Published online Jun 28, 2014. doi: 10.3748/wjg.v20.i24.7914
Revised: March 14, 2014
Accepted: April 8, 2014
Published online: June 28, 2014
Processing time: 189 Days and 11.5 Hours
AIM: To study if three clinically available small molecule kinase inhibitors (SMI), erlotinib, sunitinib and sorafenib, exert antifibrogenic effects on pancreatic stellate cells (PSC) and analyze the basis of their action.
METHODS: Cultured rat PSC were exposed to SMI. Cell proliferation and viability were assessed employing 5-bromo-2’-deoxyuridine incorporation assay and flow cytometry, respectively. 2-Deoxy-2-[18F] fluoroglucose (18F-FDG) uptake was measured to study metabolic activity. Exhibition of the myofibroblastic PSC phenotype was monitored by immunofluorescence analysis of α-smooth muscle actin (α-SMA) expression. Levels of mRNA were determined by real-time PCR, while protein expression and phosphorylation were analyzed by immunoblotting. Transforming growth factor-β1 (TGF-β1) levels in culture supernatants were quantified by ELISA.
RESULTS: All three SMI inhibited cell proliferation and 18F-FDG uptake in a dose-dependent manner and without significant cytotoxic effects. Furthermore, additive effects of the drugs were observed. Immunoblot analysis showed that sorafenib and sunitib, but not erlotinib, efficiently blocked activation of the AKT pathway, while all three drugs displayed little effect on phosphorylation of ERK1/2. Cells treated with sorafenib or sunitinib expressed less interleukin-6 mRNA as well as less collagen type 1 mRNA and protein. Sorafenib was the only drug that also upregulated the expression of matrix metalloproteinase-2 and reduced the secretion of TGF-β1 protein. All three drugs showed insignificant or discordant effects on the mRNA and protein levels of α-SMA.
CONCLUSION: The tested SMI, especially sorafenib, exert inhibitory effects on activated PSC, which should be further evaluated in preclinical studies.
Core tip: There are no specific therapies available to treat pancreatic fibrosis, a key feature of chronic pancreatitis and pancreatic cancer. Here we show that three clinically available small molecule kinase inhibitors (SMI), erlotinib, sunitinib and sorafenib, exert antifibrogenic effects in vitro by inhibiting key functions of rat pancreatic stellate cells (PSC), the main source of extracellular matrix proteins in the diseased pancreas. Furthermore, additive effects of the drugs were observed. Our studies also provide insight into molecular mechanisms of SMI action in PSC. We suggest that the antifibrotic efficiency of SMI, especially sorafenib, should be further evaluated in preclinical studies.
- Citation: Elsner A, Lange F, Fitzner B, Heuschkel M, Krause BJ, Jaster R. Distinct antifibrogenic effects of erlotinib, sunitinib and sorafenib on rat pancreatic stellate cells. World J Gastroenterol 2014; 20(24): 7914-7925
- URL: https://www.wjgnet.com/1007-9327/full/v20/i24/7914.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i24.7914
Pancreatic stellate cells (PSC) are key players in pancreatic wound healing and fibrosis[1]. In response to pancreatic injury, they transform from a quiescent into an activated phenotype that secretes large amounts of extracellular matrix (ECM) proteins. Furthermore, the cells, which form only 4%-7% of all parenchymal cells in the healthy pancreas, start to proliferate and to replace the organotypic tissue[1-3].
Under persistent pathological conditions, specifically in chronic pancreatitis (CP) and pancreatic cancer (PC), dysregulated activation of PSC and excessive deposition of ECM result in organ fibrosis[4,5]. Pancreatic fibrosis, in turn, contributes to the development of an exocrine and endocrine insufficiency of the gland[6]. Moreover, recent studies suggest that the extended stroma reaction favours progression of PC by various mechanisms[7-9]. Thus, the fibrotic wall surrounding the tumor cells provides a barrier against chemotherapeutics and immune cells. Stroma cells are also a rich source of cytokines, chemokines and growth factors that mediate chemoresistance, suppress apoptosis and stimulate proliferation of the tumor cells[9-13]. Since PC cells, on the other hand, enhance PSC activation by secreting profibrogenic mediators [e.g., the mitogen platelet-derived growth factor (PDGF) and transforming growth factor-β1 (TGF-β1), the key stimulator of ECM synthesis[10,14]], a vicious cycle of PSC activation, enhanced ECM synthesis and accelerated tumor growth may establish. In analogy, mutual paracrine effects of PSC and inflammatory cells contribute to persistent PSC activation in CP[15], which also represents a main risk factor of the tumor disease.
In recent years, attempts have been made to inhibit pancreatic fibrogenesis by interfering with PSC activation, suppressing effector functions of activated PSC, and/or terminating PSC activation through the induction of apoptosis or cellular senescence. Although some promising substances with antifibrotic activity could be identified (e.g., ligands of peroxisome proliferator-activated receptor γ[16,17], interferon-γ[18] and inhibitors of histone deacetylases[19]), there is still no specific antifibrotic therapy for clinical application available yet.
We and others have previously shown that two intracellular pathways, Ras-Raf-MEK (mitogen-activated protein kinase kinase)-ERK (extracellular signal-regulated kinase) and phosphatidylinositol (PI) 3-kinase/AKT (protein kinase B), are crucially involved in PSC activation by growth factors such as PDGF[20-22]. Most recently, various new small molecule kinase inhibitors (SMI) have become available that were developed for the specific treatment of different human malignancies. Many of these inhibitors (1) target growth factor receptors upstream of Ras-Raf-MEK-ERK and PI 3-kinase/AKT; (2) directly inhibit kinases of these pathways; or (3) do both[23-25]. The antifibrotic activity of these substances, however, has not been systematically analyzed so far.
Here, we have studied the biological and molecular effects of three clinically available SMI, erlotinib, sorafenib and sunitinib, on activated PSC. One of the three drugs, the epidermal growth factor receptor (EGFR) kinase inhibitor erlotinib, has been successfully introduced into the clinical treatment of advanced PC[26], and although its benefit with respect to patient survival is small, it represents the only molecularly targeted agent to date that reached its primary end point in a phase 3 study with PC patients[27]. Sorafenib is a multi-kinase inhibitor that targets vascular endothelial growth factor receptors (VEGFR-2 and VEGFR-3), PDGF receptor family members (PDGFR-β and KIT) and Raf kinases[28]. It is used in the treatment of hepatocellular carcinoma and advanced renal cancer[29]. Sunitinib represents another multi-kinase inhibitor and inhibits various receptor tyrosine kinases that have been implicated in tumor growth and angiogenesis, including PDGFR-α/β, VEGFR-1-3, stem cell growth factor receptor (KIT), fms-related tyrosine kinase 3, colony stimulating factor 1 receptor and RET (rearranged during transfection)[30]. The drug has been approved for treatment of metastatic renal cancer and imatinib-resistant gastrointestinal stromal tumors[31,32].
Neither sunitinib nor sorafenib are currently established in the treatment of PC (although they have been tested in clinical trials[27]), and the efficiency of erlotinib is limited. The motivation of this study, however, was to evaluate the antifibrotic efficiency of the drugs, an effect that has not assessed yet but may be exploited in future studies that combine agents with activity against cancer and stroma cells.
The results of our study show that sunitinib and especially sorafenib displayed strong inhibitory effects on activated PSC, while erlotinib was less efficient. Surprisingly, the biological effects of the drugs correlated with an AKT- but not ERK pathway inhibition.
Pancreatic stellate cells were isolated from the pancreas of male Lewis inbred rats (Charles River Laboratories, Sulzbach, Germany) by collagenase digestion of the organ and Nycodenz® (Nycomed, Oslo, Norway) density gradient centrifugation essentially as described before[20]. The cells were cultured in Iscove´s modified Dulbecco’s medium (IMDM; Biochrom, Berlin, Germany) supplemented with 17% fetal calf serum (FCS), 10 mL/L non-essential amino acids (dilution of a 100 × stock solution), 105 U/L penicillin and 100 mg/L streptomycin (all reagents from PAA Laboratories, Pasching, Austria). Upon reaching subconfluency, PSC were harvested by trypsination and recultured at equal seeding densities according to the experimental requirements. All experiments were performed with cells passaged no more than two times. Trypan blue staining was used to distinguish live from dead cells and to determine absolute cell counts.
To quantify DNA synthesis, incorporation of 5-bromo-2´-deoxyuridine (BrdU) was measured using the BrdU labelling and detection enzyme-linked immunosorbent assay kit (Roche Diagnostics, Mannheim, Germany). Therefore, cells were plated in 96-well plates at equal seeding densities and allowed to adhere overnight in complete culture medium before erlotinib, sunitinib and sorafenib [Biaffin, Kassel, Germany; solvent: dimethylsulfoxide (DMSO)] and combinations thereof were added as indicated. After 24 h, BrdU labelling was initiated by adding labelling solution at a final concentration of 10 μmol/L. Another 24 h later, labelling was stopped, and BrdU uptake was measured according to the manufacturer’s instructions.
PSC growing in 12-well plates were exposed to SMI for 48 h as indicated. Afterwards, the cells were harvested by trypsination, resuspended in buffer for flow cytometry (PBS pH 7.4; 0.5% bovine serum albumin; 0.1% sodium azide) and kept on ice until measurement. Subsequently, the samples were labelled with propidium iodide (PI: 10 mg/L; Sigma-Aldrich, Deisenhofen, Germany). PI-positive (dead) cells were quantified as previously described[33], using a FACSCalibur cytometer (BD Biosciences, Heidelberg, Germany) and Flowing Software 2.5.0 (Turku Centre for Biotechnology, Finland).
To analyze the effects of erlotinib, sunitinib and sorafenib on glucose uptake, PSC were seeded in 24-well plates at equal seeding densities and allowed to adhere overnight in complete culture medium. Afterwards, SMI and combinations thereof were added for 24 h. Next, complete culture medium was substituted by Dulbecco’s modified Eagle medium (DMEM; Fisher Scientific, Schwerte, Germany) without FCS, glucose, glutamine and phenol red (but supplemented with inhibitors as before), and incubation continued for 1 h before 2-Deoxy-2-[18F] fluoroglucose (18F-FDG) (Eckert and Ziegler f-con Europe GmbH, Berlin, Germany; 0.5 GBq/L culture medium) was added to each culture well. 30 min later, incubation was terminated by aspirating the medium and rinsing the cell layer three times with ice-cold PBS. PSC were solubilized with 100 mmol/L NaOH, and incorporated 18F activity was determined using a well counter (ISOMED 2100, Nuklear-Medizintechnik Dresden GmbH, Dresden, Germany). 18F-FDG uptake in cells treated with SMI was expressed as percent of controls exposed to the solvent DMSO only.
Protein extracts from equal numbers of PSC (pretreated as indicated) were prepared and subjected to immunoblot analysis as published before[20], using polyvinylidene fluoride membrane for protein transfer. The following primary antibodies (all from New England BioLabs, Frankfurt, Germany unless specified otherwise) were employed: anti-β-actin (#4970), anti-GAPDH (#2118), anti-phospho-AKT (P-AKT) (#4060), anti-AKT protein (#4691), anti-phospho-ERK1/2 (P-ERK1/2) (#4370), anti-ERK1/2 (#06-182, Millipore, Billerica, MA, United States), and anti-collagen I (NB600-408, Novus Biologicals, Littleton, CO, United States). The blots were developed using LI-COR reagents for an Odyssey® Infrared Imaging System as previously described[34]. The signal intensities of the investigated proteins were quantified by means of the Odyssey® software and the raw data processed as described in the corresponding figure legend.
PSC growing in 12-well plates under standard culture conditions were treated with SMI for 48 h as indicated. Subsequently, the culture supernatants were collected and stored at -80 °C until assayed. After clearing the lysates by centrifugation, TGF-β1 concentrations were measured using a commercial ELISA (#436707, BioLegend, San Diego, CA, United States) according to the manufacturer’s instructions.
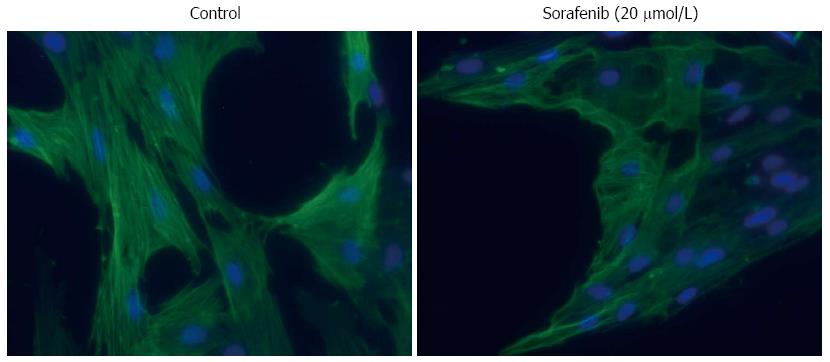
PSC were seeded onto glass coverslips and allowed to attach before they were exposed to SMI for 48 h as indicated. Afterwards, cells were fixed with ice-cold methanol followed by staining of the DNA with 4’,6-diamidino-2-phenylindole (DAPI). Next, the cells were incubated with a mouse monoclonal antibody to α-smooth muscle actin (α-SMA) (#A2547; Sigma-Aldrich). Antibody binding was determined by a fluorescein-labelled goat anti-mouse IgG (MoBiTec, Göttingen, Germany) and visualized using a fluorescence microscope (AxioScope.A1, Carl Zeiss, Jena, Germany).
Total RNA from PSC pretreated with SMI for 48 h as indicated was isolated with TriFast reagent (PEQLAB Biotechnologie, Erlangen, Germany) according to the manufacturer’s instructions. After treatment with DNAse (to remove contaminating traces of genomic DNA), 1 μg of RNA was reverse transcribed into cDNA by means of TaqMan™ Reverse Transcription Reagents and random hexamer priming. Relative quantification of target cDNA levels by real-time PCR was performed in an ABI Prism 7000 sequence detection system employing TaqMan™ Universal PCR Master Mix and the following Assay-on-Demand™ rat gene-specific fluorescently labelled TaqMan™ MGB probes (instrument and reagents: Life Technologies, Darmstadt, Germany): Rn01759928_g1 (Acta2), Rn00561420_m1 (interleukin-6; Il-6), Rn01538167_m1 (matrix metalloproteinase-2; Mmp-2), Rn00579162_m1 (Mmp-9), Rn00572010_m1 (Tgf-β1), Rn01463848_m1 (Col1a1), and Rn01527840_m1 (Hprt; house-keeping gene control). The following PCR conditions were used: 95 °C for 10 min, repeated cycles of 15 s at 95 °C/min at 60 °C. PCR reactions were performed in duplicate, and repeated 5 times with independent samples. The relative expression of each mRNA compared with Hprt was calculated according to the equation ∆Ct = Cttarget - CtHPRT. The relative amount of target mRNA in control cells and cells treated with SMI as indicated was expressed as 2-(∆∆Ct), where ∆∆Cttreatment = ∆CtSMI - ∆Ctcontrol.
Results are expressed as mean ± SE for the indicated number of separate cultures per experimental protocol. Unless indicated otherwise, statistical significance was checked using Wilcoxon’s Signed-Rank test for paired samples and the Mann-Whitney U test in case of independent samples. P < 0.05 was considered to be statistically significant.
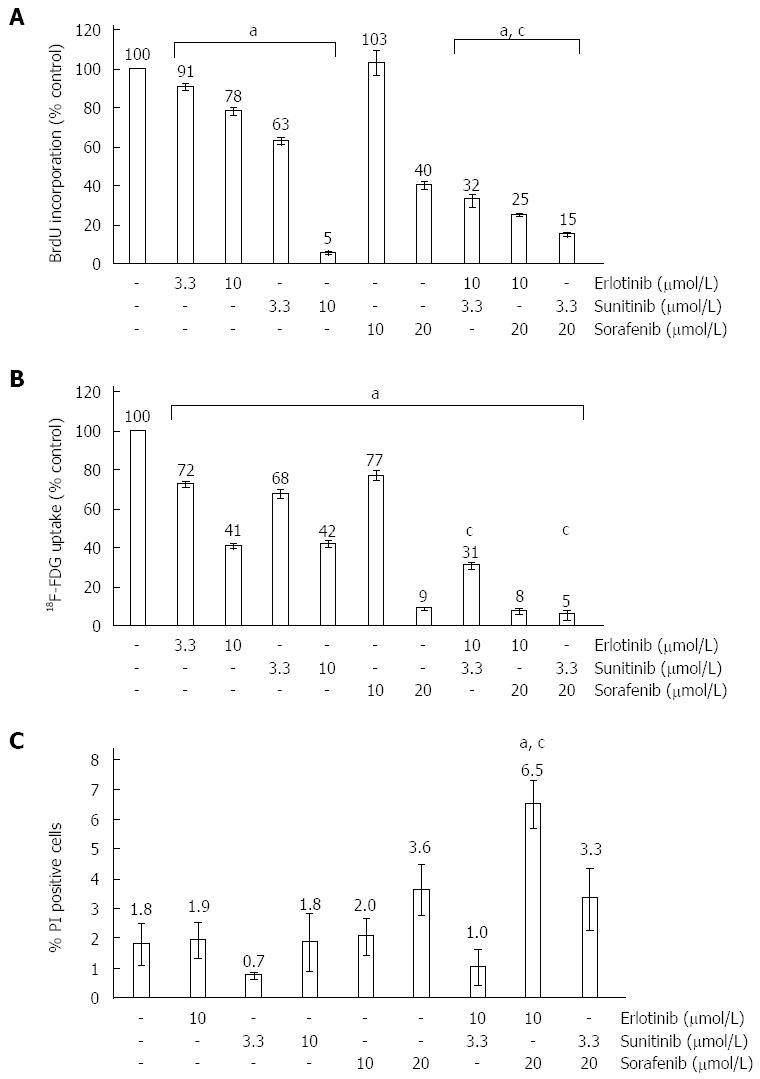
In initial experiments, the effects of erlotinib, sunitinib and sorafenib on PSC proliferation were determined by measuring incorporation of BrdU into newly synthesized DNA. At low micromolar concentrations, all three SMI inhibited DNA synthesis in a dose-dependent manner (Figure 1A), with sunitinib displaying the highest potency in this assay. Furthermore, any combination of two of the drugs exerted stronger effects than the single substances alone, suggesting an additive action.
To analyse SMI effects on cell metabolism, 18F-FDG uptake was chosen as a surrogate marker. Again, all three SMI displayed dose-dependent inhibitory effects, which were further enhanced when the drugs were combined (Figure 1B). An inhibition by more than 90% was observed in samples that were exposed to sorafenib at 20 μmol/L (alone or combined with sunitinib and erlotinib, respectively).
To assess cytotoxity of the drugs, PSC were exposed to SMI at the same concentrations as before, stained with PI and subjected to flow cytometry. As shown in Figure 1C, only the combination of erlotinib and sorafenib caused a significant increase of PI-positive dead cells, but even in this case more than 93% of PSC remained viable. Together, these data suggest that general cytotoxicity was not a major cause of the reduced BrdU incorporation and 18F-FDG uptake of SMI-treated PSC.
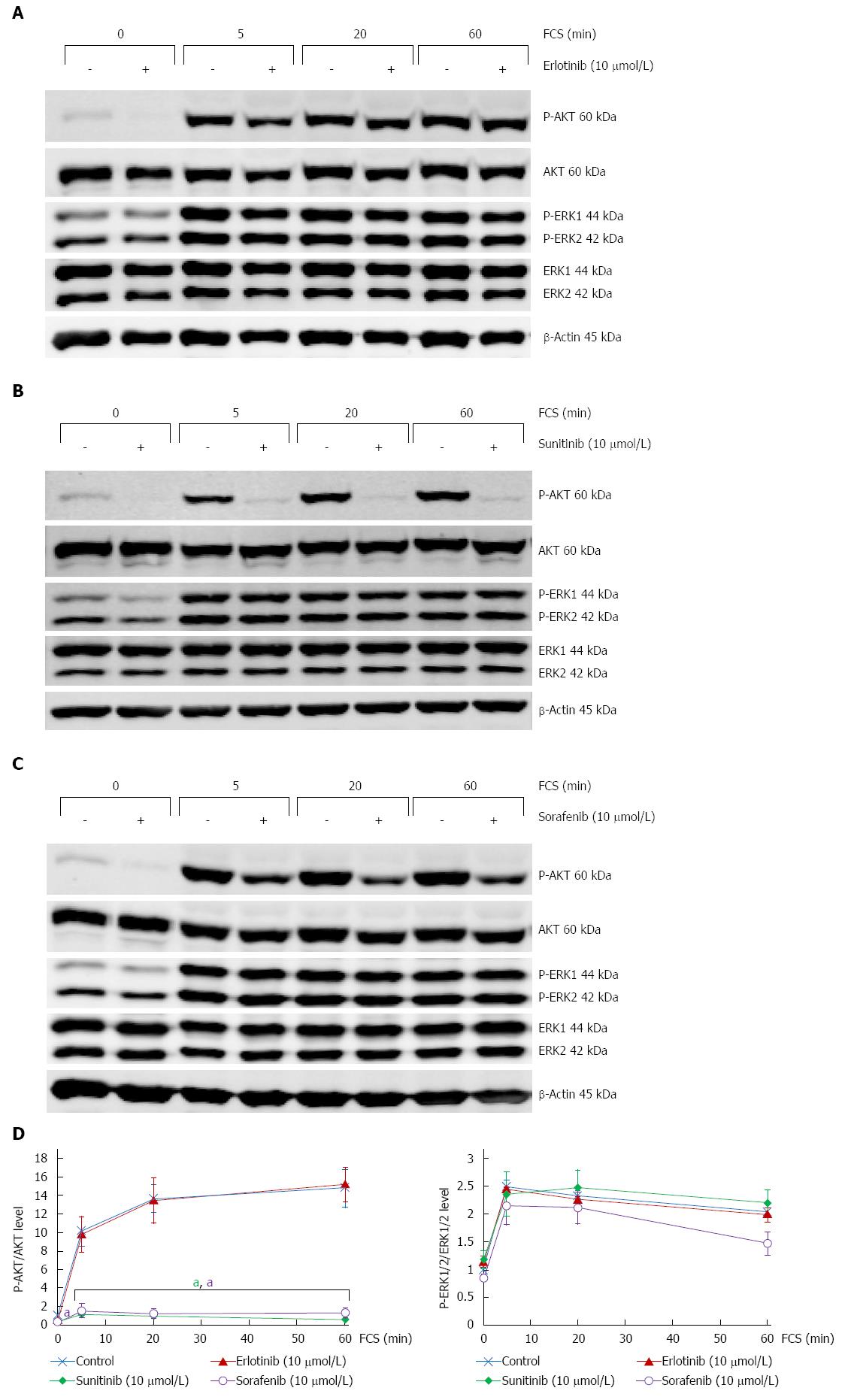
The intracellular signal transduction pathways Ras-Raf-MEK-ERK and PI 3-kinase/AKT play a key role in PSC activation and act downstream of many tyrosine kinases receptors targeted by the SMI tested in this study[20-25]. We therefore addressed the question how erlotinib, sunitinib and sorafenib affect activation of AKT and ERK in PSC, using levels of P-AKT and P-ERK1/2 as surrogate markers (Figure 2). Unexpectedly, none of the three drugs was able to prevent FCS-induced activation of ERK1/2 (Figure 2D, right panel). In contrast, both sunitinib and sorafenib efficiently blocked the increase of P-AKT levels caused by FCS-restimulation of serum-starved PSC, while erlotinib again displayed no effect (Figure 2D, left panel).
Together, these data implicate AKT in the action of sunitinib and sorafenib in PSC but point to a negligible role of the ERK pathway.
Employing real-time PCR, the gene expression profile of PSC after 48 h of SMI treatment was characterized. Figure 3 shows the results for a selected panel of genes that was chosen based on (1) their established role in PSC function and ECM metabolism; and (2) the results of pilot experiments.
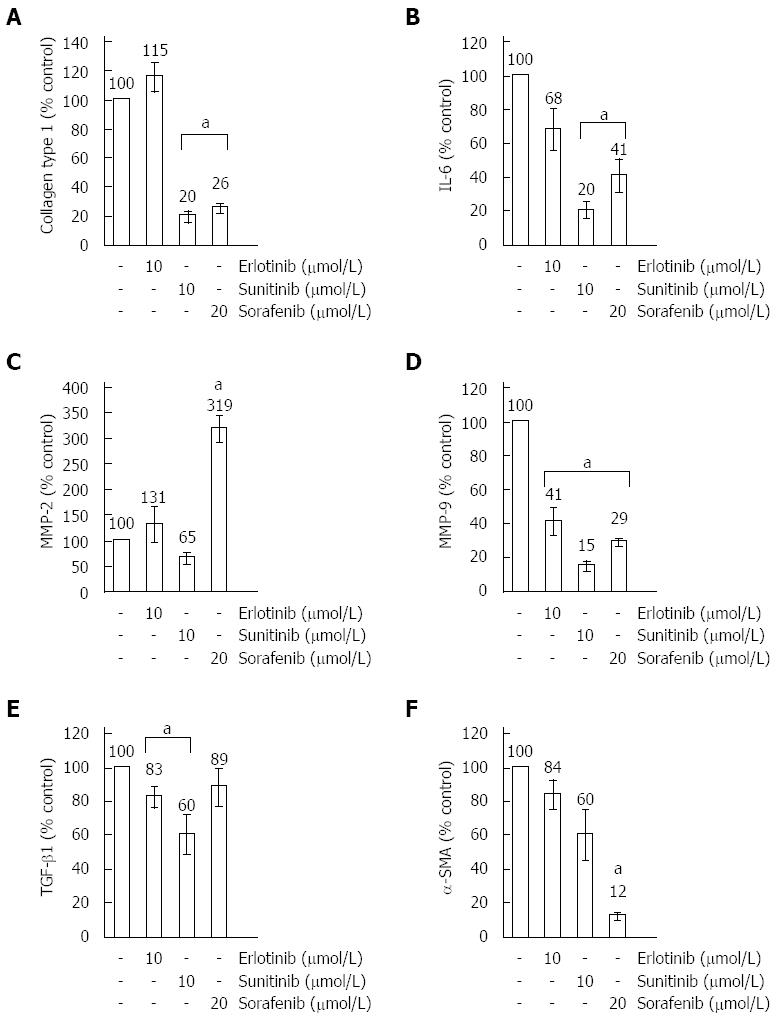
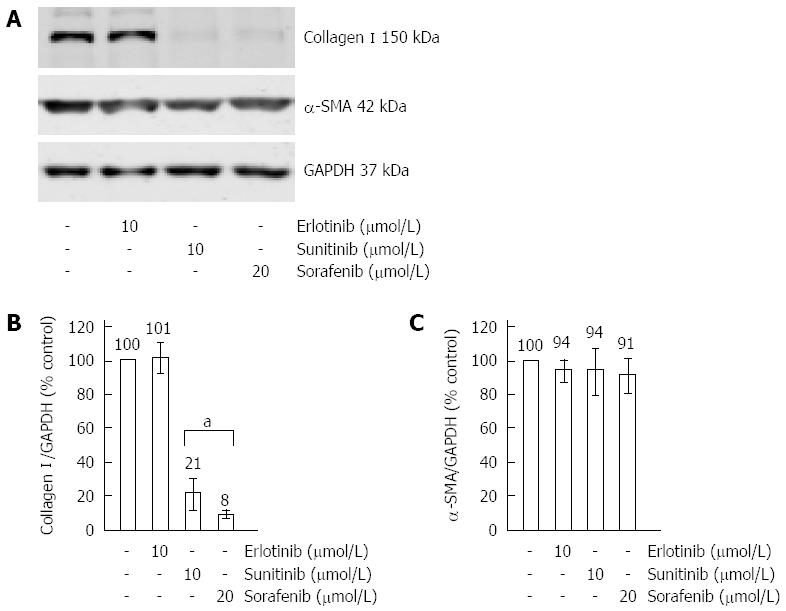
Sorafenib and sunitinib inhibited expression of type I collagen (Figure 3A) and the pro-inflammatory cytokine IL-6 (Figure 3B), which has previously been implicated in autocrine stimulation of TGF-β1 secretion[35] and enhancement of α-SMA expression in the course of PSC activation[36]. For type I collagen, additional investigations on the protein level were performed and results that are in line with the mRNA expression data were obtained (Figure 4A, B).
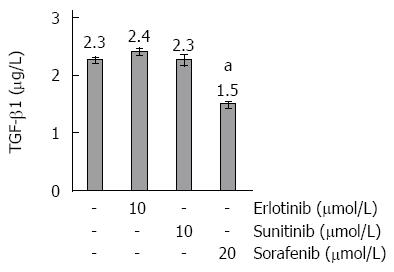
Sorafenib, but none of the other drugs, strongly induced expression of MMP-2 (Figure 3C), while all three SMI diminished the mRNA level of another collagenase secreted by activated PSC, MMP-9 (Figure 3D). Sunitinib and erlotinib reduced mRNA expression of TGF-β1, while, in this case, the effect of sorafenib was not significant (Figure 3E). However, as shown in Figure 5, the inhibitory effects of sunitinib and erlotinib at the level of mRNA did not translate into decreased TGF-β1 protein concentrations in supernatants of PSC cultures, while sorafenib significantly reduced the secretion of the cytokine. The latter effect is likely to be due to the antiproliferative action of sorafenib, since the drug diminished the rate of cell proliferation in the course of the experiment to a similar degree (data not shown).
Finally, we found that sorafenib was the only drug that displayed a strong inhibitory effect on the mRNA expression of α-SMA (Figure 3F). At the protein level (Figure 4A, C), the effects of all three drugs remained insignificant (P = 0.065 for sorafenib). To gain additional insight, immunofluorescence studies were performed and some reduction of stress fibers (bundles of α-SMA that are typical of activated PSC) in sorafenib-treated cells was observed (Figure 6). Taken together, the data nevertheless do not provide unequivocal evidence that sorafenib treatment was associated with a (partial) regression of the myofibroblastic PSC phenotype.
The results of this study indicate that three clinically available SMI, erlotinib, sunitinib and sorafenib, exert distinct inhibitory effects on activated rat pancreatic stellate cells in vitro.
Altogether, the efficacy of erlotinib was quite limited. The drug significantly reduced glucose uptake, but this effect did not translate into changes of the profibrogenic gene expression profile of the cells (except of a small decrease of TGF-β1 mRNA expression). Furthermore, the drug only weakly (although significantly) inhibited PSC proliferation. Erlotinib acts by blocking the EGFR tyrosine kinase activity[37]. Therefore, the failure of the drug to inhibit serum-induced activation of two downstream targets of the receptor, AKT and ERK1/2, was unexpected. In this study, serum and not individual growth factors were used to stimulate the cells in order to mimic biological conditions more closely. It is therefore conceivable that the contribution of the axis EGF/EGFR to the activation of Ras-Raf-MEK-ERK and PI 3-kinase/AKT pathways was small, explaining the lack of an inhibitory erlotinib effect. In any case, the molecular basis of the reduced glucose uptake in erlotinib-treated cells (possibly, a modulation of glucose transporter expression) warrants further investigation.
The multi-kinase inhibitors sunitinib and sorafenib displayed higher biological efficacies than erlotinib: At the maximum concentration tested, each drug inhibited 18F-FDG uptake and DNA synthesis by more than 50%. While sunitinib was the more potent inhibitor of cell proliferation, sorafenib exerted a stronger effect on glucose metabolism. Sunitinib and sorafenib target a partially overlapping set of tyrosine kinase receptors, e.g., of the VEGFR family[28,30]. Nevertheless, the combination of both drugs (as well as their individual combination with erlotinib) revealed additive effects regarding the inhibition of 18F-FDG uptake and DNA synthesis. Since only the simultaneous application of sorafenib and erlotinib was associated with increased cytotoxicity, combinations of SMI should be further evaluated in follow-up studies.
In contrast to erlotinib, sunitinib and sorafenib efficiently inhibited FCS-induced activation of AKT. The finding suggests that serum-dependent activation of AKT in PSC is predominantly mediated by tyrosine kinase receptors that represent targets of the two multi-kinase inhibitors. In case of ERK1/2 activation, the opposite seems to be the case: While sunitinib failed completely to block ERK1/2 phosphorylation, sorafenib displayed a small inhibitory effect only, which may reflect its additional action as a Raf kinase inhibitor. Previous studies in other types of cells have implicated both PI 3-kinase/AKT and Ras-Raf-MEK-ERK pathways in sunitinib[38-43] and sorafenib[44-48] action. The conflicting observations regarding ERK inhibition may either be caused by peculiarities of the respective cell types, or by differences of the experimental protocols that were used: Here, we have focussed on direct effects of the SMI on the investigated pathways. Therefore, preincubation time with the SMI (1 h) and time of FCS stimulation (5-60 min) were kept short. Indirect effects of SMI on ERK pathway activity (effects that require gene expression) were not addressed and can, therefore, not be excluded. At least, a prolongation of FCS stimulation for 3 more hours (4 h in total), did not change our principal findings (data not shown). In conclusion, we have observed a correlation between the inactivation of AKT (but not ERK) and the inhibition of glucose uptake and DNA synthesis by sunitinib and sorafenib. In ongoing studies, we are analyzing a possible causal relationship by employing specific inhibitors of PI 3-kinase/AKT signaling.
Both sunitinib and sorafenib modified the gene expression profile of PSC in a way that suggests less profibrogenic activity of the cells. Thus, both drugs inhibited mRNA expression of IL-6, an autocrine enhancer of PSC activation[35,36], and strongly diminished both the mRNA and protein levels of type I collagen. Interestingly, sorafenib, in contrast to the other two drugs, had no effect on the mRNA levels of TGF-β1, but was the only SMI that significantly reduced the protein concentration of this profibrogenic mediator in cell culture supernatants. Taken these data together, we consider the reduction of TGF-β1 protein levels as an indirect effect of sorafenib that is linked to its antiproliferative action.
The two gelatinases MMP-2 and MMP-9 are both secreted by activated PSC, although at different levels: In previous studies, we found that supernatants of cultured PSC contain much more active MMP-2 than MMP-9[49]. Here, we have analyzed gelatinase expression in order to assess the fibrolytic activity of SMI-treated PSC. The results are heterogeneous, since sorafenib triggered the expression of MMP-2, but all SMI (including erlotinib) diminished the mRNA levels of MMP-9. The net effects of different SMI on fibrolysis, therefore, need to be studied further.
Noteworthy, all three SMI tested in this study showed insignificant or discordant effects on the mRNA and protein levels of α-SMA. These data suggest that the drugs are not capable to reverse the myofibroblastic phenotype of activated PSC. Instead, they exert their antifibrogenic effects by inhibiting key effector functions of the cell in their activated stage.
Although cultures of PSC are considered a suitable in vitro model of pancreatic fibrogenesis, we do not claim direct clinical relevance of our findings at the current stage. One limitation refers to the fact that the in vivo bioavailability of sorafenib, the drug that showed the most consistent effects in this study, is limited by its high protein binding rate[50]. It remains to be shown, therefore, that antifibrotic effects are also achievable in vivo under the conditions of CP and PC. At least in experimental pulmonary fibrosis, however, evidence that sorafenib can ameliorate progression of the disease has recently been provided[51].
We gratefully acknowledge the excellent technical assistance of Mrs. Katja Bergmann.
Pancreatic stellate cells (PSC) are the main source of extracellular matrix proteins in pancreatic fibrosis, which is a key feature of chronic pancreatitis and pancreatic cancer and considered as an aggravating factor of both diseases. Inhibition of PSC activation has been suggested as a promising approach to inhibit pancreatic fibrogenesis, but there is still no specific therapy for clinical application available yet.
Small molecule kinase inhibitors (SMI) such as erlotinib, sunitinib and sorafenib have been introduced into clinical oncology in recent years. These drugs interfere with signal transduction pathways that are also involved in PSC activation and may therefore have an antifibrotic efficiency. This hypothesis, however, has not been experimentally tested so far.
The results of this study show for the first time that three clinically available SMI (erlotinib, sunitinib and sorafenib) inhibit PSC proliferation and glucose metabolism in a dose-dependent manner and without significant cytotoxic effects. Furthermore, the drugs displayed additive effects. By analyzing SMI effects on PSC signaling and gene expression, the authors also gained insights into the molecular mechanisms of SMI action.
Together, the data indicate that the tested SMI, in particular sorafenib, display antifibrogenic effects in vitro. The authors therefore suggest that the antifibrotic efficiency of the drugs should be evaluated further in preclinical studies.
Pancreatic fibrosis represents a process of progressive replacement of pancreatic tissue by connective tissue in the context of chronic inflammation and cancer. Pancreatic stellate cells are fibroblast-like cells that synthesize and secrete most of the extracellular matrix in the diseased organ. Erlotinib, sunitinib and sorafenib are small molecule kinase inhibitors that used in the treatment of various human malignancies.
This study explored the antifibrogenic effects of three clinically available small SMI, erlotinib, sunitinib and sorafenib on PSC and analyzed the basis of their action. It is found that these three SMI showed distinct antifibrogenic effects on PSC. It is showed that sorafenib and sunitib, but not erlotinib, efficiently blocked activation of the AKT pathway; and erlotinib and sunitinib, but not sorafenib, significantly reduced the expression of transforming growth factor-β1. It is helpful to evaluate the antifibrogenic effects of the tested SMI in preclinical studies. This is a well conducted and well written study. The experiments are described in detail, the results are shown nicely and the figures are impressive.
| 1. | Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 2. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 809] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 3. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 731] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 4. | Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 326] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 486] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 6. | Talukdar R, Saikia N, Singal DK, Tandon R. Chronic pancreatitis: evolving paradigms. Pancreatology. 2006;6:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 395] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 8. | Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 947] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 9. | Müerköster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, Klöppel G, Kalthoff H, Fölsch UR. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004;64:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (5)] |
| 11. | Qian LW, Mizumoto K, Maehara N, Ohuchida K, Inadome N, Saimura M, Nagai E, Matsumoto K, Nakamura T, Tanaka M. Co-cultivation of pancreatic cancer cells with orthotopic tumor-derived fibroblasts: fibroblasts stimulate tumor cell invasion via HGF secretion whereas cancer cells exert a minor regulative effect on fibroblasts HGF production. Cancer Lett. 2003;190:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427-7437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Schneiderhan W, Diaz F, Fundel M, Zhou S, Siech M, Hasel C, Möller P, Gschwend JE, Seufferlein T, Gress T. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grünert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Sparmann G, Glass A, Brock P, Jaster R, Koczan D, Thiesen HJ, Liebe S, Emmrich J. Inhibition of lymphocyte apoptosis by pancreatic stellate cells: impact of interleukin-15. Am J Physiol Gastrointest Liver Physiol. 2005;289:G842-G851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Masamune A, Kikuta K, Satoh M, Sakai Y, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J Biol Chem. 2002;277:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Jaster R, Lichte P, Fitzner B, Brock P, Glass A, Karopka T, Gierl L, Koczan D, Thiesen HJ, Sparmann G. Peroxisome proliferator-activated receptor gamma overexpression inhibits pro-fibrogenic activities of immortalised rat pancreatic stellate cells. J Cell Mol Med. 2005;9:670-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Baumert JT, Sparmann G, Emmrich J, Liebe S, Jaster R. Inhibitory effects of interferons on pancreatic stellate cell activation. World J Gastroenterol. 2006;12:896-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Bülow R, Fitzner B, Sparmann G, Emmrich J, Liebe S, Jaster R. Antifibrogenic effects of histone deacetylase inhibitors on pancreatic stellate cells. Biochem Pharmacol. 2007;74:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Jaster R, Sparmann G, Emmrich J, Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Masamune A, Kikuta K, Satoh M, Kume K, Shimosegawa T. Differential roles of signaling pathways for proliferation and migration of rat pancreatic stellate cells. Tohoku J Exp Med. 2003;199:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | McCarroll JA, Phillips PA, Kumar RK, Park S, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase(PI3-kinase) pathway. Biochem Pharmacol. 2004;67:1215-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Ranieri G, Pantaleo M, Piccinno M, Roncetti M, Mutinati M, Marech I, Patruno R, Rizzo A, Sciorsci RL. Tyrosine kinase inhibitors (TKIs) in human and pet tumours with special reference to breast cancer: a comparative review. Crit Rev Oncol Hematol. 2013;88:293-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | McCubrey JA, Milella M, Tafuri A, Martelli AM, Lunghi P, Bonati A, Cervello M, Lee JT, Steelman LS. Targeting the Raf/MEK/ERK pathway with small-molecule inhibitors. Curr Opin Investig Drugs. 2008;9:614-630. [PubMed] |
| 25. | Wu P, Hu YZ. PI3K/Akt/mTOR pathway inhibitors in cancer: a perspective on clinical progress. Curr Med Chem. 2010;17:4326-4341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2792] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 27. | Zagouri F, Sergentanis TN, Chrysikos D, Zografos CG, Papadimitriou CA, Dimopoulos MA, Filipits M, Bartsch R. Molecularly targeted therapies in metastatic pancreatic cancer: a systematic review. Pancreas. 2013;42:760-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 29. | Hasskarl J. Sorafenib. Recent Results Cancer Res. 2010;184:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 550] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 31. | Wood L. Sunitinib malate for the treatment of renal cell carcinoma. Expert Opin Pharmacother. 2012;13:1323-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Younus J, Verma S, Franek J, Coakley N, Sacroma Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. Sunitinib malate for gastrointestinal stromal tumour in imatinib mesylate-resistant patients: recommendations and evidence. Curr Oncol. 2010;17:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Fitzner B, Lange A, Müller S, Jaster R. Cdkn1a is a key mediator of rat pancreatic stellate cell senescence. Pancreatology. 2013;13:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Rateitschak K, Karger A, Fitzner B, Lange F, Wolkenhauer O, Jaster R. Mathematical modelling of interferon-gamma signalling in pancreatic stellate cells reflects and predicts the dynamics of STAT1 pathway activity. Cell Signal. 2010;22:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Aoki H, Ohnishi H, Hama K, Shinozaki S, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Sugano K. Existence of autocrine loop between interleukin-6 and transforming growth factor-beta1 in activated rat pancreatic stellate cells. J Cell Biochem. 2006;99:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 36. | Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 297] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 37. | Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838-4848. [PubMed] |
| 38. | Fenton MS, Marion KM, Salem AK, Hogen R, Naeim F, Hershman JM. Sunitinib inhibits MEK/ERK and SAPK/JNK pathways and increases sodium/iodide symporter expression in papillary thyroid cancer. Thyroid. 2010;20:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 39. | Di Desidero T, Fioravanti A, Orlandi P, Canu B, Giannini R, Borrelli N, Man S, Xu P, Fontanini G, Basolo F. Antiproliferative and proapoptotic activity of sunitinib on endothelial and anaplastic thyroid cancer cells via inhibition of Akt and ERK1/2 phosphorylation and by down-regulation of cyclin-D1. J Clin Endocrinol Metab. 2013;98:E1465-E1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Saito Y, Tanaka Y, Aita Y, Ishii KA, Ikeda T, Isobe K, Kawakami Y, Shimano H, Hara H, Takekoshi K. Sunitinib induces apoptosis in pheochromocytoma tumor cells by inhibiting VEGFR2/Akt/mTOR/S6K1 pathways through modulation of Bcl-2 and BAD. Am J Physiol Endocrinol Metab. 2012;302:E615-E625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Yeramian A, Sorolla A, Velasco A, Santacana M, Dolcet X, Valls J, Abal L, Moreno S, Egido R, Casanova JM. Inhibition of activated receptor tyrosine kinases by Sunitinib induces growth arrest and sensitizes melanoma cells to Bortezomib by blocking Akt pathway. Int J Cancer. 2012;130:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res. 2010;8:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 43. | Ikezoe T, Yang Y, Nishioka C, Bandobashi K, Nakatani H, Taguchi T, Koeffler HP, Taguchi H. Effect of SU11248 on gastrointestinal stromal tumor-T1 cells: enhancement of growth inhibition via inhibition of 3-kinase/Akt/mammalian target of rapamycin signaling. Cancer Sci. 2006;97:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012;176:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, Evers BM. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951-4958. [PubMed] |
| 46. | Lu X, Tang X, Guo W, Ren T, Zhao H. Sorafenib induces growth inhibition and apoptosis of human chondrosarcoma cells by blocking the RAF/ERK/MEK pathway. J Surg Oncol. 2010;102:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Kharaziha P, Rodriguez P, Li Q, Rundqvist H, Björklund AC, Augsten M, Ullén A, Egevad L, Wiklund P, Nilsson S. Targeting of distinct signaling cascades and cancer-associated fibroblasts define the efficacy of Sorafenib against prostate cancer cells. Cell Death Dis. 2012;3:e262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Carlo-Stella C, Locatelli SL, Giacomini A, Cleris L, Saba E, Righi M, Guidetti A, Gianni AM. Sorafenib inhibits lymphoma xenografts by targeting MAPK/ERK and AKT pathways in tumor and vascular cells. PLoS One. 2013;8:e61603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Jaster R, Hilgendorf I, Fitzner B, Brock P, Sparmann G, Emmrich J, Liebe S. Regulation of pancreatic stellate cell function in vitro: biological and molecular effects of all-trans retinoic acid. Biochem Pharmacol. 2003;66:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Villarroel MC, Pratz KW, Xu L, Wright JJ, Smith BD, Rudek MA. Plasma protein binding of sorafenib, a multi kinase inhibitor: in vitro and in cancer patients. Invest New Drugs. 2012;30:2096-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Chen YL, Zhang X, Bai J, Gai L, Ye XL, Zhang L, Xu Q, Zhang YX, Xu L, Li HP. Sorafenib ameliorates bleomycin-induced pulmonary fibrosis: potential roles in the inhibition of epithelial-mesenchymal transition and fibroblast activation. Cell Death Dis. 2013;4:e665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
P- Reviewers: Hahm KB, Gong ZJ S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM