Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8458
Revised: December 18, 2013
Accepted: April 1, 2014
Published online: July 14, 2014
Processing time: 294 Days and 7.8 Hours
Pancreatic ductal adenocarcinoma is the 4th leading cause of cancer deaths in the United States. The majority of patients are candidates only for palliative chemotherapy, which has proven largely ineffective in halting tumor progression. One proposed mechanism of chemoresistance involves signaling via the mesenchymal-epithelial transition factor protein (MET), a previously established pathway critical to cell proliferation and migration. Here, we review the literature to characterize the role of MET in the development of tumorigenesis, metastasis and chemoresistance, highlighting the potential of MET as a therapeutic target in pancreatic cancer. In this review, we characterize the role of c-Met in the development of tumorigenesis, metastasis and chemoresistance, highlighting the potential of c-Met as a therapeutic target in pancreatic cancer.
Core tip: As one of the leading causes of cancer-related deaths, pancreatic cancer remains elusive to our current therapeutic options. These modest advances in current therapies for pancreatic cancer have led to the recognition and development of targeted therapies toward tyrosine kinase receptors such as the c-Met receptor. In this review, we characterize the role of c-Met in the development of tumorigenesis, metastasis and chemoresistance, highlighting the potential of c-Met as a therapeutic target in pancreatic cancer.
- Citation: Delitto D, Vertes-George E, Hughes SJ, Behrns KE, Trevino JG. c-Met signaling in the development of tumorigenesis and chemoresistance: Potential applications in pancreatic cancer. World J Gastroenterol 2014; 20(26): 8458-8470
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8458
Pancreatic cancer is the 4th leading cause of cancer deaths in the United States[1]. Currently, surgical resection is the only treatment option with the potential of cure. However, only 17% of patients are surgical candidates upon diagnosis and surgical resection in combination with chemotherapy and radiation therapy results in a 5-year survival of approximately 23% in specialized centers focused on pancreatic cancer[2]. While chemotherapy has the potential to delay tumor progression, innate or acquired chemoresistance and subsequent tumor resurgence is the norm[3,4]. Biologically diverse mechanisms have been identified to be involved in the chemoresistant phenotype, ranging from genetic and epigenetic changes to microenvironmental adaptation[4,5]. The goal of this review is to focus on the signaling of the mesenchymal-epithelial transition factor protein (MET) in pancreatic cancer.
The mesenchymal-epithelial transition factor gene (c-met) encodes for a membrane-bound receptor tyrosine kinase (RTK) expressed predominantly by epithelial cells. MET is activated and signals downstream pathways following induction of phosphorylation in response to binding of its ligand, hepatocyte growth factor (HGF), also referred to as scatter factor. These ligands are secreted by cells of mesenchymal origin. The resulting HGF/MET pleiotropic signaling cascade activates mediators of cell proliferation and motility and has been heavily implicated in tumorigenesis via identification of amplification, activating mutation, and/or overexpression of MET in most solid organ neoplasms. Here, we review the literature to characterize the role of MET in the development of tumorigenesis, invasion, metastasis and chemoresistance, highlighting the potential of MET as a therapeutic target in pancreatic cancer.
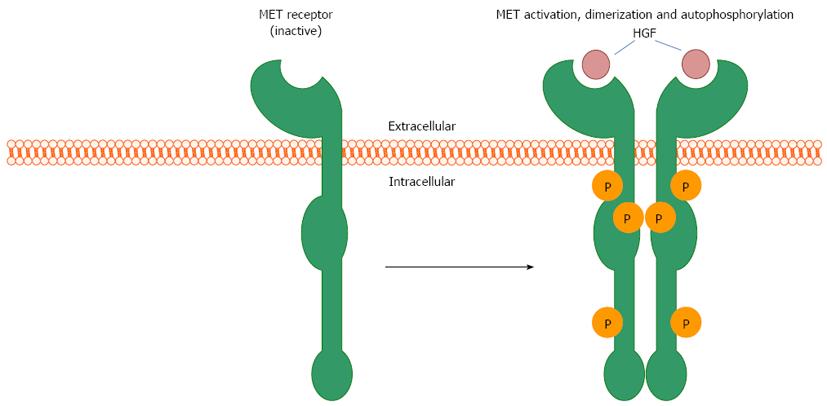
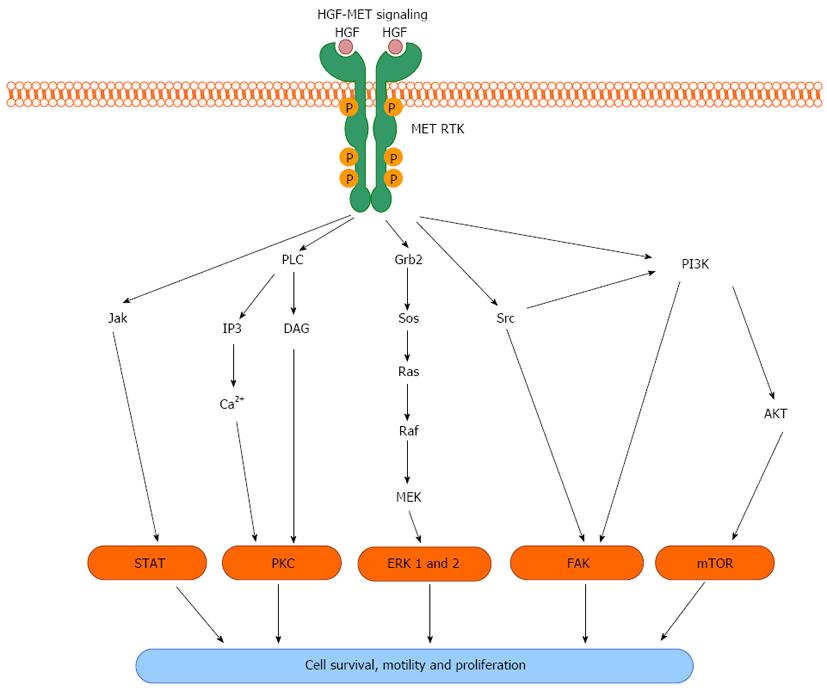
MET activation propagates a complex system of intracellular signaling cascades that act to affect cell proliferation and migration. HGF is secreted by mesenchymal cells in close proximity to MET-expressing epithelial cells during embryogenesis or in response to tissue injury, thus functioning as a paracrine signaling mechanism that promotes cell proliferation and migration. MET is translated as a 180 kDa protein that is subsequently cleaved to form a heterodimer consisting of a short alpha (approximately 40 kDa) and long beta (approximately 140 kDa) chain of residues. The mature protein is then transported to and inserted in the plasma membrane. Upon HGF ligand binding to MET, autophosphorylation at multiple tyrosine residues within the cytoplasmic domain occurs, catalyzed by intrinsic ATPase activity. This results in changes in the tertiary structure of MET facilitating the formation of a signaling complex including GAB1 and GRB2 proteins that subsequently activates multiple downstream pathways (Figure 1). Known effector molecules of this signaling cascade include Src, mitogen-activated kinase, extracellular signal-regulated kinase 1 and 2, phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), signal transducer and activator of transcription (STAT), nuclear-factor-κB, and mammalian target of rapamycin[6-9]. MET-mediated induction of these pathways acts to positively influence cell proliferation, migration, and survival (Figure 2). Via these down-stream effectors, HGF-MET signaling plays a crucial role in important physiologic processes including embryonic development, organ regeneration and wound healing.
MET is essential for embryonic development and hgf- or c-met-null embryos die in utero[10]. In early embryonic development, HGF and its receptor MET are co-expressed by progenitor cells, suggesting autocrine signaling is an early homeostatic mechanism for stem cell survival[11]. HGF-MET signaling is necessary to ensure the growth and survival of placental trophoblast cells as well as embryonic hepatocytes. MET signaling is also necessary for the proper migration of muscle progenitor cells, development of the embryonic nervous system, and epithelial branching morphogenesis[12,13]. Later in development, paracrine HGF-MET signaling is critical for properly orchestrating organogenesis. Assays evaluating the ability of epithelial cells to form tubules in vitro, a process which recapitulates organ development, demonstrate that HGF signaling induces cells to undergo an epithelial-to-mesenchymal (EMT) transition. This transition allows host cells to relocate during embryonic development. Ultimately, these cells reclaim their epithelial identity, but the EMT marks a critical event in organogenesis.[11]
Inflammation and wound healing following injury are also highly dependent on HGF-MET signaling. HGF increases dramatically following renal or hepatic damage, inducing a diverse array of anti-apoptotic responses[9,14,15]. In cases of chronic or repetitive injury, HGF acts to oppose fibrosis by inducing apoptosis of myofibroblasts and by antagonizing transforming growth factor-β (TGF-β)[9,13,16]. Peptic ulcer disease represents a specific example of MET’s protective effect. The loss of HGF signaling in a murine model led to decreased gastric mucosal cell proliferation and delayed healing from mucosal injury[17]. In fact, HGF-MET signaling has been implicated as essential to the protection, regeneration, and anti-fibrotic activity of cutaneous, pulmonary, hepatic, and gastrointestinal tissues in response to injury[13].
With respect to pancreatic endocrine physiology, the beta cell, responsible for insulin secretion, is dependent on HGF-MET signaling to hypertrophy and proliferate in response to persistent hyperglycemia[18]. In effect, MET is essential for the hyperinsulinemia seen in Type II diabetes. c-met knockdown mice exhibit increased beta cell apoptosis during development and are more susceptible to streptozotocin-induced diabetes[19]. Additionally, c-met knockdown mice displayed reduced beta cell expansion during pregnancy leading to an increase in gestational diabetes[20]. Multiple investigations have confirmed that these knockdown mice have decreased glucose tolerance and reduced insulin secretion after stimulation[21,22]. In fact, stimulation of the HGF/MET pathway has been suggested to encourage beta cell proliferation after islet cell transplantation. Thus, MET plays a critical role in pancreatic neuroendocrine cell proliferation and development.
Relatively little data is available concerning MET signaling and normal pancreatic exocrine development. A recent investigation by Anderson et al[23] examined the phenotype of a point mutation in c-met that impaired localization and activation of MET. Zebrafish with this mutation exhibited mislocalization of pancreatic ductal cells compared with wild-type animals. Interestingly, ductal proliferation was unaffected. Further, inhibition of MET proteindownstream signaling with PI3K and STAT inhibitors produced a similar phenotype, suggesting an essential role for MET in migration and localization of embryonic pancreatic ductal cells.
In summary, physiologic HGF-MET signaling is essential for appropriate embryonic development and organ repair. The function of the HGF/MET pathway observed in multiple organ systems appears to drive cell proliferation and mobility. Unfortunately, dysregulation of this pathway clearly could result in tumor initiation and/or progression. Amplification, mutation or overexpression of c-met become deleterious, contributing to malignant transformation and metastasis. Activating and sustaining HGF-MET signaling in this pathologic context drives tumor progression and is responsible, at least in part, to the development of chemoresistance.
Excessive MET activity is a feature of many cancers, although inciting mechanisms appear to be tumor-specific[24]. c-met received early attention as a proto-oncogene when activating mutant alleles were implicated in cases of hereditary papillary renal cell carcinoma[25]. The resulting MET receptor was constitutively activated, undergoing spontaneous ligand-independent phosphorylation[11]. In an analysis of seven families with hereditary papillary renal carcinoma, four displayed activating c-met mutations, all of which were located in the tyrosine kinase domain of the MET protein[25]. Sporadic c-met mutations have also been described in gastric carcinomas, glioblastomas, and squamous cell carcinomas of the head and neck[11,12,26]. Furthermore, aberrant positive feedback systems involving autocrine and paracrine signaling in the HGF-MET axis contribute to tumorigenic phenotypes in melanomas, osteosarcomas, breast cancer and gliomas[26]. One retrospective histopathologic analysis observed MET overexpression in 87% of renal cell carcinoma specimens[27]. Additionally, a strong correlation between MET expression and the esophageal metaplasia-dysplasia-adenocarcinoma continuum has been shown in surgical specimens from patients with esophageal adenocarcinoma[28]. In fact, c-met amplification occurs in approximately 9% of esophageal cancers[29]. These investigations provide compelling evidence that c-Met is a potent oncogene.
The association between MET activity and neoplastic progression has been investigated in animal models. Hypoxia-induced tumor cell invasion is dependent upon upregulated MET signaling, suggesting another mechanism driving growth and metastasis[30,31]. Overexpression of wild-type MET in hepatocytes led to spontaneous hepatocellular carcinoma development that regressed upon MET inactivation[30,32]. Thus, overexpression of non-mutated MET is sufficient to induce tumor development. Moreover, inhibition of MET caused established tumors to regress, suggesting that MET signaling is necessary for tumor growth and maintenance. Subsequent animal models have proposed that the frequency of many carcinomas and lymphomas is greatly increased by MET overexpression[33]. Non-neoplastic cell lines forced to constitutively express HGF or MET become highly tumorigenic when implanted in vivo[34,35]. Therefore, while MET activity may not be the inciting mechanism in the formation of many cancers, overexpression in pre-clinical models appears to confer a more aggressive phenotype.
In fact, MET expression has been correlated with more aggressive disease and worse clinical outcomes in many cancers. In NSCLC, MET overexpression correlates with an unfavorable prognosis and has been implicated as a primary mechanism of resistance to epidermal growth factor receptor (EGFR) inhibitor therapy[36,37]. In hepatocellular carcinoma the expression level of MET is directly correlated to metastatic behavior and inversely correlated to the level of tumor differentiation and patient survival[38-41]. In a prospective cohort analysis of 554 patients with renal cell carcinoma, a particular single nucleotide polymorphism (SNP) in c-met was associated with a decline in median recurrence-free survival from 50 to 19 mo[42]. While the functional outcome of this SNP remains to be elucidated, an activating point mutation is highly suspected. Likewise, MET overexpression is a HER2/neu-independent prognostic marker for node-positive breast cancer, signifying increased tumor aggressiveness[43]. MET expression significantly correlated with the depth of invasion and regional lymph node metastasis in colorectal cancer[44]. Thus, the list of solid organ neoplasms for which upregulation of HGF-MET signaling portends a more aggressive phenotype is extensive[45,46]. Taken together, this data demonstrates that dysregulation of the HGF-MET pathway contributes to tumor progression. This data also has implications regarding the status of the HGF-MET pathway on the effectiveness of certain biologic therapies, a concept we will expand upon later.
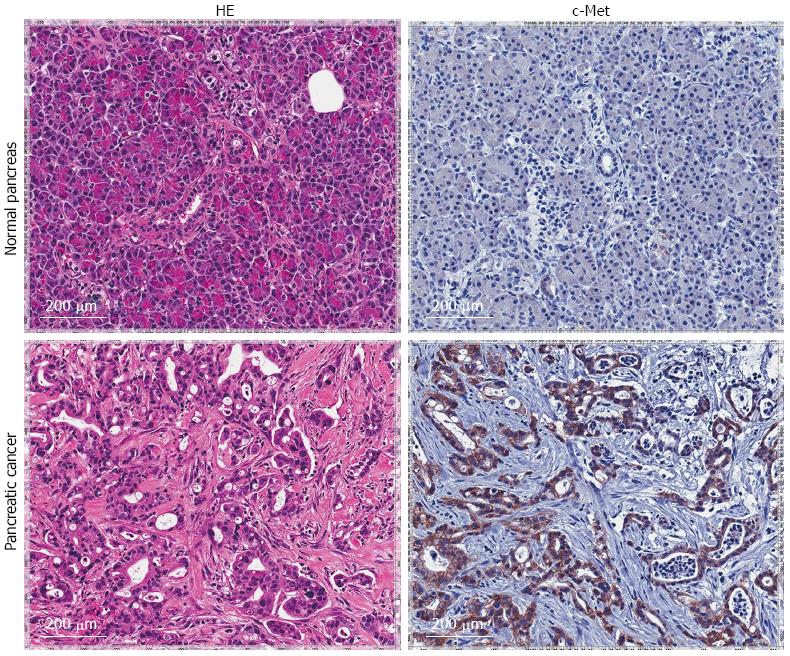
Concerning pancreatic adenocarcinoma, evidence is accumulating that correlates dysregulated MET activity with an aggressive phenotype. In a recent investigation thirty-six pancreatic tumor samples were analyzed and MET expression levels were directly proportional to tumor grade[47]. Similar histopathologic analyses showed an approximate five to seven-fold increase in MET protein expression levels in pancreatic cancer compared to normal pancreas samples[48,49]. Histopathologic evaluation of our own resected patient population support these findings (Figure 3). A larger collection of pancreatic tumor specimens subsequently confirmed increased MET protein expression compared with normal controls and MET protein overexpression significantly correlated with increased TNM stage[50]. In fact, secreted HGF protein from surrounding stromal tissue has been correlated with MET overexpression in patients with pancreatic cancer and associated with worsened overall survival[51]. Given the known pathophysiologic actions of MET in cancer and a well-demonstrated overexpression pattern in pancreatic adenocarcinoma, inhibition would seem a logical therapeutic avenue.
Unfortunately, targeting MET alone as a therapeutic strategy appears to be overly optimistic. Despite convincing evidence of primarily MET-induced tumors, a growing body of evidence supports secondary MET involvement in a synergistic crosstalk with other RTKs such as EGFR, vascular endothelial growth factor receptor and insulin-like growth factor-1 receptor (IGF-1R) to promote malignant cell migration, invasion, and chemoresistance[52-55]. In hepatocellular carcinoma cells, EGFR co-immunoprecipitates with MET and activated EGFR leads to ligand-independent activation of the MET pathway[36]. MET and IGF-1R synergistically promote migration and invasion in pancreatic adenocarcinoma. Down-regulation of MET via adenoviral infection with a MET ribozyme abrogated the effects of IGF-1, suggesting co-dependence of IGF-1R and MET in directing tumor invasion and migration[56]. These complex, multifactorial interactions among RTKs play a key role in growth and maintenance of a variety of tumor types and are under intense scrutiny for potential therapeutic value or mechanisms of therapeutic resistance. These discoveries will be essential to the evolving reality of personalized cancer treatment strategies.
The microenvironment of a tumor may be as instrumental to the progression of disease as the tumor itself. In fact, stromal support in the form of angiogenesis, mitogenic signaling and cytoskeletal attachments are necessary for tumors to grow and metastasize in vivo. As previously mentioned, HGF secretion by stromal cells mediates MET activity in a paracrine manner. Additionally, HGF-MET signaling encourages angiogenesis by inducing VEGF expression by cancer cells[57,58]. However, neovascularization alone is not sufficient for metastasis to occur.
Recall that in embryonic development and tissue repair, MET plays an essential, physiologic role in cellular migration and subsequent organogenesis. Unfortunately, overexpression of MET and its subsequent downstream pathways, including PI3K and Src, similarly enable growth and invasion of malignant cell populations. An initial step in tumor migration involves clearing a path through the extracellular matrix (ECM). This is accomplished primarily by the actions of secreted matrix metalloproteinases (MMPs), which digest surrounding ECM. Not surprisingly, MMPs have been shown to be upregulated by MET signaling[24].
Cells must also respond to chemotactic factors in the ECM for effective migration. As previously mentioned, an EMT endows epithelial cells with certain properties of mesenchymal cells that enable migration. Furthermore, it has recently been proposed that the EMT may be coupled with a transition to a more stem-cell-like state, suggesting further importance of the EMT to metastasis and tumor progression[59]. In embryogenesis, MET controls the EMT necessary to enable myogenic progenitor cell migration[9]. Additionally, EMT is further driven by Wnt signaling, a pathway that is also stimulated by MET via glycogen synthase kinase 3-β[60]. The mechanism by which MET governs the EMT directly in tumor metastases remains to be elucidated.
Finally, malignant cells must take up residence in a distant organ as a metastatic focus. Remarkably, HGF-MET signaling plays a role both in cellular dissociation within the primary tumor and cellular re-association within the metastatic niche[24]. HGF triggers destabilization of adherens junctions within the primary tumor through FAK-mediated integrin signaling[61]. As tumor cells invade and metastasize, failure of proper interaction with foreign microenvironments leads to programmed cell death. HGF-MET signaling upregulates cytoskeleton adhesion receptors and enables tumor cells to effectively engage their new surroundings and elude apoptosis, thereby facilitating metastatic development[24]. Thus, in addition to fostering primary tumor growth, MET appears to act at multiple regulatory points in the development of metastatic disease.
A growing body of evidence suggests that a hierarchy exists in cancer cell populations, a notion initially discovered in hematopoietic malignancies. Cancer stem cells (CSCs) actually comprise a small minority of tumor cells but appear to be the only group capable of unlimited self-renewal and formation of xenografts. Interestingly, these cells appear to have a limited potential for further differentiation[62,63]. CSC populations have subsequently been identified in a variety of solid organ neoplasms including brain, breast, melanoma, pancreas, prostate and colon. While CSC identification is specific to each tumor type, common themes include cell surface markers such as CD24, CD44, CD133, epithelial surface antigen (ESA), chemokine receptor type 4, and urokinase plasminogen activator (Table 1)[64-72]. Importantly, in pancreatic cancer stem cell (PCSC) populations, MET overexpression conferred an equally tumorigenic phenotype to CD44+/CD24+/ESA+ cells[73]. Restated, MET overexpression alone may sustain a pancreatic cancer stem cell phenotype.
| CSC marker | Proposed function |
| CD44 | ECM binding, organization of actin cytoskeleton, modulation of mitogenic signaling[112] |
| CD24 | P-Selectin binding, cell migration[113] |
| ESA | Mediation of epithelial intercellular adhesion[114] |
| CD133 | Activation of Wnt signaling and angiogenesis[115,116] |
| CXCR4 | Receptor of SDF-1, hematopoietic stem cell homing, invasion[117] |
| MET | Receptor of HGF, promotes cell growth, proliferation, migration[11] |
| u-PA | ECM degradation, cell migration[118] |
Conversely, MET overexpression may prompt cancer cells to dedifferentiate into CSCs. MET activation in prostate cancer cells induces a stem-like phenotype and endows cells with more invasive potential[74]. In head and neck squamous cell carcinoma, cells overexpressing MET can recapitulate the heterogeneity of parental tumors in vivo and exhibit increased self-renewal, invasion, and metastasis[75]. In glioblastomas, overexpression of MET leads to a stem-like phenotype resistant to terminal differentiation signals[76]. Regardless of the origin of CSCs, MET overexpression is associated with a stem-cell-like phenotype in a wide range of cancers.
Chemoresistance is an important factor contributing to the high mortality rate of most cancers and is germane to treatment failure in pancreatic cancer. With few exceptions, tumor metastasis precludes surgical therapy and leaves chemotherapy as the only therapeutic option. In borderline cases, neoadjuvant chemotherapy protocols may offer opportunities for attempts at a surgical resection. After surgery, adjuvant chemotherapy protocols are beneficial in avoiding recurrence, especially in more aggressive tumor types. Unfortunately, the development of chemoresistance is a real oncologic dilemma. In the face of chemoresistant tumor populations, no effective treatments exist. Therefore, understanding the molecular regulators of chemoresistance has major implications in therapeutic intervention. Several lines of evidence converge to suggest that MET overexpression may confer a chemoresistant phenotype.
We have outlined the close relationship between MET and CSCs. In fact, CSCs have been shown to be largely responsible for chemoresistant phenotypes in glioblastomas, hematopoietic, pancreatic and colorectal cancers[77-83]. Mechanisms range from reducing drug delivery to repairing cytotoxic injury and ultimately result in tumor cell repopulation[77-83]. Furthermore, a higher proportion of cells bearing CSC markers has been associated with poor outcomes in glioblastomas, breast and pancreatic cancer[84-86]. Thus, investigative directions have become particularly focused on identifying factors that drive and sustain CSCs. Given the significance of HGF-MET signaling in PCSC populations, the role of MET in this process would seem to be particularly relevant in pancreatic cancer.
The activation of the HGF-MET axis has been directly implicated in acquiring and maintaining chemoresistance in several tumor cell populations (Table 2). HGF stimulation protects NSCLC cells from cisplatin toxicity, in part mediated by downregulation of apoptosis-inducing factor[87]. c-met amplification is associated with NSCLC resistance to the EGFR inhibitor Gefitinib via modulation of the PI3K pathway[88]. Multiple investigations have revealed that MET inhibition sensitizes ovarian carcinoma to carboplatin plus paclixatel, whereas MET overexpression imparts chemoresistance[89,90]. Furthermore, stimulation of the HGF-MET pathway confers protection against chemotherapeutic agents by upregulation of PI3K/Akt signaling in multiple myeloma, glioblastoma and gastric adenocarcinoma[91-93]. Our group has found that pharmacologic MET inhibition using a small molecule inhibitor sensitizes esophageal adenocarcinoma cells to pyrimidine analog chemotherapy (unpublished data). Additionally, preclinical studies have demonstrated that overexpression of MET has also been associated with EMT-like changes in acquired-gemcitabine-resistant pancreatic cancer cells[94]. These findings are not surprising as pancreatic cancer is known for rapid acquisition of chemoresistant behavior and also MET overexpression. Additionally, MET inhibition in pancreatic adenocarcinoma leads to gemcitabine sensitization[95]. Although consisting largely of in vitro data, these investigations demonstrate a strong correlation between MET overexpression and chemoresistance in a variety of malignancies.
| Cancer type | Chemotherapy | Mechanism of HGF-MET signaling in chemoresistance |
| Multiple myeloma | Bortezomib | MET overexpression: Apoptotic resistance via PI3K-Akt activation[92] |
| Glioblastoma | Radiation, cisplatin, camptothecin, adriamycin, and taxol groups | Addition of HGF: Anti-apoptotic effects via PI3K-Akt dependent pathways[91] |
| Rhabdomyosarcoma | Vincristine/etoposide, radiation | Addition of HGF: Enhanced migration, MMP secretion, PI3K-Akt activation[119] |
| Non-small cell lung carcinoma | Cisplatin | Addition of HGF: Downregulation of apoptosis-inducing factor (AIF)[87] |
| Non-small cell lung carcinoma | Erlotinib | c-met amplification: Activation of EGFR, preservation of PI3K-Akt activation[88] |
| Gastric adenocarcinoma | Adriamycin | Addition of HGF: Anti-apoptotic effects via PI3K-Akt upregulation[93] |
| Pancreatic adenocarcinoma | Gemcitabine | MET overexpression: Anti-apoptotic effects via PI3K-Akt activation, induction of EMT-like changes[94,95] |
| Ovarian adenocarcinoma | Carboplatin/paclitaxel | MET overexpression: Apoptotic resistance via PI3K-Akt activation[89,90] |
The mechanism by which MET overexpression confers chemoresistance in pancreatic cancer likely involves the mesenchymal support network. Tumors most heavily invested with stroma are often those most refractory to chemotherapy[4]. Stroma is the predominant source of HGF, suggesting MET activation is, at least in part, a result of paracrine signaling. In breast cancer, HGF-MET signaling augments tumor cell adhesion to ECM components by upregulating integrin synthesis and inducing conformational changes that activate integrins[24,96]. This integrin-mediated adhesion is actually a mechanism by which tumor cells can oppose the cytotoxic effect of chemotherapy[97]. Indeed, studies have shown that integrin expression, specifically αβ, is upregulated in cases of relapsed leukemia. This finding suggests that increased integrin expression may contribute to generating minimal residual disease, defined as tumor cell persistence following therapy[4]. Further investigation is necessary to characterize the mechanism by which MET-driven integrin upregulation imparts chemoresistance and whether this principle is applicable to other tumor types. However, disruption of the HGF-MET axis may result in biochemical dissociation from the protective mesenchymal environment, thereby imparting sensitivity to cytotoxic therapies.
Data specific to the pancreatic cancer microenvironment regarding MET signaling is forthcoming. Animal models that utilize VEGF inhibitors to impart ischemia actually result in increased tumor growth and invasion but inhibition of MET abrogates this proliferative response to hypoxia[98]. As previously mentioned, PCSCs can be defined by comparatively high MET expression. Pharmacologic inhibition of MET in PCSC populations blocked self-renewal capacity, reduced the overall PCSC population and significantly slowed tumor growth in vivo[99]. Treatment with MetMAb, a monovalent antibody against MET, has shown decreased pancreatic tumor growth in orthotopic models in vivo[100]. Further, recent preclinical data suggest cabozantinib, a novel small molecule MET inhibitor, overcomes gemcitabine resistance. These studies will likely lead to phase 3 clinical trials using this inhibitor in pancreatic cancer patients[101].
Finally, the interplay between RTKs and the potential for redundancy deserves emphasis when discussing therapeutic intervention. MET and other RTKs are involved in a complex signaling network that may exist as a redundant system with controlled feedback. For example, MET induction has been associated with anti-EGFR therapy and resultant MET overexpression confers resistance to EGFR inhibitors in lung and colorectal cancer[88,102-104]. Thus, MET inhibition may potentiate therapeutic effects aimed against other RTKs, and vice versa. In fact, effective siRNA inhibition of c-Met transcripts in NSCLC confers sensitization to gefitinib, an inhibitor of EGFR[88]. Further, concomitant administration of EGFR and MET inhibitors eliminated NSCLC cells more effectively than either drug alone[55,105]. Similarly, MET inhibition led to increased sensitivity of her2-positive breast cancer cells to trastuzumab[106]. Not surprisingly, combination RTK inhibition is quickly becoming the standard in targeted oncologic chemotherapies involving MET inhibition.
In summary, c-met encodes a versatile RTK crucial to physiologic cell proliferation, organogenesis and wound healing. Its mechanism of action involves multiple anti-apoptotic, pro-mitogenic, and pro-motility downstream effectors. Unfortunately, dysregulated HGF-MET signaling is implicated in multiple oncologic mechanisms, including tumor growth, invasion and chemoresistance. Not surprisingly, clinical studies have consistently revealed MET overexpression as a negative prognostic indicator in a wide variety of malignancies.
HGF-MET signaling mediates mesenchymal-cell-mediated mitogenic support to developing tumor cell populations. MET activity enhances ECM degradation and integrin-mediated adhesion. In addition to promoting mobility and invasion, this appears to confer a protective microenvironment conducive to the development of chemoresistant clones. MET signaling is a marker of cancer stem cell populations, a recently characterized subgroup of cancer cells resistant to cytotoxic therapies.
A better understanding of tumor growth signaling pathways and chemoresistant mechanisms carries the potential of immense therapeutic value, especially in aggressive tumors such as pancreatic adenocarcinoma. Strategies include targeting chemoresistant CSCs, limiting acquired resistance with combination therapy, and developing methods of biochemically dissociating tumor cells from their mitogenic microenvironments. Each of these mechanisms has been associated with HGF-MET signaling. Not surprisingly, a series of MET inhibitors and more nonspecific RTK inhibitors are currently under investigation (Table 3)[107-111]. The evidence presented makes a compelling case for further insights into HGF-MET signaling as a therapeutic target in pancreatic cancer.
| Drug | Target(s) | Impact |
| Cabozantinib | MET | Induced apoptosis in gemcitabine-resistant pancreatic cancer cell lines, currently in phase I clinical trials[101] |
| Crizotinib | ALK, MET | Inhibited growth of gemcitabine resistant pancreatic cancer cell lines[95], FDA approved for ALK-expressing NSCLC and myofibroblastic sarcomas |
| Foretinib | MET, VEGFR | Inhibited tumor growth in lung metastasis animal model but failed to show benefit in multiple phase II clinical trials[110,120,121] |
| Tivantinib | MET | Inhibited growth in multiple cancer cell lines via MET targeting as well as inhibition of microtubule formation[122] |
| E7050 | MET, VEGFR | Inhibited growth in xenograft models of lung, gastric and pancreatic cancer[123] |
| PF-04217903 | MET | Inhibited growth and metastasis of pancreatic neuroendocrine tumors[124] |
| SU11274 | MET | Inhibited growth and proliferation in colon cancer cell lines[125] |
| T-1840383 | MET, VEGFR | Inhibited tumor growth in a variety of murine xenograft models[126] |
| 1. | Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, Miller B, Williams M, Ward E, Wingo PA. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107:1711-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 624] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 712] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 5. | Fodale V, Pierobon M, Liotta L, Petricoin E. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J. 2011;17:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Aparicio IM, Garcia-Marin LJ, Andreolotti AG, Bodega G, Jensen RT, Bragado MJ. Hepatocyte growth factor activates several transduction pathways in rat pancreatic acini. Biochim Biophys Acta. 2003;1643:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Osada S, Carr BI. Critical role of extracellular signal-regulated kinase (ERK) phosphorylation in novel vitamin K analog-induced cell death. Jpn J Cancer Res. 2000;91:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Treviño JG, Pillai S, Kunigal S, Singh S, Fulp WJ, Centeno BA, Chellappan SP. Nicotine induces inhibitor of differentiation-1 in a Src-dependent pathway promoting metastasis and chemoresistance in pancreatic adenocarcinoma. Neoplasia. 2012;14:1102-1114. [PubMed] |
| 9. | Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 990] [Article Influence: 61.9] [Reference Citation Analysis (1)] |
| 10. | Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 796] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 11. | Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26 Suppl 1:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 14. | Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608-10613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Kosai K, Matsumoto K, Nagata S, Tsujimoto Y, Nakamura T. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem Biophys Res Commun. 1998;244:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Nakahira R, Mizuno S, Yoshimine T, Nakamura T. The loss of local HGF, an endogenous gastrotrophic factor, leads to mucosal injuries in the stomach of mice. Biochem Biophys Res Commun. 2006;341:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Araújo TG, Oliveira AG, Carvalho BM, Guadagnini D, Protzek AO, Carvalheira JB, Boschero AC, Saad MJ. Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Endocrinology. 2012;153:5760-5769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Mellado-Gil J, Rosa TC, Demirci C, Gonzalez-Pertusa JA, Velazquez-Garcia S, Ernst S, Valle S, Vasavada RC, Stewart AF, Alonso LC. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-cell death and accelerates the onset of diabetes. Diabetes. 2011;60:525-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, Casinelli GP, Alonso LC, Vasavada RC, García-Ocana A. Loss of HGF/c-Met signaling in pancreatic β-cells leads to incomplete maternal β-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61:1143-1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Dai C, Huh CG, Thorgeirsson SS, Liu Y. Beta-cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol. 2005;167:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A. Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes. 2005;54:2090-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Anderson RM, Delous M, Bosch JA, Ye L, Robertson MA, Hesselson D, Stainier DY. Hepatocyte growth factor signaling in intrapancreatic ductal cells drives pancreatic morphogenesis. PLoS Genet. 2013;9:e1003650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 583] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 25. | Schmidt L, Duh FM, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1126] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 26. | Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, Stefani AD, Valente G, Giordano S, Cortesina G, Comoglio PM. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 247] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Natali PG, Prat M, Nicotra MR, Bigotti A, Olivero M, Comoglio PM, Di Renzo MF. Overexpression of the met/HGF receptor in renal cell carcinomas. Int J Cancer. 1996;69:212-217. [PubMed] |
| 28. | Herrera LJ, El-Hefnawy T, Queiroz de Oliveira PE, Raja S, Finkelstein S, Gooding W, Luketich JD, Godfrey TE, Hughes SJ. The HGF receptor c-Met is overexpressed in esophageal adenocarcinoma. Neoplasia. 2005;7:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Bandla S, Pennathur A, Luketich JD, Beer DG, Lin L, Bass AJ, Godfrey TE, Litle VR. Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg. 2012;93:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1036] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 31. | Sennino B, Ishiguro-Oonuma T, Schriver BJ, Christensen JG, McDonald DM. Inhibition of c-Met reduces lymphatic metastasis in RIP-Tag2 transgenic mice. Cancer Res. 2013;73:3692-3703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 228] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Graveel C, Su Y, Koeman J, Wang LM, Tessarollo L, Fiscella M, Birchmeier C, Swiatek P, Bronson R, Vande Woude G. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc Natl Acad Sci USA. 2004;101:17198-17203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Jeffers M, Rong S, Anver M, Vande Woude GF. Autocrine hepatocyte growth factor/scatter factor-Met signaling induces transformation and the invasive/metastastic phenotype in C127 cells. Oncogene. 1996;13:853-856. [PubMed] |
| 35. | Rong S, Segal S, Anver M, Resau JH, Vande Woude GF. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA. 1994;91:4731-4735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 278] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Park S, Choi YL, Sung CO, An J, Seo J, Ahn MJ, Ahn JS, Park K, Shin YK, Erkin OC. High MET copy number and MET overexpression: poor outcome in non-small cell lung cancer patients. Histol Histopathol. 2012;27:197-207. [PubMed] |
| 37. | Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3:S21-S35. [PubMed] |
| 38. | Daveau M, Scotte M, François A, Coulouarn C, Ros G, Tallet Y, Hiron M, Hellot MF, Salier JP. Hepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinoma. Mol Carcinog. 2003;36:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu MY, Xu Y, Song ZJ, Wang ZJ, Wu JC. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Tavian D, De Petro G, Benetti A, Portolani N, Giulini SM, Barlati S. u-PA and c-MET mRNA expression is co-ordinately enhanced while hepatocyte growth factor mRNA is down-regulated in human hepatocellular carcinoma. Int J Cancer. 2000;87:644-649. [PubMed] |
| 41. | Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology. 1997;25:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Schutz FA, Pomerantz MM, Gray KP, Atkins MB, Rosenberg JE, Hirsch MS, McDermott DF, Lampron ME, Lee GS, Signoretti S. Single nucleotide polymorphisms and risk of recurrence of renal-cell carcinoma: a cohort study. Lancet Oncol. 2013;14:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Lengyel E, Prechtel D, Resau JH, Gauger K, Welk A, Lindemann K, Salanti G, Richter T, Knudsen B, Vande Woude GF. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 44. | Takeuchi H, Bilchik A, Saha S, Turner R, Wiese D, Tanaka M, Kuo C, Wang HJ, Hoon DS. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res. 2003;9:1480-1488. [PubMed] |
| 45. | Torres KE, Zhu QS, Bill K, Lopez G, Ghadimi MP, Xie X, Young ED, Liu J, Nguyen T, Bolshakov S. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res. 2011;17:3943-3955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Varkaris A, Corn PG, Gaur S, Dayyani F, Logothetis CJ, Gallick GE. The role of HGF/c-Met signaling in prostate cancer progression and c-Met inhibitors in clinical trials. Expert Opin Investig Drugs. 2011;20:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Gardian K, Janczewska S, Durlik M. Microenvironment elements involved in the development of pancreatic cancer tumor. Gastroenterol Res Pract. 2012;2012:585674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Ebert M, Yokoyama M, Friess H, Büchler MW, Korc M. Coexpression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res. 1994;54:5775-5778. [PubMed] |
| 49. | Yu J, Ohuchida K, Mizumoto K, Ishikawa N, Ogura Y, Yamada D, Egami T, Fujita H, Ohashi S, Nagai E. Overexpression of c-met in the early stage of pancreatic carcinogenesis; altered expression is not sufficient for progression from chronic pancreatitis to pancreatic cancer. World J Gastroenterol. 2006;12:3878-3882. [PubMed] |
| 50. | Zhu GH, Huang C, Qiu ZJ, Liu J, Zhang ZH, Zhao N, Feng ZZ, Lv XH. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig Dis Sci. 2011;56:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 51. | Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, Miyazaki K. The hypoxic environment in tumor-stromal cells accelerates pancreatic cancer progression via the activation of paracrine hepatocyte growth factor/c-Met signaling. Ann Surg Oncol. 2007;14:2600-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Garofalo M, Romano G, Di Leva G, Nuovo G, Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med. 2012;18:74-82. [PubMed] |
| 53. | Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806-8811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 278] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Peghini PL, Iwamoto M, Raffeld M, Chen YJ, Goebel SU, Serrano J, Jensen RT. Overexpression of epidermal growth factor and hepatocyte growth factor receptors in a proportion of gastrinomas correlates with aggressive growth and lower curability. Clin Cancer Res. 2002;8:2273-2285. [PubMed] |
| 55. | Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Bauer TW, Somcio RJ, Fan F, Liu W, Johnson M, Lesslie DP, Evans DB, Gallick GE, Ellis LM. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol Cancer Ther. 2006;5:1676-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1012] [Cited by in RCA: 1038] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 58. | Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911-5918. [PubMed] |
| 59. | Brabletz T. To differentiate or not--routes towards metastasis. Nat Rev Cancer. 2012;12:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 503] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 60. | Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G, Sottile A, Naldini L, Comoglio PM. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 61. | Flinder LI, Wierød L, Rosseland CM, Huitfeldt HS, Skarpen E. FAK regulates Cdk2 in EGF-stimulated primary cultures of hepatocytes. J Cell Physiol. 2013;228:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4932] [Article Influence: 170.1] [Reference Citation Analysis (1)] |
| 63. | Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 921] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 64. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7800] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 65. | Asuthkar S, Gondi CS, Nalla AK, Velpula KK, Gorantla B, Rao JS. Urokinase-type plasminogen activator receptor (uPAR)-mediated regulation of WNT/β-catenin signaling is enhanced in irradiated medulloblastoma cells. J Biol Chem. 2012;287:20576-20589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Asuthkar S, Stepanova V, Lebedeva T, Holterman AL, Estes N, Cines DB, Rao JS, Gondi CS. Multifunctional roles of urokinase plasminogen activator (uPA) in cancer stemness and chemoresistance of pancreatic cancer. Mol Biol Cell. 2013;24:2620-2632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320-4333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 68. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2163] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 69. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2455] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 70. | Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153-3161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 71. | O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3068] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 72. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [PubMed] |
| 73. | Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218-2227.e5. [PubMed] |
| 74. | van Leenders GJ, Sookhlall R, Teubel WJ, de Ridder CM, Reneman S, Sacchetti A, Vissers KJ, van Weerden W, Jenster G. Activation of c-MET induces a stem-like phenotype in human prostate cancer. PLoS One. 2011;6:e26753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Sun S, Wang Z. Head neck squamous cell carcinoma c-Met⁺ cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129:2337-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 76. | Li Y, Li A, Glas M, Lal B, Ying M, Sang Y, Xia S, Trageser D, Guerrero-Cázares H, Eberhart CG. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci USA. 2011;108:9951-9956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 77. | Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4467] [Cited by in RCA: 4858] [Article Influence: 242.9] [Reference Citation Analysis (0)] |
| 78. | Brennan SK, Meade B, Wang Q, Merchant AA, Kowalski J, Matsui W. Mantle cell lymphoma activation enhances bortezomib sensitivity. Blood. 2010;116:4185-4191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2102] [Cited by in RCA: 1962] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 80. | Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 440] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 81. | Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, García-García E, López-Ríos F, Matsui W. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 82. | Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1379] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 83. | Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 421] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 84. | Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3173] [Cited by in RCA: 3109] [Article Influence: 163.6] [Reference Citation Analysis (0)] |
| 85. | Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 86. | Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 442] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 87. | Chen JT, Huang CY, Chiang YY, Chen WH, Chiou SH, Chen CY, Chow KC. HGF increases cisplatin resistance via down-regulation of AIF in lung cancer cells. Am J Respir Cell Mol Biol. 2008;38:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3442] [Cited by in RCA: 3720] [Article Influence: 195.8] [Reference Citation Analysis (0)] |
| 89. | Marchion DC, Bicaku E, Xiong Y, Bou Zgheib N, Al Sawah E, Stickles XB, Judson PL, Lopez AS, Cubitt CL, Gonzalez-Bosquet J. A novel c-Met inhibitor, MK8033, synergizes with carboplatin plus paclitaxel to inhibit ovarian cancer cell growth. Oncol Rep. 2013;29:2011-2018. [PubMed] |
| 90. | Tang MK, Zhou HY, Yam JW, Wong AS. c-Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2. Neoplasia. 2010;12:128-138. [PubMed] |
| 91. | Bowers DC, Fan S, Walter KA, Abounader R, Williams JA, Rosen EM, Laterra J. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 2000;60:4277-4283. [PubMed] |
| 92. | Que W, Chen J. Knockdown of c-Met inhibits cell proliferation and invasion and increases chemosensitivity to doxorubicin in human multiple myeloma U266 cells in vitro. Mol Med Rep. 2011;4:343-349. [PubMed] |
| 93. | Takeuchi K, Ito F. Suppression of adriamycin-induced apoptosis by sustained activation of the phosphatidylinositol-3’-OH kinase-Akt pathway. J Biol Chem. 2004;279:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629-3637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 95. | Avan A, Quint K, Nicolini F, Funel N, Frampton AE, Maftouh M, Pelliccioni S, Schuurhuis GJ, Peters GJ, Giovannetti E. Enhancement of the antiproliferative activity of gemcitabine by modulation of c-Met pathway in pancreatic cancer. Curr Pharm Des. 2013;19:940-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 96. | Trusolino L, Cavassa S, Angelini P, Andó M, Bertotti A, Comoglio PM, Boccaccio C. HGF/scatter factor selectively promotes cell invasion by increasing integrin avidity. FASEB J. 2000;14:1629-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Zutter MM. Integrin-mediated adhesion: tipping the balance between chemosensitivity and chemoresistance. Adv Exp Med Biol. 2007;608:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Lynn KD, Brekken RA. Anti-VEGF therapy revived by c-Met inhibition, but is c-Met the answer? Cancer Discov. 2012;2:211-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Herreros-Villanueva M, Zubia-Olascoaga A, Bujanda L. c-Met in pancreatic cancer stem cells: therapeutic implications. World J Gastroenterol. 2012;18:5321-5323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Jin H, Yang R, Zheng Z, Romero M, Ross J, Bou-Reslan H, Carano RA, Kasman I, Mai E, Young J. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360-4368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 101. | Hage C, Rausch V, Giese N, Giese T, Schönsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 102. | Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932-20937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1380] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 103. | Liska D, Chen CT, Bachleitner-Hofmann T, Christensen JG, Weiser MR. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin Cancer Res. 2011;17:472-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 104. | Tanaka A, Sueoka-Aragane N, Nakamura T, Takeda Y, Mitsuoka M, Yamasaki F, Hayashi S, Sueoka E, Kimura S. Co-existence of positive MET FISH status with EGFR mutations signifies poor prognosis in lung adenocarcinoma patients. Lung Cancer. 2012;75:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Chen G, Noor A, Kronenberger P, Teugels E, Umelo IA, De Grève J. Synergistic effect of afatinib with su11274 in non-small cell lung cancer cells resistant to gefitinib or erlotinib. PLoS One. 2013;8:e59708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 106. | Shattuck DL, Miller JK, Carraway KL, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 107. | Bagai R, Fan W, Ma PC. ARQ-197, an oral small-molecule inhibitor of c-Met for the treatment of solid tumors. IDrugs. 2010;13:404-414. [PubMed] |
| 108. | Blumenschein GR, Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol. 2012;30:3287-3296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 109. | Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med. 2010;16:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 110. | Shah MA, Wainberg ZA, Catenacci DV, Hochster HS, Ford J, Kunz P, Lee FC, Kallender H, Cecchi F, Rabe DC. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One. 2013;8:e54014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 111. | Venepalli NK, Goff L. Targeting the HGF-cMET Axis in Hepatocellular Carcinoma. Int J Hepatol. 2013;2013:341636. [PubMed] |
| 112. | Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1645] [Cited by in RCA: 1850] [Article Influence: 80.4] [Reference Citation Analysis (15)] |
| 113. | Aigner S, Ruppert M, Hubbe M, Sammar M, Sthoeger Z, Butcher EC, Vestweber D, Altevogt P. Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol. 1995;7:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 114. | Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 394] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 115. | Akita M, Tanaka K, Matsumoto S, Komatsu K, Fujita K. Detection of the Hematopoietic Stem and Progenitor Cell Marker CD133 during Angiogenesis in Three-Dimensional Collagen Gel Culture. Stem Cells Int. 2013;2013:927403. [PubMed] |
| 116. | Barcelos LS, Duplaa C, Kränkel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 117. | Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523-4530. [PubMed] |
| 118. | Gorantla B, Asuthkar S, Rao JS, Patel J, Gondi CS. Suppression of the uPAR-uPA system retards angiogenesis, invasion, and in vivo tumor development in pancreatic cancer cells. Mol Cancer Res. 2011;9:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 119. | Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003;63:7926-7935. [PubMed] |
| 120. | Qian F, Engst S, Yamaguchi K, Yu P, Won KA, Mock L, Lou T, Tan J, Li C, Tam D. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009;69:8009-8016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 284] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 121. | Seiwert T, Sarantopoulos J, Kallender H, McCallum S, Keer HN, Blumenschein G. Phase II trial of single-agent foretinib (GSK1363089) in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2013;31:417-424. [PubMed] |
| 122. | Katayama R, Aoyama A, Yamori T, Qi J, Oh-hara T, Song Y, Engelman JA, Fujita N. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res. 2013;73:3087-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 123. | Nakagawa T, Tohyama O, Yamaguchi A, Matsushima T, Takahashi K, Funasaka S, Shirotori S, Asada M, Obaishi H. E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci. 2010;101:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 124. | Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 337] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 125. | Gao SH, Liu C, Wei J, Feng Y. Effect of c-Met inhibitor SU11274 on human colon cancer cell growth. Chin Med J (Engl). 2013;126:2705-2709. [PubMed] |
| 126. | Awazu Y, Nakamura K, Mizutani A, Kakoi Y, Iwata H, Yamasaki S, Miyamoto N, Imamura S, Miki H, Hori A. A novel inhibitor of c-Met and VEGF receptor tyrosine kinases with a broad spectrum of in vivo antitumor activities. Mol Cancer Ther. 2013;12:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
P- Reviewers: Bramhall S, Barreto S, Chowdhury P, Symeonidis NG S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN