ± 0.73 | |
| ≥ 6 | 90 | 3.57 ± 0.98 | |
| Type | |||
| Mess type | 104 | 3.13 ± 0.87 | 0.390 |
| Ulceration | 148 | 3.21 ± 0.89 | |
| Infiltrative | 3 | 2.58 ± 0.13 | |
| Tumor location | |||
| Left | 155 | 3.06 ± 0.82 | 0.020 |
| Right | 89 | 3.38 ± 0.97 | |
| Transverse | 11 | 2.99 ± 0.55 | |
| Perineural invasion | |||
| Yes | 7 | 3.40 ± 0.59 | 0.488 |
| No | 248 | 3.16 ± 0.88 | |
| Vascular invasion | |||
| Yes | 13 | 3.29 ± 0.94 | 0.619 |
| No | 242 | 3.16 ± 0.87 | |
| CEA (ng/mL) | |||
| < 5 | 161 | 3.08 ± 0.89 | 0.038 |
| ≥ 5 | 94 | 3.32 ± 0.84 | |
| AFP (ng/mL) | |||
| < 5 | 231 | 3.17 ± 0.88 | 0.747 |
| ≥ 5 | 24 | 3.11 ± 0.82 | |
| mGPS | |||
| 0 | 163 | 3.00 ± 0.77 | 0.000 |
| 1 | 71 | 3.37 ± 0.94 | |
| 2 | 21 | 3.80 ± 1.00 | |
| WBC count | |||
| < 5 × 109/L | 61 | 2.68 ± 0.56 | 0.000 |
| ≥ 5 × 109/L | 194 | 3.32 ± 0.90 | |
| NLR | |||
| < 5 | 238 | 3.13 ± 0.86 | 0.013 |
| ≥ 5 | 17 | 3.69 ± 1.02 | |
| PLR | |||
| < 150 | 134 | 2.96 ± 0.75 | 0.000 |
| 150-300 | 105 | 3.41 ± 0.96 | |
| > 300 | 15 | 3.40 ± 0.83 | |
To determine the prognostic significance, preoperative fibrinogen and other clinicopathological factors were correlated with the 5-year disease-free survival (DFS) and overall survival (OS) rates of the patient cohort (Table 2). Results show that TNM stage, tumor grade, vascular invasion status, mGPS score, preoperative fibrinogen, WBC count, NLR, PLR, and CEA levels were all significantly correlated with both OS and DFS rates (all P < 0.05). In addition, AFP and BMI were significantly correlated with OS (P < 0.05). In contrast, no significant relationship was observed between prognosis and age, nationality, smoking status, gross tumor type, tumor location, or perineural invasion status.
| Parameters | n | 5-yr DFS | χ2 | Pvalue | 5-yr OS | χ2 | Pvalue |
| Age (yr) | |||||||
| < 60 | 119 | 49.3% | 0.301 | 0.583 | 64.90% | 0.616 | 0.432 |
| ≥ 60 | 136 | 47.7% | 61.80% | ||||
| Nationality | |||||||
| Han | 216 | 51.3% | 3.185 | 0.203 | 64.60% | 3.477 | 0.176 |
| Uygur | 30 | 34.4% | 48.80% | ||||
| Others | 9 | 33.3% | 76.20% | ||||
| BMI (kg/m2) | |||||||
| < 18.5 | 8 | 37.5% | 3.905 | 0.272 | 50% | 9.296 | 0.026 |
| 18.5-23.99 | 115 | 46.5% | 55.60% | ||||
| 24-27.99 | 102 | 54.4% | 74.00% | ||||
| ≥ 28 | 30 | 39.7% | 59.30% | ||||
| Smoking | |||||||
| Never | 173 | 49.1% | 0.079 | 0.961 | 65.00% | 0.153 | 0.927 |
| Light | 18 | 50.0% | 60.20% | ||||
| Heavy | 64 | 47.7% | 61.40% | ||||
| Grade | |||||||
| Low | 203 | 52.7% | 5.948 | 0.015 | 67.10% | 4.482 | 0.034 |
| High | 52 | 32.7% | 49.30% | ||||
| TNM | |||||||
| I | 29 | 78.7% | 38.724 | 0.000 | 86.20% | 28.420 | 0.000 |
| II | 139 | 57.3% | 70.60% | ||||
| III | 87 | 25.2% | 43.90% | ||||
| Type | |||||||
| Mess type | 104 | 54.3% | 2.523 | 0.283 | 68% | 3.088 | 0.213 |
| Ulceration | 148 | 44.1% | 59.30% | ||||
| Infiltrative | 3 | 27.2% | 0 | ||||
| Tumor location | |||||||
| Left | 155 | 51.8% | 2.992 | 0.224 | 67.20% | 5.563 | 0.062 |
| Right | 89 | 42.0% | 54.30% | ||||
| Transverse | 11 | 54.5% | 81.80% | ||||
| Perineural invasion | |||||||
| Yes | 7 | 42.9% | 0.551 | 0.458 | 42.90% | 2.108 | 0.147 |
| No | 248 | 48.6% | 63.90% | ||||
| Vascular invasion | |||||||
| Yes | 13 | 15.4% | 8.432 | 0.004 | 30.80% | 7.747 | 0.005 |
| No | 242 | 50.3% | 65.10% | ||||
| CEA (ng/mL) | |||||||
| < 5 | 161 | 56.6% | 18.630 | 0.000 | 71.20% | 15.269 | 0.000 |
| ≥ 5 | 94 | 33.6% | 49.10% | ||||
| AFP (ng/mL) | |||||||
| < 5 | 231 | 49.7% | 3.568 | 0.059 | 65.20% | 5.117 | 0.024 |
| ≥ 5 | 24 | 35.0% | 43.20% | ||||
| mGPS | |||||||
| 0 | 163 | 58.9% | 79.236 | 0.000 | 77.80% | 90.552 | 0.000 |
| 1 | 71 | 60.2% | 49.80% | ||||
| 2 | 21 | 0.0% | 4.80% | ||||
| WBC count | |||||||
| < 5 × 109/L | 61 | 60.9% | 4.032 | 0.045 | 76.70% | 4.870 | 0.027 |
| ≥ 5 × 109/L | 194 | 44.8% | 59.30% | ||||
| NLR | |||||||
| < 5 | 238 | 50.1% | 6.060 | 0.014 | 64.70% | 4.754 | 0.029 |
| ≥ 5 | 17 | 25.0% | 43.80% | ||||
| PLR | |||||||
| < 150 | 134 | 55.6% | 11.095 | 0.004 | 71.20% | 11.772 | 0.003 |
| 150-300 | 105 | 43.0% | 57.20% | ||||
| > 300 | 15 | 20.0% | 33.30% | ||||
| Fibrinogen (g/L) | |||||||
| < 2.61 | 74 | 77.0% | 26.717 | 0.000 | 87.20% | 20.255 | 0.000 |
| ≥ 2.61 | 181 | 36.9% | 53.60% |
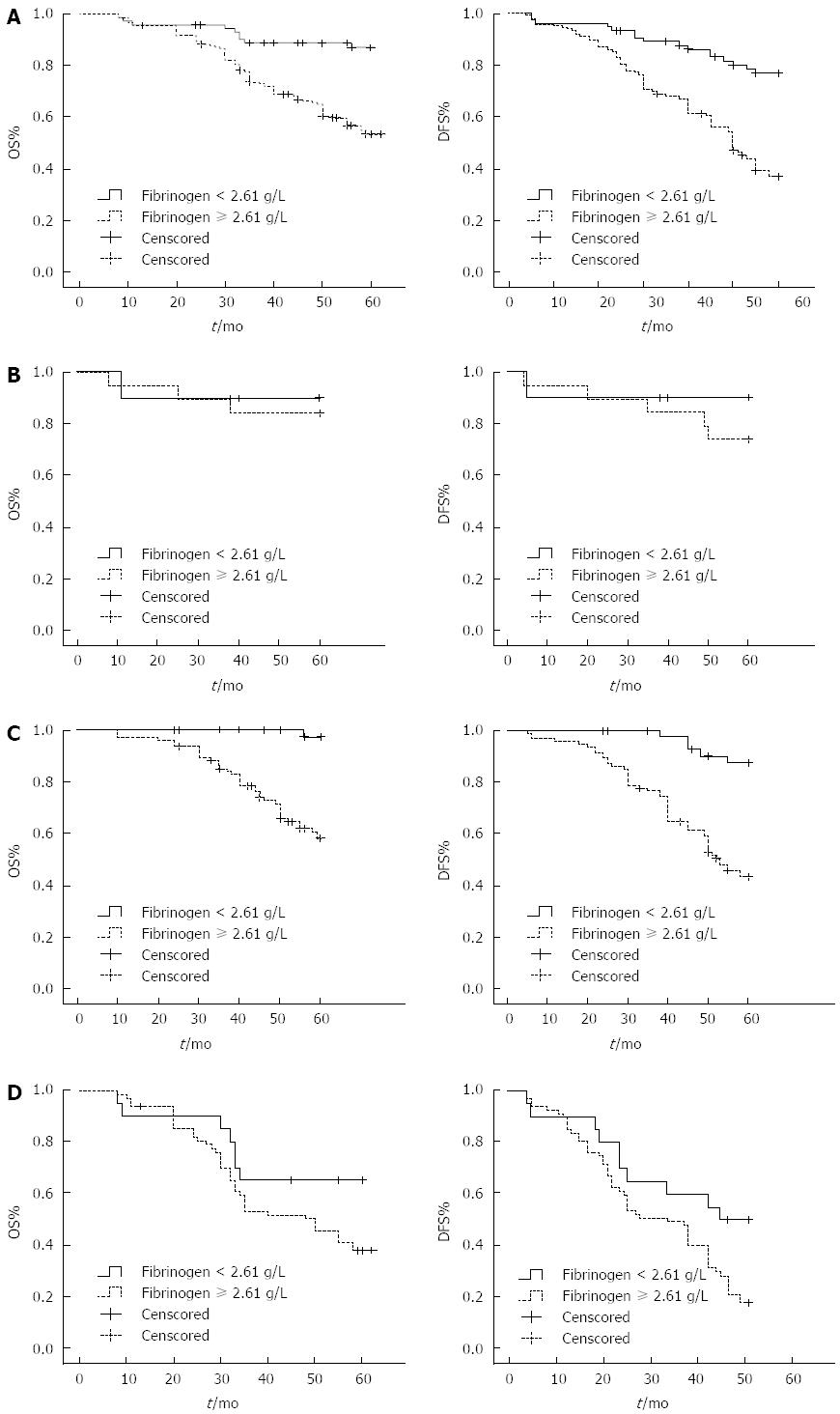
Given the correlation observed between preoperative fibrinogen and DFS and OS rates, the effect of fibrinogen levels on patient survival was examined. Patients were divided into hyperfibrinogen (> 2.61 g/L) or hypofibrinogen (< 2.61 g/L) groups. Kaplan-Meier survival analyses were performed to determine 5-year OS and DFS rates for each group. Among the entire cohort, both OS and DFS rates were higher in the hypofibrinogen group compared with the hyperfibrinogen group (Figure 1A; P < 0.05). Furthermore, hyperfibrinogen was associated with significantly reduced OS and DFS rates in stage II and III colon cancer patients (Figure 1C, D; P < 0.05), but not in patients with stage I disease (Figure 1B). However, the lack of a significant association with stage I disease may be related to the relatively small sample size for this patient group.
Cox regression analyses showed that preoperative fibrinogen, TNM stage, mGPS score, CEA and AFP levels were significantly associated with both 5-year OS and 5-year DFS (P < 0.05). For DFS, the strongest predictive factor was preoperative fibrinogen levels (RR: 2.940; 95%CI: 1.707-5.064, P < 0.05), followed by TNM stage, mGPS, AFP and CEA levels. Similarly, fibrinogen was the strongest predictor for OS (RR: 3.324; 95%CI: 1.620-6.821, P < 0.05), followed by mGPS, TNM stage, AFP and CEA (Table 3). These data indicate that preoperative fibrinogen plays an important role in the prognosis of colon cancer patients who receive radical surgery.
| Parameters | 5-yr OS | 5-yr DFS | ||||
| Pvalue | RR | 95%CI | Pvalue | RR | 95%CI | |
| PLR | 0.330 | 0.825 | 0.560-1.215 | 0.274 | 0.835 | 0.604-1.154 |
| NLR | 0.262 | 1.541 | 0.724-3.282 | 0.109 | 1.701 | 0.889-3.255 |
| WBC count | 0.384 | 1.318 | 0.708-2.452 | 0.547 | 1.161 | 0.715-1.886 |
| AFP | 0.002 | 2.583 | 1.406-4.744 | 0.008 | 2.111 | 1.218-3.656 |
| CEA | 0.010 | 1.779 | 1.146-2.762 | 0.002 | 1.762 | 1.223-2.539 |
| Vascular invasion | 0.456 | 1.359 | 0.606-3.047 | 0.528 | 1.260 | 0.614-2.585 |
| Fibrinogen | 0.001 | 3.324 | 1.620-6.821 | 0.000 | 2.940 | 1.707-5.064 |
| BMI | 0.401 | 0.888 | 0.674-1.171 | - | - | - |
| TNM | 0.000 | 2.583 | 1.704-3.916 | 0.000 | 2.405 | 1.722-3.360 |
| Grade | 0.538 | 0.851 | 0.511-1.420 | 0.892 | 0.970 | 0.629-1.497 |
| mGPS | 0.000 | 2.968 | 2.137-4.122 | 0.000 | 2.400 | 1.799-3.201 |
Recent evidence has indicated that there are links between inflammation and cancer development[8]. In addition to the genetic basis of cancer, the host inflammatory response can play an important role in tumor progression[9,10]. In particular, inflammatory cytokines are associated with a number of cancer types and contribute to tumorigenesis by promoting tumor cell proliferation, invasion, or metastasis, which can have dramatic effects on patient prognosis. Several inflammatory markers that are readily detectable in plasma are currently being investigated as prognostic factors for cancer patients, as well as for their role in tumor biology. These markers include monocyte chemo-attractant proteins[11,12], WBC count, NLR[13,14], PLR[11,15], and fibrinogen[16], among others. However, there is currently no consensus concerning their efficacy to assess patient prognosis[1-3].
Fibrinogen is synthesized in the liver as a 350 kDa glycoprotein. Fibrinogen plays an important role in blood coagulation, thrombosis, wound healing, and platelet aggregation. Moreover, recent studies suggest fibrinogen is associated with cancer development (Table 4). As a component of the extracellular matrix, fibrinogen can affect cell behavior through interactions with other matrix proteins, or the cell itself. Fibrinogen has been associated with increased tumor growth and metastatic potential, although the precise mechanism remains unclear. One potential mechanism involves fibrinogen’s influence on tumor cell proliferation, migration, and signaling through interactions with multiple integrin and non-integrin receptors. Another possible mechanism is the promotion of tumor angiogenesis, as fibrinogen has been shown to cooperate with growth factors, including vascular endothelial and fibroblast growth factors, to stimulate angiogenesis[17-19]. Furthermore, the fibrinolytic system derived from fibrinogen also plays a facilitating role in both angiogenesis and the proliferation process of tumor cells[20].
| Ref. | Country | n | Pre-therapeutic plasma fibrinogen (g/L) | Cancer type | PrognosisDFS/OS |
| Takeuchi et al[1] (2007) | Japan | 105 | 3.86 ± 1.02 | Esophageal cancer | No, neither |
| Polterauer et al[2] (2009) | Austria | 313 | 4.17 ± 1.30 | Cervical cancer | Yes, both |
| Polterauer et al[3] (2009) | Austria | 422 | 4.50 ± 1.50 | Ovarian cancer | Yes, both |
| Ghezzi et al[4] (2010) | Italy | 336 | hyper: 40.8% | Endometrial cancer | Yes, both |
| Zhao et al[5] (2012) | China | 160 | 4.72 ± 1.46 | Non-small cell lung cancer | DFS, no; OS, yes |
| Son et al[6] (2013) | South Korea | 624 | 3.25 ± 0.88 | Non-metastatic colon cancer | Yes, both |
| Zhu et al[7] (2013) | China | 275 | 3.92 (0.68-9.80) | Non-small cell lung cancer | Yes, both |
Several lines of evidence indicate that fibrinogen can promote tumor cell metastasis. The fibrinolytic system stimulates adhesion between tumor cells and platelets or endothelial cells, thereby promoting the dissemination of tumor cells into the bloodstream[21]. Studies using fibrinogen-deficient mice have indicated that fibrinogen is an important factor in the metastasis of circulating tumor cells[22]. In addition, fibrinogen can suppress natural killer cell-mediated tumor cell clearance, leading to an increase in the number of metastatic cells[23]. Taken together, these data suggest that fibrinogen is involved in tumor cell invasion and metastasis, further supporting its potential as a prognostic factor for cancer patients.
In clinical studies, pre-treatment fibrinogen levels have been associated with clinicopathological factors in a number of cancers. Fibrinogen has been correlated with the depth of tumor invasion, tumor size, and WBC and platelet counts[1]. Elevated fibrinogen levels are associated with lymph node metastasis and distant metastasis. Fibrinogen has also been correlated with clinicopathological factors such as age and BMI[3]. In our study, we analyzed additional clinicopathological parameters in colon cancer patients, and found that preoperative fibrinogen levels correlated with several cancer-relevant factors, including age, smoking status, tumor size, tumor location, TNM stage, mGPS score, WBC count, NLR, and PLR. The results of this study also support previous reports demonstrating that smoking, which confers a high risk of tumor development, is correlated with serum fibrinogen levels[24]. However, the mechanisms linking smoking and fibrinogen in cancer patients remain unclear.
Tumor progression is the result of complex interactions between tumor cells and the host environment[25] and the host inflammatory response. Tumors can occur at sites of inflammation, such as during hepatitis virus or H. pylori infection, and can also trigger the release of pro-inflammatory cytokines to promote an inflammatory microenvironment that favors tumorigenesis[26,27]. The elevation of fibrinogen levels in colon cancer patients may be the result of tissue damage and subsequent inflammatory responses[28]. For example, tumors can induce a hypercoagulable and hypoxic state through a variety of mechanisms, including the stimulation of an inflammatory response[18,21,29]. Thus, it is likely that elevated fibrinogen reflects the host-tumor response[15]. However, tumor cells can produce endogenous fibrinogen, suggesting that other sources may also contribute[18]. Consistent with this evidence, results from this study indicate that serum fibrinogen is closely related with other inflammatory markers, such as the mGPS score, WBC count, NLR and PLR, suggesting that fibrinogen reflects the inflammatory status of colon cancer tumors.
A systemic inflammatory response prior to surgery may reflect the presence of an additional disease or a change in the patient’s physiology[30]. Several studies have demonstrated that fibrinogen levels can serve as an independent prognostic factor for cancer patients. For example, fibrinogen levels were closely associated with both 5-year DFS and OS in a cohort of 313 cervical cancer patients[2]. A similar analysis of a cohort of 422 ovarian cancer patients led to the same conclusion[3]. In colon cancer patients, systemic inflammatory markers can predict patient survival following radical resection[15] and provide independent prognostic risk factors for tumor specificity and OS for these patients[31].
A Cox regression statistical analysis in the present study showed that preoperative fibrinogen level was closely associated with prognosis of patients with colon cancer, which is in contrast to the results of Takeuchi et al[1], who found that serum fibrinogen was not associated with prognosis in 105 esophageal cancer patients. Furthermore, survival analyses indicated that both the 5-year OS and DFS of the entire cohort, as well as of stage II and stage III patients, were significantly higher in the hypofibrinogen group compared to the hyperfibrinogen group. These results are consistent with previous studies investigating inflammation and colon cancer, and suggest that fibrinogen can serve as a useful prognostic marker for colon cancer patients.
In conclusion, this study demonstrates that fibrinogen is closely associated with several clinicopathological features and 5-year survival rates of colon cancer patients. Furthermore, elevated preoperative fibrinogen levels were found to predict poor survival in colon cancer patients. These data suggest that preoperative fibrinogen is a promising prognostic marker for colon cancer patients receiving radical surgery. Moreover, fibrinogen-based therapies may provide a novel therapeutic approach for treating colon cancer.
Inflammation, including inflammatory cytokines, plays an important role in the incidence, invasion, and metastasis of several tumors. However, as an inflammation precursor, the role of fibrinogen in tumors, particularly colon cancer, remains unclear.
Increasing evidence indicates that the host inflammatory response plays an important role in tumor development and progression. Inflammatory mediators and cytokines secreted by inflammatory cells are elevated in tumors and promote proliferation, invasion or metastasis, which consequently have an impact on patient prognosis. These inflammatory markers, including monocyte chemo-attractant proteins, white blood cell counts, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and fibrinogen, are easily detected in plasma and therefore could represent useful prognostic markers. However, the prognostic value of inflammatory markers, particularly fibrinogen, remains unclear.
While there have been a number of studies on the relationship of fibrinogen and cancer, few have analyzed this relationship in colon cancer patients. In this study, the prognostic significance of preoperative fibrinogen in colon cancer patients was investigated. The results illustrate that preoperative fibrinogen is negatively correlated with prognosis in colon cancer patients who received radical surgery, suggesting that preoperative fibrinogen is a promising prognostic marker for colon cancer patients.
The study indicates that preoperative fibrinogen is an independent prognostic factor for colon cancer patients that undergo radical surgery. Consequently, preoperative fibrinogen may serve as a potential prognostic marker for colon cancer patients. Moreover, anti-fibrinogen therapies may provide a novel strategy for treating colon cancer.
Fibrinogen is a plasma glycoprotein that is involved in blood clot formation. Levels of this protein have been associated with tumor progression and metastasis in many cancer types.
This study comprehensively analyzes the prognostic significance of preoperative fibrinogen levels in patients with colon cancer. The results suggest that preoperative fibrinogen is a promising independent prognostic factor for patients with colon cancer, and may provide a new target for colon cancer therapy.
| 1. | Takeuchi H, Ikeuchi S, Kitagawa Y, Shimada A, Oishi T, Isobe Y, Kubochi K, Kitajima M, Matsumoto S. Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol. 2007;22:2222-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Polterauer S, Seebacher V, Hefler-Frischmuth K, Grimm C, Heinze G, Tempfer C, Reinthaller A, Hefler L. Fibrinogen plasma levels are an independent prognostic parameter in patients with cervical cancer. Am J Obstet Gynecol. 2009;200:647.e1-647.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Polterauer S, Grimm C, Seebacher V, Concin N, Marth C, Tomovski C, Husslein H, Leipold H, Hefler-Frischmuth K, Tempfer C. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: a multicenter study. Oncologist. 2009;14:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 4. | Ghezzi F, Cromi A, Siesto G, Giudici S, Serati M, Formenti G, Franchi M. Prognostic significance of preoperative plasma fibrinogen in endometrial cancer. Gynecol Oncol. 2010;119:309-313. [PubMed] |
| 5. | Zhao J, Zhao M, Jin B, Yu P, Hu X, Teng Y, Zhang J, Luo Y, Zhang L, Zheng S. Tumor response and survival in patients with advanced non-small-cell lung cancer: the predictive value of chemotherapy-induced changes in fibrinogen. BMC Cancer. 2012;12:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Son HJ, Park JW, Chang HJ, Kim DY, Kim BC, Kim SY, Park SC, Choi HS, Oh JH. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20:2908-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 7. | Zhu JF, Cai L, Zhang XW, Wen YS, Su XD, Rong TH, Zhang LJ. High plasma fibrinogen concentration and platelet count unfavorably impact survival in non-small cell lung cancer patients with brain metastases. Chin J Cancer. 2014;33:96-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 8. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11517] [Article Influence: 479.9] [Reference Citation Analysis (2)] |
| 9. | McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Tenesa A, Theodoratou E, Din FV, Farrington SM, Cetnarskyj R, Barnetson RA, Porteous ME, Campbell H, Dunlop MG. Ten common genetic variants associated with colorectal cancer risk are not associated with survival after diagnosis. Clin Cancer Res. 2010;16:3754-3759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 475] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 11. | Brown DJ, Milroy R, Preston T, McMillan DC. The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol. 2007;60:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 768] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 13. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 861] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 751] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 16. | Seebacher V, Polterauer S, Grimm C, Husslein H, Leipold H, Hefler-Frischmuth K, Tempfer C, Reinthaller A, Hefler L. The prognostic value of plasma fibrinogen levels in patients with endometrial cancer: a multi-centre trial. Br J Cancer. 2010;102:952-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 199] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost. 2008;6:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Fitzgerald DJ. Fibrinogen receptor and platelet signalling. Blood Coagul Fibrinolysis. 1999;10 Suppl 1:S77-S79. [PubMed] |
| 20. | Gerner C, Steinkellner W, Holzmann K, Gsur A, Grimm R, Ensinger C, Obrist P, Sauermann G. Elevated plasma levels of crosslinked fibrinogen gamma-chain dimer indicate cancer-related fibrin deposition and fibrinolysis. Thromb Haemost. 2001;85:494-501. [PubMed] |
| 21. | Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302-3309. [PubMed] |
| 22. | Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 767] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 23. | Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Everett CJ, Wells BJ, Frithsen IL, Koopman RJ. Smoking, fibrinogen and cancer mortality. J Natl Med Assoc. 2007;99:328-333. [PubMed] |
| 25. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5856] [Article Influence: 234.2] [Reference Citation Analysis (1)] |
| 26. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8560] [Article Influence: 475.6] [Reference Citation Analysis (0)] |
| 27. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8435] [Article Influence: 527.2] [Reference Citation Analysis (9)] |
| 28. | Beer JH, Haeberli A, Vogt A, Woodtli K, Henkel E, Furrer T, Fey MF. Coagulation markers predict survival in cancer patients. Thromb Haemost. 2002;88:745-749. [PubMed] |
| 29. | Lawrence SO, Simpson-Haidaris PJ. Regulated de novo biosynthesis of fibrinogen in extrahepatic epithelial cells in response to inflammation. Thromb Haemost. 2004;92:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009;249:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Richards CH, Leitch EF, Horgan PG, Anderson JH, McKee RF, McMillan DC. The relationship between patient physiology, the systemic inflammatory response and survival in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2010;103:1356-1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
P- Reviewers: Chen KF, García MT, Murtaza I, Wasserberg N S- Editor: Zhai HH L- Editor: Cant MR E- Editor: Wang CH