Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8558
Revised: December 31, 2013
Accepted: April 8, 2014
Published online: July 14, 2014
Processing time: 272 Days and 15.8 Hours
AIM: To longitudinally investigate cytokine gene expression and protein levels in Th17 and Treg cells, to observe T-cell phenotypes during hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACHBLF) and to analyze changes in Th17 and Treg phenotypes during disease progression.
METHODS: We measured the expression of seven Th17/Treg differentiation-related genes and serum concentrations of the corresponding cytokines in 18 ACHBLF, 18 chronic hepatitis B (CHB) disease controls and 10 healthy controls (HCs) by real-time quantitative PCR and enzyme linked immunosorbent assay. Peripheral Th17 and Treg cell frequencies were analyzed by flow cytometry.
RESULTS: From the onset of ACHBLF, patients presented with a conductive Th17 differentiation cytokine environment accompanied by high Th17 frequency and high serum IL-17 levels, which were sustained throughout the disease course. The Treg-related cytokine IL-2 and Foxp3 were also up-regulated from disease onset, and Foxp3 gene expression showed a gradually increasing trend during ACHBLF. The circular phenotype of Treg and Th17 cells showed changes from the onset of ACHGLF. At disease onset, Th17 frequency increased significantly compared with both CHB and HCs, but Treg cell frequency decreased significantly compared with CHB. During the ACHBLF event, Th17 frequency remained higher compared with HCs, but decreased sharply from the peak point to the recovery point; Treg cell frequency increased gradually during the ACHBLF event. Treg and Th17 cell counts correlated with ACHBLF development; in all patients, serum IL-17 levels significantly correlated with patient serum ALT levels. In survivors, Th17 frequency at the onset point and the Treg to Th17 ratio at the peak point correlated with the patient’s model for end stage liver disease (MELD) plus sodium (MELD-Na) score. The Treg to Th17 ratio and the Th17 frequency at onset were significant predictors of patient survival. Low Treg/Th17 cell ratios at the onset predicted poor survival. Survivors exhibited an initial decrease in the circulating Treg/Th17 ratio from the onset to the peak time, and subsequently displayed a continuous increase.
CONCLUSION: Treg and Th17 cells showed changes in genes, protein levels and T cell phenotypes during ACHBLF events. An increased Treg/Th17 ratio was associated with the survival of ACHBLF patients.
Core tip: In this study, we longitudinally investigated Foxp3+Treg cells and IL-17+Th cells by measuring gene levels, protein levels and T-cell phenotypes during HBV-related acute-on-chronic liver failure (ACHBLF) progression. From the onset of ACHBLF, there were changes in Foxp3+Treg and Th17 cell frequencies. An increased Treg/Th17 ratio was associated with the survival of ACHBLF patients.
- Citation: Liang XS, Li CZ, Zhou Y, Yin W, Liu YY, Fan WH. Changes in circulating Foxp3+ regulatory T cells and interleukin-17-producing T helper cells during HBV-related acute-on-chronic liver failure. World J Gastroenterol 2014; 20(26): 8558-8571
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8558.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8558
Hepatitis B virus (HBV) is a major human pathogen, infecting 350 million people worldwide. Adults infected with HBV can either exhibit acute self-limited HBV infection or progress to chronic infection, while viral infection in utero or early in life generally results in chronic hepatitis B (CHB)[1]. Some CHB patients may rapidly progress towards liver failure, a condition referred to as acute-on-chronic liver failure (ACLF)[2]. HBV-related acute-on-chronic liver failure (ACHBLF) is one of the most severe consequences of HBV infection. ACHBLF refers to liver failure occurring in patients with CHB, CHB-related liver cirrhosis, or chronic asymptomatic HBV carriers[3]. In China, as a result of the high prevalence of HBV infection, ACHBLF cases account for more than 80% of ACLF cases[4,5]. The precise mechanisms underlying the deterioration of liver function occurring in ACLF remain unclear; however, the impairment of cellular immunity is believed to be a contributing factor[6-9].
Recently, a novel and unique pro-inflammatory T cell subset, interleukin-17 (IL-17)-producing CD4+T helper cells (Th17), was identified. Several key cytokines, including IL-1β, IL-6, tumor necrosis factor alpha (TNF-α) and IL-23, create a cytokine milieu that regulates the differentiation and expansion of human Th17 cells[10-13]. Th17 and its related cytokines may play an important role in the pathogenesis of HBV infection[6-9,14-16].
Conversely, another CD4+T cell subset, Foxp3+ regulatory T cells (Treg), which are characterized by their constitutive expression of CD25 and Foxp3 and by immunological suppression[17-21], can restrain the immune response, thus limiting liver damage[9,22-24]. In chronic HBV infection, an imbalance between Tregs and effector T cells has recently been described by several groups. These studies suggest that either the number or functional imbalance of Tregs in the blood or liver may be a reason for persistent HBV infection and disease progression[9,24-26].
Although increased Th17 and Treg frequencies in patients with CHB have been reported, little is known about the relationship between circulating Th17 and Tregs and their role in ACHBLF development. Recently, we studied the changes in Treg and Th17 cell balance in the development of acute and chronic hepatitis B virus infection and found that in ACHBLF patients, peripheral blood Th17 cell frequency increased significantly compared with that seen in healthy controls (HCs), but the frequency of Treg cells did not increase synchronously, creating an imbalance between Treg and Th17 cells in ACHBLF[9]. Zhai et al[27] also found that at the onset of ACHBLF, the frequency of Th17 cells increased in ACHBLF patients and that the ratio of Th17 to Treg cells correlated with patient survival. However, Th17 and Treg cell dynamics in ACHBLF patients have not been previously reported.
Our study longitudinally analyzed the frequency of Treg and Th17 cells and related cytokine protein and gene expression in a cohort of 18 patients with ACHBLF. Our data document changes in Treg and Th17 cell populations during ACHBLF.
A total of 18 patients with ACHBLF have been described in our recent study[9]. In brief, inclusion criteria included (1) HBV-related liver cirrhosis or chronic HBV infection based on a histopathologic diagnosis or compatible laboratory data and sonographic findings; (2) recent development of jaundice, ascites, hemodynamic instability and/or encephalopathy grade III-IV, compatible with the definition of hepatic decompensation and necessitating further treatment in the ICU; (3) no evidence of hepatocellular carcinoma or other metastatic liver tumors that could affect liver function; and (4) no immunosuppressive medication within the last 3 mo prior to study entry[9,28]. All of the patients were longitudinally followed. Table 1 summarizes the clinical data acquired from the investigations of these patients at the onset point (1-2 wk after clinical onset), peak point (the time of peak total bilirubin level, 2-3 wk after clinical onset), and recovery point (total bilirubin levels decreased by more than 30%, typically 7-8 wk after clinical onset)[29]. The disease control group was composed of 18 age-, gender- and Child-Pugh stage-matched patients with CHB. The diagnostic criteria for CHB were as follows: positive for hepatitis B surface antigen (HBsAg) and anti-hepatitis B core (anti-HBc) for more than 6 mo; negative for antibodies to hepatitis C virus, hepatitis D virus, hepatitis G virus, and HIV-1 and -2; no other causes of chronic liver damage; and persistently elevated serum ALT levels and positive serum HBV DNA for at least 6 mo. Ten healthy adults (age range: 24-42 years, 7 females and 3 males) were included as HCs. All patients and controls were Chinese. The study was approved by the local medical ethics committee of Shanghai Changhai Hospital, and informed consent was acquired from each individual.
| No. | Age (yr) | Sex | Onset | Peak | Recovery | |||||||||||||||
| Week after clinical onset | ALT (U/L) | TBIL (μmol/L) | HBV DNA(Log 10 copies/mL) | PTA | MELD-Na | Week after clinical onset | ALT(U/L) | TBIL(μmol/L) | HBV DNA(Log 10 copies/mL ) | PTA | MELD-Na | Week after clinical onset | ALT(U/L) | TBIL(μmol/L) | HBV DNA(Log 10 copies/mL) | PTA | MELD-Na | |||
| 1 | 58 | M | 1 | 112 | 497.3 | 5.69 | 48.01% | 24.70 | 2 | 72 | 239.5 | 4.58 | 51.36% | 22.56 | 7 | 59 | 152.3 | 3.21 | 50.38% | 21.19 |
| 2 | 48 | M | 1 | 65 | 309.5 | 5.21 | 38.30% | 18.38 | 2 | 64 | 267.5 | 4.25 | 45.56% | 20.45 | 8 | 68 | 152.8 | 3.21 | 41.97% | 15.04 |
| 3 | 45 | M | 1 | 1002 | 400.5 | 3.67 | 21.65% | 26.86 | 3 | 316 | 576.8 | 2.08 | 16.06% | 58.53 | Died | |||||
| 4 | 50 | F | 1 | 302 | 520.1 | 6.36 | 16.35% | 52.47 | 2 | Died | ||||||||||
| 5 | 59 | M | 1 | 435 | 170.6 | 5.22 | 40.36% | 16.84 | 3 | 62 | 60.2 | 4.15 | 60.13% | 11.99 | 5 | 44 | 45.6 | 4 | 62.26% | 10.25 |
| 6 | 50 | M | 2 | 145 | 620.0 | 3.29 | 46.40% | 31.14 | 3 | 165 | 613.7 | 3.29 | 46.81% | 27.99 | 7 | 129 | 546.3 | 3.29 | 36.72% | 30.55 |
| 7 | 38 | M | 1 | 92 | 449.5 | 4.57 | 24.24% | 26.48 | 2 | 89 | 481.9 | 3.29 | 22.78% | 25.77 | 6 | 68 | 456.6 | 3.04 | 28.57% | 26.08 |
| 8 | 30 | M | 1 | 504 | 293.6 | 7.14 | 42.65% | 21.78 | 3 | 148 | 403.4 | 3.12 | 42.65% | 25.07 | 8 | 59 | 350.0 | 3.27 | 53.44% | 24.05 |
| 9 | 42 | M | 1 | 282 | 439.1 | 5.88 | 42.31% | 18.93 | 2 | 151 | 449.6 | 3.29 | 37.50% | 21.70 | 8 | 83 | 471.9 | 3.12 | 34.78% | 26.08 |
| 10 | 61 | M | 1 | 81 | 465.6 | 4.37 | 27.53% | 29.51 | 2 | 61 | 405.0 | 3.11 | 27.53% | 32.25 | 7 | 33 | 174.0 | 2.54 | 45.99% | 15.93 |
| 11 | 44 | M | 2 | 53 | 599.8 | 2.67 | 28.12% | 25.36 | 3 | 58 | 381.5 | 2.23 | 27.53% | 46.89 | 8 | 46 | 297.0 | 2.12 | 22.02% | 37.87 |
| 12 | 55 | F | 1 | 35 | 182.8 | 4.30 | 25.78% | 20.31 | 2 | 46 | 208.5 | ND | 27.24% | 20.78 | 7 | 60 | 197.8 | 2.23 | 23.70% | 22.58 |
| 13 | 52 | M | 1 | 213 | 381.1 | 5.89 | 10.96% | 39.84 | 2 | 584 | 540.2 | ND | 4.70% | 62.03 | Died | |||||
| 14 | 48 | M | 1 | 355 | 364.7 | 6.07 | 32.43% | 25.63 | 2 | 63 | 546.2 | 2.12 | 29.20% | 29.60 | 8 | 75 | 127.0 | 1.25 | 49.44% | 24.03 |
| 15 | 45 | F | 1 | 21 | 508.8 | 3.35 | 40.99% | 33.77 | 2 | 31 | 481.2 | 2.98 | 44.07% | 38.17 | 8 | 22 | 158.8 | 2.12 | 59.46% | 15.23 |
| 16 | 30 | M | 1 | 93 | 395.0 | 3.19 | 18.67% | 29.42 | 2 | 86 | 353.8 | 3.19 | 19.14% | 36.92 | 8 | 76 | 169.9 | 2.86 | 21.84% | 12.40 |
| 17 | 53 | M | 2 | 138 | 541.5 | 2.67 | 30.73% | 28.79 | 3 | 107 | 639.6 | 2.32 | 27.53% | 50.07 | Died | |||||
| 18 | 33 | M | 1 | 138 | 216.0 | 5.68 | 47.212% | 22.43 | 2 | 63 | 259.2 | 4.11 | 74.58% | 16.17 | 6 | 89 | 132.9 | 2.89 | 89.80% | 10.38 |
Peripheral blood mononuclear cells (PBMCs) were isolated from the heparinized peripheral blood of the studied subjects by standard Ficoll-Paque (GE Healthcare, Uppsala, Sweden) density centrifugation. Total RNA was extracted from PBMCs using the RNeasy kit (Qiagen, Valencia, CA, and United States). All samples were treated with DNaseI to eliminate potential genomic DNA contamination. The quality and quantity of the RNA were determined using an ultraviolet spectrophotometer (NANODROP1000, Thermo). Target RNA was reverse-transcribed using the Omniscript RT Kit (Qiagen, Valencia, CA, United States). All samples were treated according to identical protocols and in parallel. RNA and cDNA were stored at -80 °C until further processing.
The panels of genes of interest (GOIs) are listed in Table 2. The GAPDH gene was selected as an endogenous reference. Candidate primer sets were first designed by Primer Express Software (Applied Biosystems, Foster City, CA, United States) and then validated manually to satisfy the following criteria: (1) amplicon length between 50 and 150 base pairs; (2) amplification efficiencies, as determined by a template linear dilution method as described previously[30], were approximately equal for the GOIs and 18S rRNA; and (3) primers were verified to generate a single product specific to target genes by both blast algorithm (http://www.ncbi.nlm.nih.gov/blast/) and melting curve analysis. The expression of GOIs was determined by RQ-PCR using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) in a final volume of 50 μL with the following thermal conditions: 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Samples were assayed in triplicate. The expression ratio of each GOI between different groups was analyzed using the delta comparative cycle threshold method[31].
| Genes | GenBank accession number | Primers (5' to 3') | Reported function |
| IL-1β | NM_000576 | Forward: GCTGATGGCCCTAAACAGATGAA | Induce human Th17 polarization[17] |
| Reverse: TGAAGCCCTTGCTGTAGTGGTG | |||
| IL-6 | NM_000600 | Forward: AAGCCAGAGCTGTGCAGATGAGTA | Induce human Th17 polarization[17] |
| Reverse: TGTCCTGCAGCCACTGGTTC | |||
| IL-23/p19 | NM_016584 | Forward: GCAGCCTGAGGGTCACCACT | Unique subunit of IL-23 |
| Reverse: GGCGGCTACAGCCACAAA | |||
| IL-17A | NM_002190 | Forward: TGTCCACCATGTGGCCTAAGAG | Main effective cytokine of Th17 cells[7] |
| Reverse: GTCCGAAATGAGGCTGTCTTTGA | |||
| TGF-β1 | NM_000660 | Forward: AGCGACTCGCCAGAGTGGTTA | Induce mouse Th17 and Treg polarization, suppress human Th17 polarization[9,10,17] |
| Reverse: GCAGTGTGTTATCCCTGCTGTCA | |||
| IL-2 | NM_000586 | Forward: CAACTCCTGTCTTGCATTGCACTAA | Induce human and mouse Treg polarization[10] |
| Reverse: AATGTGAGCATCCTGGTGAGTTTG | |||
| FoxP3 | NM_014009 | Forward: GTTCACACGCATGTTTGCCTTC | Master regulatory transcription factors of Treg lineage[21] |
| Reverse: CACAAAGCACTTGTGCAGACTCAG |
For Th17 cell examination, the PBMCs were isolated from peripheral blood. PBMCs (2 × 106) were stimulated further for 5 h with 50 ng/mL phorbol myristate acetate, 1 mmol/L ionomycin (both from Sigma, St Louis, MO, United States) and 10 mg/mL brefeldin A (TocrisCookson, Bristol, United Kingdom) in complete RPMI-1640 (Invitrogen, Carlsbad, CA, United States) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, United States). Upon harvest, cells were first surface-stained with fluorescein isothiocyanate (FITC)-conjugated anti-human CD4 antibodies for 20 min, fixed and permeabilized with Perm/Fix solution, and then stained intracellularly with phycoerythrin (PE)-conjugated anti-human IL-17A.
For Treg cell examination, peripheral blood (100 μL) was first surface-stained with FITC-conjugated anti-human CD4 antibodies and allophycocyanin (APC)-conjugated anti-human CD25 antibodies for 30 min, then lysed with FACSTM lysing solution (BD PharMingen) and treated with eBioscience fix/perm mixture (eBiosciences) according to the manufacturer’s instructions. Finally, the cells were incubated with PE-conjugated anti-human Foxp3 antibodies overnight. Isotope controls were used to ensure antibody specificity. Flow cytometry was performed using a FACSCalibur (Becton Dickinson, San Jose, CA). FACS data were analyzed using CellQuest software (Becton Dickinson Rutherford, NJ)[28]. All antibodies were purchased from BD Biosciences (San Jose, CA, United States).
Serum concentrations of IL-1β, IL-6, IL-23, IL-17A, TGF-β1, and IL-2 were measured by commercially available enzyme linked immunosorbent assay kits (R and D Systems, Minneapolis, MN, United States) according to the protocols provided by the manufacturer. All samples were assessed in triplicate.
The levels of HBsAg, HBeAg, anti-HBs, anti-HBc, anti-HBe, anti-HCV, anti-HDV, anti-HGV, anti-HIV-1, and anti-HIV-2 were measured using commercially available kits (Abbot Laboratories, North Chicago, IL) in our clinical lab. Serum HBV-DNA levels were measured by fluorescent quantitative PCR with commercially available kits (PE/B/MJ/L, Shenzhen, China) according to the manufacturer’s instructions. The threshold of the HBV DNA detection limit was 500 copies/mL.
All data were analyzed using IBM SPSS statistic version 19 (IBM, Com). The Kruskal-Wallis test was used to evaluate the differences among more than 2 groups. The Mann-Whitney U test was used to evaluate the difference between two groups. Spearman’s correlation test was used to assess the correlation of immune factors and clinical characters. The area under the receiver operating characteristic (ROC) curve was used to compute predictive values of different factors on survival rates. For all tests, two-sided P < 0.05 was considered significant.
The clinical and biochemical details of the studied patients are listed in Table 1. We measured the expression of 7 genes and the serum concentrations of six corresponding cytokines, which have been reported previously to contribute to the differentiation of Th17 and Treg cells[32,33]. The name, GenBank accession number, primer sequences and reported function of each gene are presented in Table 2.
Patients with ACHBLF received conservative management, including nutritional support, hepatoprotective drugs, antiviral therapy (lamivudine 100 mg/d) and prevention and control of complications, but did not receive any immune-modulating therapy. Patients were divided subsequently into non-survivor (NS) patients (who died or received a liver graft) and survivors (who survived or whose total bilirubin level decreased by more than 30% by the end of study) according to an earlier report[29]. Four patients died during the study, and one patient received a liver graft.
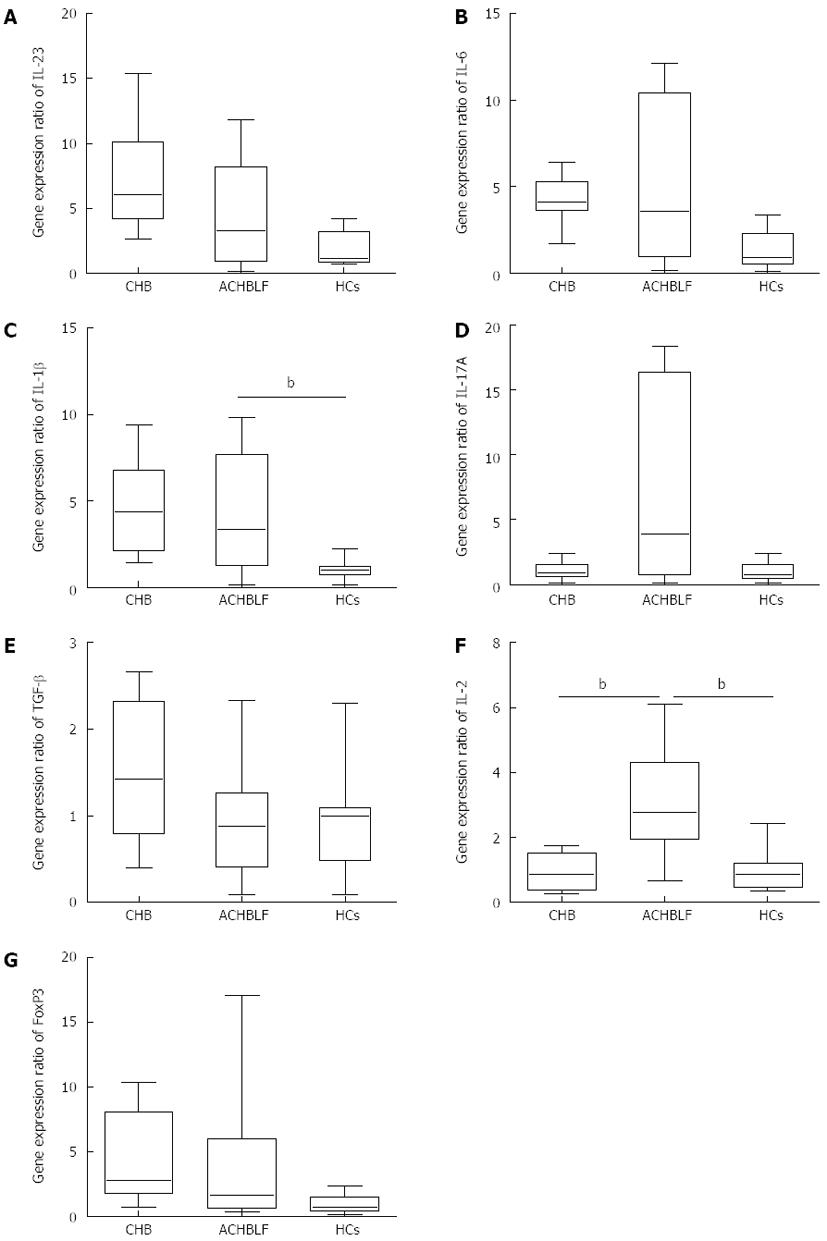
Compared with HCs, the gene expression of the pro-Th17 cytokines IL-1β, IL-6 and IL-23/p29 was up-regulated, especially IL-1β, at the onset of ACHBLF (Figure 1A-C). We also observed higher expression of IL-17A (Figure 1D). However, there were no significant differences between the ACHBLF and CHB patients.
Although the expression of TGF-β1 in the patients with ACHBLF did not differ from that of the HCs and CHB patients (Figure 1E), IL-2 gene expression was up-regulated significantly compared with the HCs and CHB patients (Figure 1F). We also observed higher levels of Foxp3 gene expression (Figure 1G).
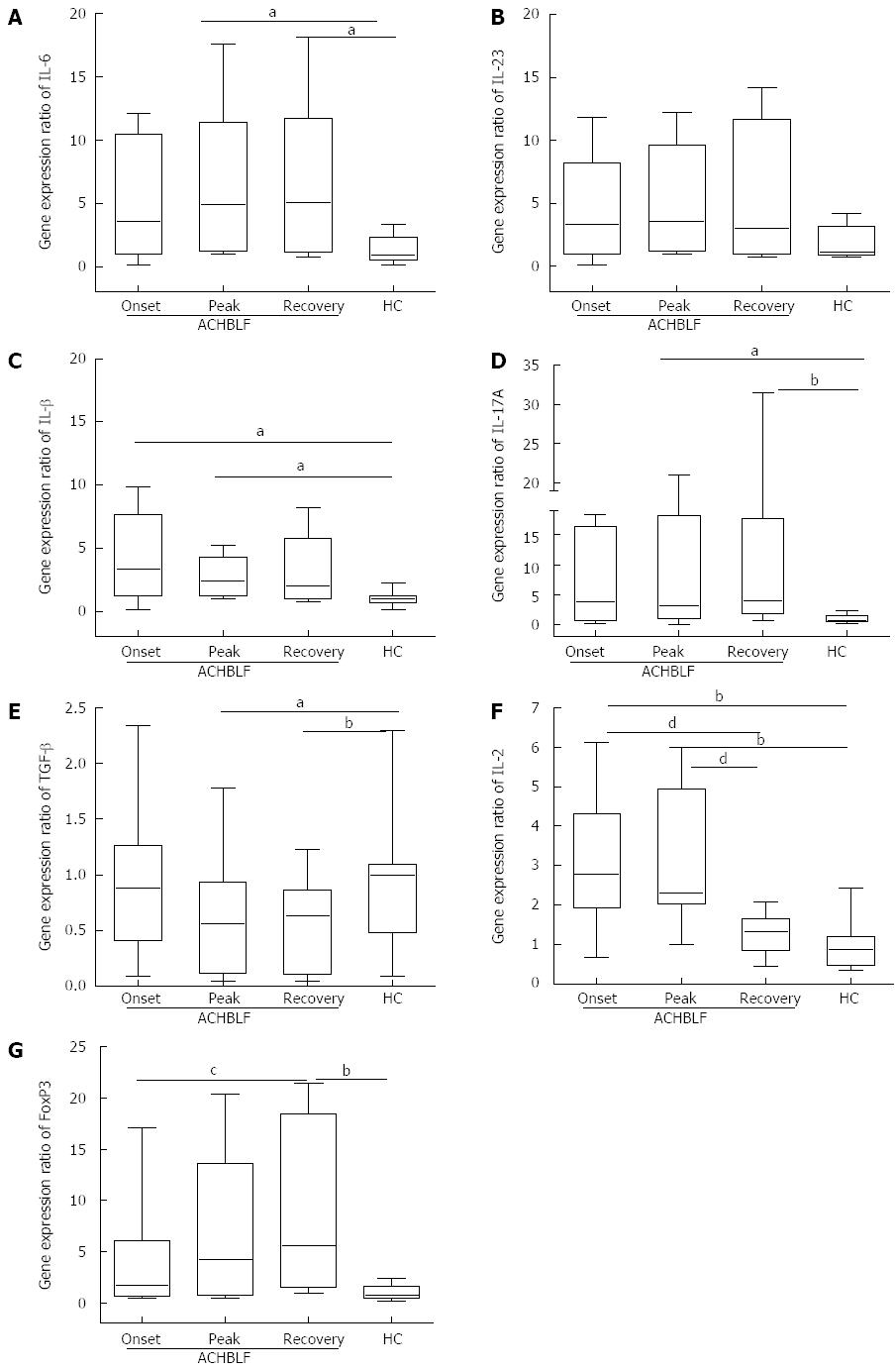
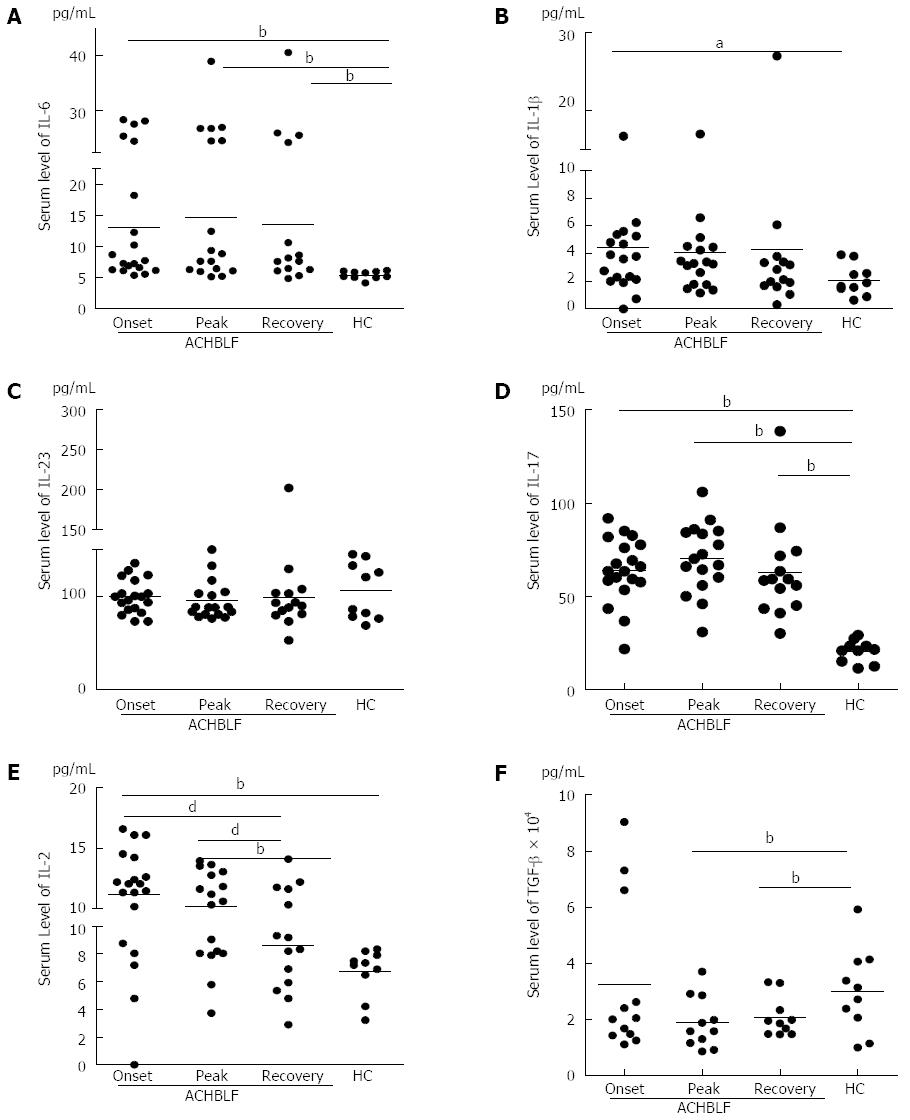
During ACHBLF, the differentiation environment was conducive to Th17 cell differentiation (Figure 2A-C and Figure 3A-C), which was demonstrated by significantly elevated IL-6 and IL-1β levels both at the gene and protein expression level (Figures 2A, C and 3A, B). However, Treg cell-related cytokine expression levels gradually decreased during ACHBLF (Figures 2E, F and 3E, F). Corresponding to this Th17 differentiation environment, patients possessed higher IL-17A levels at both the gene and protein levels during the ACHBLF event (Figure 2D and Figure 3D). Interestingly, Foxp3 gene expression was up-regulated gradually during the ACHBLF event. Foxp3 gene levels at the recovery point were significantly higher than HCs and at onset time point (Figure 2G).
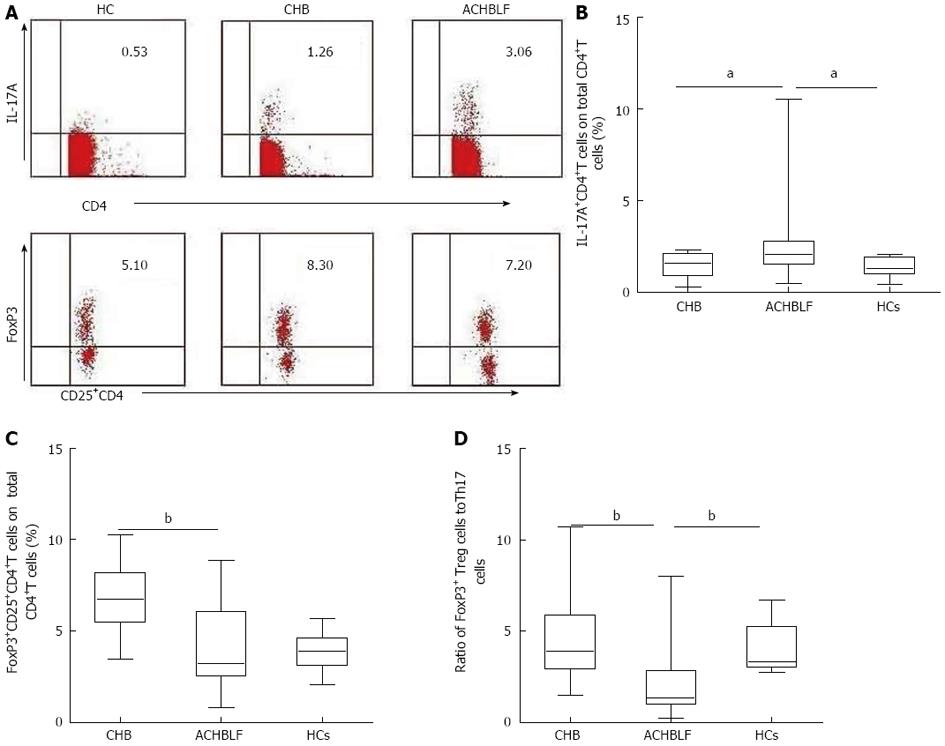
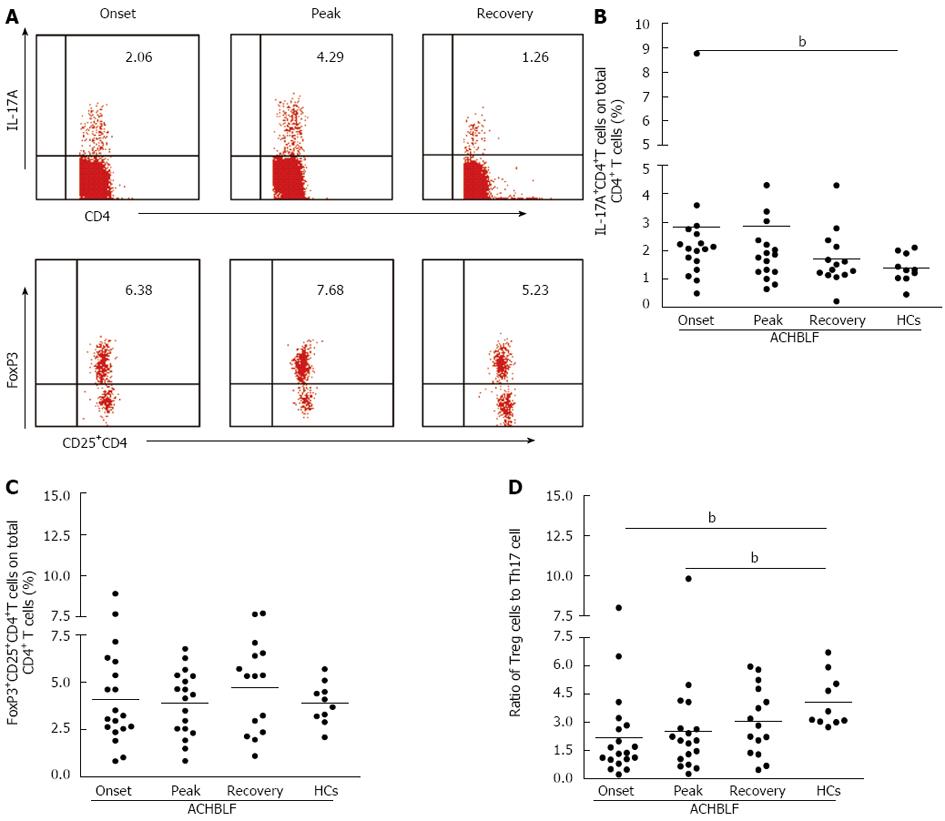
Based on our knowledge of the cytokine milieu in patients with ACHBLF at the onset point, we further evaluated the Treg and Th17 cell subsets in PBMCs, defined as the percentage of Th17 cells and Treg cells in the total CD4+ T cell population using flow cytometry (Figure 4A). The Th17 cell population was increased in patients with ACHBLF (2.83% ± 2.61%) compared with patients with CHB (1.48% ± 0.71%, P = 0.04,) and HCs (1.37% ± 0.51%, P = 0.01; Figure 4B), whereas Treg decreased in ACHBLF patients (4.08% ± 2.28%) compared with CHB patients (6.71% ± 1.76%, P = 0.001) (Figure 4C).
Given the nonsynchronous changes of Treg and Th17 cells at the onset of ACHBLF, to better understand the relationship between these two types of immune cells, we used the Treg/Th17 ratio. The Treg/Th17 ratio of ACHBLF at the onset point was decreased significantly (2.83% ± 2.60%) compared with HCs (4.09% ± 1.39%, P = 0.003) and the CHB patients (4.67% ± 2.30%, P = 0.007) (Figure 4D).
These data indicate that a significant imbalance in the numbers of circulating CD4+T cells occurs in patients with ACHBLF.
To characterize the changes in the circulating CD4+T subset, all 18 patients with ACHBLF were longitudinally followed. Intracellular IL-17 and FoxP3 staining in different stages of ACHBLF were tested using flow cytometry (Figure 5A). As shown in Figure 5B, during ACHBLF events, patients had a sustained higher frequency of Th17 cells in the peripheral blood compared with HCs. The frequency of Th17 cells at the peak point was increased slightly compared with at the onset point, but there was no significant difference between the two clinical phases. However, at the recovery time point, the frequency of Th17 cells (1.69% ± 0.97%) was decreased sharply from the peak time point (2.84% ± 2.92%). The frequency of Treg cells was not changed significantly during the entire clinical phase (Figure 5C).
We further analyzed the changes in the ratio of Tregs to Th17 cells during ACHBLF events. The ratio of Treg to Th17 cells was increased gradually from the onset point to the recovery point, and at the recovery point, the ratio of Treg to Th17 (3.07% ± 1.80%) was similar to that of the HCs (4.09% ± 1.39%, P = 0.17) (Figure 5D).
To identify the relationship between host immune changes and disease development, we used total bilirubin (TBIL), alanine aminotransferase (ALT) and a model for end stage liver disease (MELD) plus sodium (MELD-Na) score to evaluate disease development[34,35]. We performed correlation analyses between these main biochemical measures and the MELD-Na score and Th17 frequency, Treg frequency and the ratio of Treg to Th17 cells at the onset, peak, and recovery phases of the disease among our 18 ACHBLF patients. The data revealed that all of these host immune characteristics positively or negatively correlated with disease development (Table 3). However, in all patients, only serum IL-17 levels at peak point markedly correlated with the ALT level (r = -0.56, P = 0.02). We further analyzed patients in the survival group and found that the ratio of Treg to Th17 at the peak point positively correlated with ALT levels (r = 0.58, P = 0.04). Furthermore, we also found that in the survival group, changes in the Treg to Th17 ratio from the onset to the peak point markedly correlated with TBIL levels (r = -0.57, P = 0.04).
| Onset | Peak | Recovery | |||||||||||||||||||||||
| Th17 | Treg | Treg/Th17 | IL-17 | Th17 | Treg | Treg/Th17 | IL-17 | Th17 | Treg | Treg/Th17 | IL-17 | ||||||||||||||
| r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | ||
| Total patients | ALT | 0.17 | 0.49 | -0.02 | 0.92 | -0.004 | 0.98 | -0.17 | 0.48 | -0.20 | 0.44 | 0.11 | 0.68 | 0.42 | 0.10 | -0.56 | 0.02 | -0.17 | 0.56 | -0.33 | 0.24 | 0.00 | 1.00 | -0.24 | 0.41 |
| TBIL | 0.06 | 0.81 | -0.21 | 0.38 | -0.20 | 0.41 | -0.28 | 0.25 | 0.04 | 0.88 | -0.11 | 0.68 | -0.04 | 0.88 | -0.30 | 0.23 | -0.30 | 0.29 | -0.15 | 0.59 | -0.09 | 0.76 | -0.36 | 0.19 | |
| MELD-Na | 0.09 | 0.71 | -0.02 | 0.91 | -0.18 | 0.46 | -0.01 | 0.96 | 0.02 | 0.95 | -0.09 | 0.74 | 0.07 | 0.79 | -0.06 | 0.82 | -0.50 | 0.06 | -0.01 | 0.98 | 0.15 | 0.60 | -0.27 | 0.34 | |
| Survivors | ALT | -0.11 | 0.71 | 0.17 | 0.56 | 0.25 | 0.40 | -0.12 | 0.68 | -0.42 | 0.15 | 0.12 | 0.73 | 0.58 | 0.04 | -0.53 | 0.06 | -0.19 | 0.52 | -0.17 | 0.57 | 0.19 | 0.53 | -0.29 | 0.32 |
| TBIL | -0.17 | 0.57 | 0.09 | 0.75 | 0.14 | 0.63 | -0.33 | 0.26 | -0.29 | 0.34 | -0.34 | 0.25 | 0.01 | 0.99 | -0.14 | 0.65 | -0.35 | 0.23 | 0.05 | 0.85 | 0.06 | 0.83 | -0.42 | 0.14 | |
| MELD-Na | -0.54 | 0.05 | 0.008 | 0.98 | 0.30 | 0.32 | 0.01 | 0.96 | -0.08 | 0.80 | -0.33 | 0.27 | -0.10 | 0.75 | 0.24 | 0.44 | -0.55 | 0.05 | 0.18 | 0.54 | 0.31 | 0.29 | -0.32 | 0.27 | |
Because Treg, Th17, and IL-17 levels and the ratio of Treg to Th17 were shown to be associated with ACHBLF development, we further evaluated the early predictive value of these factors on ACHBLF outcome. We evaluated the value of these factors at onset and at peak point in predicting ACHBLF survival by the end of a 28-d follow-up period. ROC curve analysis showed that Th17 frequency and the ratio of Treg to Th17 at the onset point were significant in predicting 28-d ACHBLF survival. The Th17 frequency was a negative predictor and the Treg to Th17 ratio was a positive predictor [the area under the ROC was 0.1 (95%CI: 0.000-0.248, P = 0.01) for Th17 frequency at onset and 0.846 (95%CI: 0.65-1.00, P = 0.02) for the ratio of Treg to Th17 at onset]. There was no significant predictive value of Treg frequency during the event, IL-17 levels during the event, and changes in the ratio of Treg to Th17 from the onset to peak point (Table 4).
| Variables | Area | Standard error | Asymptotic sign | 95%CI | |
| Low boundary | Upper boundary | ||||
| Treg frequency at onset | 0.577 | 0.163 | 0.622 | 0.258 | 0.896 |
| Th17 frequency at onset | 0.108 | 0.081 | 0.012 | 0.000 | 0.266 |
| Ratio of Treg to Th17 at onset | 0.846 | 0.100 | 0.027 | 0.650 | 1.000 |
| Serum IL-17 level at onset | 0.554 | 0.136 | 0.730 | 0.288 | 0.820 |
| Treg frequency at peak | 0.523 | 0.166 | 0.882 | 0.197 | 0.849 |
| Th17 frequency at peak | 0.462 | 0.168 | 0.805 | 0.132 | 0.791 |
| Ratio of Treg to Th17 at peak | 0.585 | 0.160 | 0.588 | 0.270 | 0.899 |
| Ratio of Treg to Th17 change from onset to peak | 0.308 | 0.132 | 0.218 | 0.049 | 0.566 |
| Serum IL-17 level at peak | 0.785 | 0.108 | 0.068 | 0.573 | 0.997 |
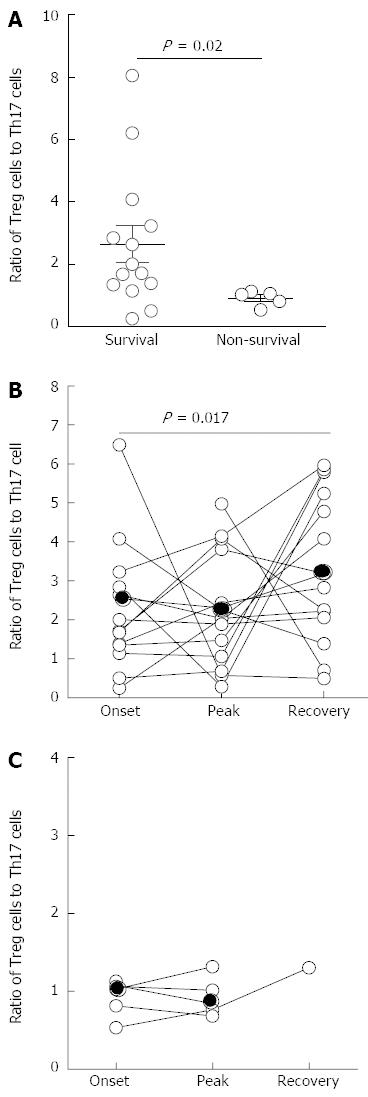
To further investigate the predictive value of the Treg to Th17 frequency ratio during ACHBLF events on the survival of ACHBLF patients, we divided the ACHBLF patients into survivors and non-survivors (NS)[29]. As shown in Figure 6A, we found that the ratio of Treg to Th17 frequency at the onset point in NS patients (0.91% ± 0.24%) was significantly lower than that in survivors (2.64% ± 2.19%, P = 0.02). Furthermore, we also found that survivors exhibited an initial decrease in the circulating ratio of Treg to Th17 frequency from the onset point to the peak point. Subsequently, survivors displayed a continuous increase of the Treg to Th17 ratio, accompanied by a total bilirubin level decrease of more than 30% (Figure 6B). However, dead/transplanted patients lacked these sequential responses compared with survivors. The Treg to Th17 ratio remained persistently low in these patients (Figure 6C). These data suggest that the restoration of the balance of Treg and Th17 cells may represent a prognostic marker for a favorable clinical result in ACHBLF patients.
In the present study, we demonstrated that a Treg and Th17 immune imbalance exists in ACHBLF patients by measuring gene, protein and T cell phenotypes. We also longitudinally identified the pattern of dynamic change in Treg and Th17 cells during ACHBLF. We found that compared with CHB, ACHBLF patients possessed a favorable Th17 differentiation environment, accompanied by a sustained higher Th17 frequency and IL-17 levels. The results suggest that Th17 and related pro-inflammatory cytokine up-regulation played a vital role in disease progression from CHB to ACHBLF and also played a critical role in ACHBLF development. Zhang et al[7] previously found that circulating Th17 cells increased with disease progression from CHB to ACHBLF. Another CD4+T cell subset, Tregs, plays a critical role in HBV persistence during CHB events. We found that when disease progressed from CHB to ACHBLF, although the Treg differentiation cytokine IL-2 was up-regulated, circulating Treg frequency began to decrease and showed lower levels during ACHBLF events compared with CHB events. These data suggest that Treg down-regulation may promote liver inflammation and accelerate disease progression from CHB to ACHBLF and ACHBLF development.
The development of ACHBLF involves dysregulation of both the innate and adaptive immune systems. Many different cell types, including T lymphocytes, monocytes and dendritic cells, are primed in ACHBLF. These cells are believed to play a pivotal role in the pathogenesis of ACHBLF[4,6,8,12,26]. Treg and Th17 cells are both involved in the pathogenesis of HBV infection. Tregs and Th17 cells are closely associated with each other; the two types of T cells not only share the same origin but are also mutually antagonistic in function. Thus, the balance between the two types of T cells could impact inflammation control and autoimmune inflammation[36,37]. Many studies have found that an imbalance between Th17 and Treg cells is closely related to the development of a number of diseases, including HBV infection[9,27,36-39]. Recently, two different groups have demonstrated imbalances between Treg and Th17 cells in ACHBLF patients and that this imbalance plays an important role in the progression of ACHBLF[27,40]. Furthermore, several studies have demonstrated that antiviral therapy induces a viral load reduction that partly restores antiviral immunity in patients with CHB[41-44]. We also previously reported that for acute and chronic HBV infected patients, the balance between Treg and Th17 cell frequency was different. Treg and Th17 immune imbalance only exists in CHB and ACHBLF[9]. In this study, we further demonstrated that immune imbalance of Treg and Th17 cells exists in ACHBLF measured by both gene and protein levels from the clinical onset. Furthermore, the imbalance of Treg to Th17 changed along the progression of the disease and correlated with the outcome of the disease. Furthermore, to avoid a quick viral load reduction that induces immune restoration in the early part of the ACHBLF event, in this study, all patients accepted lamivudine therapy to improve the long-term prognosis of ACHBLF.
Although it is now recognized that an imbalance between Th17 and Tregs exists in ACHBLF patients, our knowledge about the dynamic interplay between Tregs and Th-17 cells during ACHBLF events is limited. In this study, we longitudinally followed ACHBLF patients and demonstrated that the immune imbalance between Treg and Th17 dynamically changed along ACHBLF progression, but mechanistic data were absent in our study. At onset, the ratio of Treg to Th17 cells, with a significant Th17 increase, was decreased significantly compared with CHB patients and HCs. Then, the Treg to Th17 ratio dynamically increased from the onset to the recovery point, and finally, the ratio of Treg to Th17 was increased to similar levels as HCs at the recovery point. These data suggest that the Treg and Th17 balance modulates liver damage and the outcome of ACHBLF.
Based on the role of Th17 and Treg in ACHBLF and the relationship between the two types of T cells, we studied the relationship between the two types of T cells and ACHBLF progression. We used the MELD-Na score to evaluate ACHBLF disease development, ALT and TBIL to evaluate liver inflammation, and the ratio of Treg to Th17 to represent the interplay of the two types of T cells. We found that in all patients, the frequency of the two types of T cells and their ratio during ACHBLF events correlated with the ALT, TBIL and MELD-Na score positively or negatively, but only serum IL-17 levels at peak point significantly correlated with ALT levels (Table 4). However, in survivors, we found that Th17 frequency at onset and recovery showed a relationship with MELD-Na (Table 4), and the ratio of Treg to Th17 at the peak point significantly correlated with ALT levels. These data suggested that MELD-Na is an improved model for the prediction of mortality in patients with end-stage liver disease awaiting liver transplantation but cannot exactly represent the stage of ACHBLF, especially in patients whose acute liver damage developed quickly[45].
The mortality of ACHBLF is high and there are few early predictors for survival in ACHBLF. Zhai et al[27] found that the ratio of Th17 to Treg cells was inversely associated with survival in HBV-related ACLF patients. In this study, we not only found that a lower ratio of circulating Treg to Th17 frequency at the onset predicts a poor survival rate but also found that survivors exhibited an initial decrease in the circulating ratio of Treg to Th17 cells from onset to peak time and subsequently displayed a continuous increase in the ratio of Treg to Th17 cells, accompanied by a total bilirubin level decrease of more than 30%. According to these data, we speculate that the interplay between Tregs and Th-17 is important for maintaining the balance between a limited immune response and pathological damage.
In summary, our findings suggest that an immune imbalance between Treg and Th17 cells exists in ACHBLF patients, and the immune imbalance dynamically changes with disease progression. The ratio of Treg to Th17 cell frequency is dynamically associated with survival in ACHBLF patients. A low Treg to Th17 cell ratio at onset predicts poor survival. Survivors exhibit an initial decrease in the circulating ratio of Treg to Th17 cells from onset to peak time and subsequently display a continuous increase in the ratio of Treg to Th17 cells.
We would like to thank all of the patients enrolled in this study for their kind understanding and support.
FoxP3+Treg cells and IL-17+Th cells are both involved in the progression of hepatitis B virus (HBV) infection. During chronic HBV infection there is an imbalance between the two related types of immune T cells. Little is known about the change in the pattern of the two types of T cells during HBV-related acute-on-chronic liver failure (ACHBLF) progression.
Studies have previously demonstrated that there is an imbalance between FoxP3+Treg and IL-17+Th cells during ACHBLF. The ratio of Th17 to Treg cells is inversely associated with survival in HBV-related ACLF patients.
In this study, the authors longitudinally investigated FoxP3+Treg cells and IL-17+Th cells using gene expression and protein levels and also T-cell phenotypes during ACHBLF. At the onset of ACHBLF, patients presented with an environment conducive for Th17 differentiation, but Treg cell-related cytokines showed a gradually increasing trend over time. FoxP3+Treg and IL-17+T cells showed changes in gene, protein level and T cell phenotypes during ACHBLF events. An increased Treg/Th17 ratio was associated with survival in ACHBLF patients.
ACHBLF is one of the most severe consequences of HBV infection. The mortality of ACHBLF is high, and there are few early predictors for survival in ACHBLF. The ratio of Treg to Th17 cell frequency is dynamically associated with survival in ACHBLF patients. A low Treg to Th17 cell ratio at disease onset predicts poor survival, and survivors exhibit an initial decrease in the circulating ratio of Treg to Th17 cells from onset to peak time; therefore, monitoring the changes in the Treg to Th17 cell ratio could predict survival in ACHBLF.
ACHBLF refers to acute liver failure occurring in patients with chronic HBV infection (CHB), CHB-related liver cirrhosis, or chronic asymptomatic HBV carriers. ACHBLF manifests as jaundice and coagulopathy, complicated within 4 wk by ascites and/or encephalopathy.
The manuscript aimed to longitudinally investigate the expression levels of Treg cells in patients with ACHBLF and to analyze changes in Th17 and Treg cells during the follow-up period. Overall, the data are interesting. Although the numbers of patients are small, they are well characterized. However, an absence of mechanistic data was observed in this manuscript.
| 1. | Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22 Suppl 2:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1225] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 3. | Management scheme of diagnositic and therapy criteria of viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |
| 4. | Zou Z, Xu D, Li B, Xin S, Zhang Z, Huang L, Fu J, Yang Y, Jin L, Zhao JM. Compartmentalization and its implication for peripheral immunologically-competent cells to the liver in patients with HBV-related acute-on-chronic liver failure. Hepatol Res. 2009;39:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ke WM, Ye YN, Huang S. Discriminant function for prognostic indexes and probability of death in chronic severe hepatitis B. J Gastroenterol. 2003;38:861-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 405] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL, Shi F, Shi M, Wang HF. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Wu W, Li J, Chen F, Zhu H, Peng G, Chen Z. Circulating Th17 cells frequency is associated with the disease progression in HBV infected patients. J Gastroenterol Hepatol. 2010;25:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Xue-Song L, Cheng-Zhong L, Ying Z, Mo-Bin W. Changes of Treg and Th17 cells balance in the development of acute and chronic hepatitis B virus infection. BMC Gastroenterol. 2012;12:43. [PubMed] |
| 10. | Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3407] [Cited by in RCA: 3638] [Article Influence: 173.2] [Reference Citation Analysis (0)] |
| 11. | Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3769] [Cited by in RCA: 4212] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 12. | Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3074] [Cited by in RCA: 3409] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 13. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1583] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 14. | Ge J, Wang K, Meng QH, Qi ZX, Meng FL, Fan YC. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol. 2010;30:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Wang LY, Meng QH, Zou ZQ, Fan YC, Han J, Qi ZX, Ge J, Xu AL, Wang SK, Wang K. Increased frequency of circulating Th17 cells in acute-on-chronic hepatitis B liver failure. Dig Dis Sci. 2012;57:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Yang B, Wang Y, Zhao C, Yan W, Che H, Shen C, Zhao M. Increased Th17 cells and interleukin-17 contribute to immune activation and disease aggravation in patients with chronic hepatitis B virus infection. Immunol Lett. 2013;149:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 852] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 18. | Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 203] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 844] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 20. | Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2532] [Cited by in RCA: 2575] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 21. | Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1234] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 22. | Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739-747. [PubMed] |
| 23. | Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008;123:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 412] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 25. | Xu Z, Ren X, Liu Y, Li X, Bai S, Zhong Y, Wang L, Mao P, Wang H, Xin S. Association of hepatitis B virus mutations in basal core promoter and precore regions with severity of liver disease: an investigation of 793 Chinese patients with mild and severe chronic hepatitis B and acute-on-chronic liver failure. J Gastroenterol. 2011;46:391-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Franzese O, Kennedy PT, Gehring AJ, Gotto J, Williams R, Maini MK, Bertoletti A. Modulation of the CD8+-T-cell response by CD4+ CD25+ regulatory T cells in patients with hepatitis B virus infection. J Virol. 2005;79:3322-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Zhai S, Zhang L, Dang S, Yu Y, Zhao Z, Zhao W, Liu L. The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol. 2011;24:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Diagnostic and treatment guidelines for liver failure]. Zhonghua Gan Zang Bing Zazhi. 2006;14:643-646. [PubMed] |
| 29. | Zhao J, Zhang JY, Yu HW, He YL, Zhao JJ, Li J, Zhu YK, Yao QW, Wang JH, Liu HX. Improved survival ratios correlate with myeloid dendritic cell restoration in acute-on-chronic liver failure patients receiving methylprednisolone therapy. Cell Mol Immunol. 2012;9:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med (Berl). 2006;84:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 648] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 31. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 139272] [Article Influence: 5570.9] [Reference Citation Analysis (3)] |
| 32. | Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 33. | Hemdan NY, Birkenmeier G, Wichmann G. Key molecules in the differentiation and commitment program of T helper 17 (Th17) cells up-to-date. Immunol Lett. 2012;148:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 574] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 35. | Yuen MF, Sablon E, Hui CK, Li TM, Yuan HJ, Wong DK, Doutreloigne J, Bogaerts V, Wong BC, Fan ST. Prognostic factors in severe exacerbation of chronic hepatitis B. Clin Infect Dis. 2003;36:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Li J, Wang L, Wang S, Zhu H, Ye P, Xie A, Shen B, Liu C, Guo C, Fu Q. The Treg/Th17 imbalance in patients with idiopathic dilated cardiomyopathy. Scand J Immunol. 2010;71:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Li N, Bian H, Zhang J, Li X, Ji X, Zhang Y. The Th17/Treg imbalance exists in patients with heart failure with normal ejection fraction and heart failure with reduced ejection fraction. Clin Chim Acta. 2010;411:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Li J, Qiu SJ, She WM, Wang FP, Gao H, Li L, Tu CT, Wang JY, Shen XZ, Jiang W. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One. 2012;7:e39307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739-747. [PubMed] |
| 40. | Niu Y, Liu H, Yin D, Yi R, Chen T, Xue H, Zhang S, Lin S, Zhao Y. The balance between intrahepatic IL-17(+) T cells and Foxp3(+) regulatory T cells plays an important role in HBV-related end-stage liver disease. BMC Immunol. 2011;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | You J, Sriplung H, Geater A, Chongsuvivatwong V, Zhuang L, Li YL, Lei H, Liu J, Chen HY, Tang BZ. Impact of viral replication inhibition by entecavir on peripheral T lymphocyte subpopulations in chronic hepatitis B patients. BMC Infect Dis. 2008;8:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Boni C, Penna A, Bertoletti A, Lamonaca V, Rapti I, Missale G, Pilli M, Urbani S, Cavalli A, Cerioni S. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J Hepatol. 2003;39:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Akbar SM, Horiike N, Chen S, Michitaka K, Abe M, Hiasa Y, Matsuura B, Onji M. Mechanism of restoration of immune responses of patients with chronic hepatitis B during lamivudine therapy: increased antigen processing and presentation by dendritic cells. J Viral Hepat. 2011;18:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | TrehanPati N, Kotillil S, Hissar SS, Shrivastava S, Khanam A, Sukriti S, Mishra SK, Sarin SK. Circulating Tregs correlate with viral load reduction in chronic HBV-treated patients with tenofovir disoproxil fumarate. J Clin Immunol. 2011;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Sun QF, Ding JG, Xu DZ, Chen YP, Hong L, Ye ZY, Zheng MH, Fu RQ, Wu JG, Du QW. Prediction of the prognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat. 2009;16:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
P- Reviewers: Chen DY, Yan YQ S- Editor: Ma YJ L- Editor: Logan S E- Editor: Zhang DN