Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7497
Revised: January 17, 2014
Accepted: April 1, 2014
Published online: June 21, 2014
Processing time: 266 Days and 19.3 Hours
AIM: To review pediatric cases of orofacial granulomatosis (OFG), report disease characteristics, and explore the association between OFG and Crohn’s disease.
METHODS: We conducted a systematic review according to the PRISMA guidelines. We searched Medline, LILACS, Virtual Health Library, and Web of Knowledge in September 2013 for cases of OFG in the pediatric age range (< 18 years), with no language limitations. All relevant articles were accessed in full text. The manual search included references of retrieved articles. We extracted data on patients’ characteristics, disease characteristics, association with other diseases, and treatment. We analyzed the data and reported the results in tables and text.
RESULTS: We retrieved 173 reports of OFG in children. Mean age at onset was 11.1 ± 3.8 years (range: 2.0-18 years). Prevalence in males was significant higher than in females (P < 0.001), with a male:female ratio of 2:1. Gastrointestinal signs or symptoms were present in 26.0% of children at the time of OFG diagnosis. Overall, 70/173 (40.4%) children received a concomitant diagnosis of Crohn’s disease. In about half (51.4%) of the cases the onset of OFG anticipated the diagnosis of Crohn’s disease, with a mean time between the two diagnoses of 13.1 ± 11.6 mo (range: 3-36 mo). Overall, 21/173 (12.1%) of the children with OFG had perianal disease, while 11/173 (6.4%) had a family history of Crohn’s disease. Both perianal disease and a family history of Crohn’s disease were significantly associated with a higher risk of Crohn’s disease diagnosis in children with OFG [relative risk (RR) = 3.10, 95% confidence interval (CI): 2.46-3.90; RR = 2.74, 95%CI: 2.24-3.36, P < 0.0001 for both). Treatment of OFG included steroids (70.8% of children) and other immunosuppressive drugs (42.7%), such as azathioprine, thalidomide and infliximab.
CONCLUSION: High prevalence of Crohn’s disease in children with OFG suggests that OFG may be a subtype of Crohn’s disease.
Core tip: This systematic review of children with orofacial granulomatosis (OFG) resulted in the following main findings: (1) 40.4% of children with OFG were affected by Crohn’s disease during their life; (2) 12.1% of children with OFG had perianal disease; (3) 6.4% had a positive family history for Crohn’s disease; (4) both OFG and Crohn’s disease were more prevalent in boys; and (5) both diseases had a long-term course, and treatment resembled the treatment used for Crohn’s disease. Taken together, these findings suggest that OFG may be a subtype of Crohn’s disease.
- Citation: Lazzerini M, Bramuzzo M, Ventura A. Association between orofacial granulomatosis and Crohn’s disease in children: Systematic review. World J Gastroenterol 2014; 20(23): 7497-7504
- URL: https://www.wjgnet.com/1007-9327/full/v20/i23/7497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i23.7497
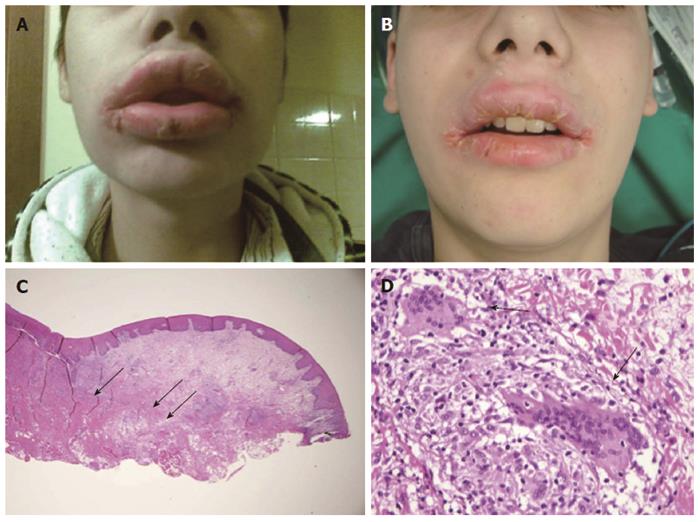
The term orofacial granulomatosis (OFG) is conventionally used to describe patients with granulomatous lesions affecting the orofacial tissues[1,2]. The disease is uncommon but is increasingly being recognized. Lip swelling and facial swelling are the most common clinical signs of OFG, often presenting with a spectrum of other features (Figure 1)[3,4]. Over time the majority of patients tend to develop additional lesions, and the lip or facial swelling can become indurated, permanent, and significantly debilitating[1-4].
The pathogenesis of OFG is still uncertain. Different theories suggested a possible role for allergy, infections, and genetic predisposition[1,2].
More recently, it has been hypothesized that OFG may be a subtype of Crohn’s disease. This hypothesis is based on the following data: (1) histologically OFG is characterized by noncaseating epithelioid cell granulomas that are indistinguishable from the features of Crohn’s disease[1,2]; (2) concurrent intestinal Crohn’s disease has been described in 20-50% of adult patients with OFG[5,6]; and (3) both OFG and Crohn’s disease have a similar clinical course, that is, long-term with a series of recurrent attacks[1-4].
To the best of our knowledge, no systematic review of pediatric cases of OFG has previously been published. The objective of this work was to systematically review pediatric cases of OFG, and evaluate the association between OFG and Crohn’s disease.
This systematic review was conducted according to the PRISMA guidelines[6]. We searched Medline, LILACS, Virtual Health Library, and Web of Knowledge in September 2013 for cases of OFG in the pediatric age range (< 18 years), with no language limitations. The search strategy is reported in Table 1. All relevant articles were accessed in full text. The manual search included references of retrieved articles. We extracted data on patients’ characteristics, disease characteristics, association with other diseases, and treatment. We analyzed the data and reported the results in the tables and text. Cases of granulomatous perioral dermatitis, Melkersson-Rosenthal syndrome (i.e., cases characterized by facial nerve palsy), and OFG-like lesions after organ transplant were excluded from this review.
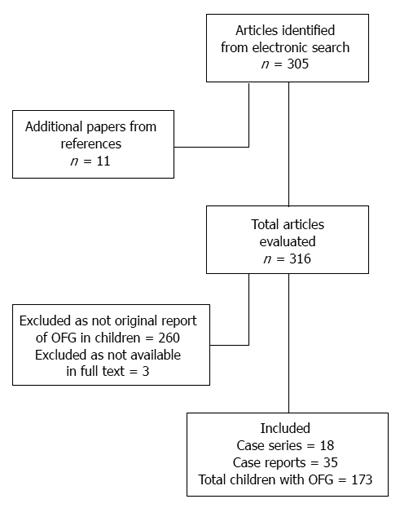
The process of study selection is reported in Figure 2. We retrieved 18 case series[7-23] and 35 case reports[24-56], for a total of 173 children with OFG. One article could not be found in full text[57].
The mean age at OFG onset was 11.1 ± 3.8 years (range: 2.0-18 years). Although not all reports detailed the sex of the patients, OFG appeared to be significantly more prevalent in boys than in girls (P < 0.001, Table 1), with a male to female ratio of 2:1. The disease was reported more commonly in children of Caucasian origin.
The primary clinical feature of children with OFG was lip swelling (93.3%), with involvement of either one or both lips (Table 2). About half of the children presented some intraoral manifestations, such as ulcers, gingival hyperemia or hypertrophy, oral cobble-stoning lesions, or tongue abnormalities. Both angular cheilitis and perioral swelling were each present in about 20% of children. Gastrointestinal signs or symptoms (perianal disease, and/or abdominal pain, diarrhea, or intestinal bleeding) were present in 26.0% of the cases at time of OFG diagnosis.
| Clinical features | Value (n = 104)2 |
| Lips | |
| Present | 104 (100.0) |
| Lip swelling | 97 (93.3) |
| Only upper lip | 27 (26.0) |
| Only lower lip | 15 (14.4) |
| Both lips | 26 (25.0) |
| Preset but unspecified | 29 (27.9) |
| Angular cheilitis | 22 (21.1) |
| Intra-oral manifestation1 | |
| Present | 50 (48.1) |
| Oral ulcerations | 24 (23.1) |
| Gingival hyperemia/hypertrophy | 22 (21.1) |
| Tongue abnormalities | 8 (7.7) |
| Oral cobble-stoning lesions | 8 (7.7) |
| Facies | |
| Perioral/cheek swelling | 19 (18.3) |
| Neck | |
| Cervical lymphadenopathy1 | 6 (5.8) |
| Gastrointestinal | |
| Present | 27 (26.0) |
| Perianal disease1 | 21 (20.2) |
| Abdominal pain1 | 7 (6.7) |
| Diarrhea1 | 7 (6.7) |
| Intestinal bleeding1 | 6 (5.8) |
| Nutritional status | |
| Impaired growth1 | 5 (4.8) |
| Obesity | 1 (1.0) |
| Ocular | |
| Conjunctivitis1 | 1 (1.0) |
| Genitalia | |
| Vulvar oedema1 | 2 (2.0) |
| Scrotal swelling1 | 1 (1.0) |
The diagnosis of OFG was made more often by a team comprising different specialists (65.3%), rather than by one single type of physician (24.7%, P < 0.001, Table 3). Stomatologists and dentists were the specialists who were more frequently involved in the diagnosis of OFG (64.7%), followed by gastroenterologists (49.7%), while dermatologists and pediatricians were involved in the diagnosis in only 22.0% and 18.5% of cases, respectively. A consistent delay (months to years) in reaching the final diagnosis of OFG was frequently reported, with a single patient reporting up to 5 years delay[18,42,52].
| Characteristics | Value (n = 173) |
| Physicians involved in the diagnosis | |
| Stomatologist/dentist/maxillo surgeon | 112 (64.7) |
| Gastroenterologist | 86 (49.7) |
| Dermatologist | 38 (22.0) |
| Pediatrician | 32 (18.5) |
| Otorhinolaryngologist | 13 (7.5) |
| Allergologist | 4 (2.3) |
| Plastic surgeon | 3 (1.7) |
| Internal Medicine | 1 (0.6) |
| Composition of the team | |
| One single specialty | 60 (34.7) |
| More than one specialty | 113 (65.3) |
| Differential diagnosis considered at time of OFG presentation | |
| Crohn’s disease | 137 (79.2) |
| Sarcoidosis | 36 (20.8) |
| Tuberculosis | 25 (14.4) |
| Allergy | 19 (11.0) |
| Infection | 17 (9.8) |
| C1q esterase deficiency | 10 (5.8) |
| Melkersson–Rosenthal syndrome | 4 (2.3) |
| Autoimmunity | 3 (1.7) |
| Vasculitis | 1 (0.6) |
| Foreign body | 1 (0.6) |
| Enteropathic acrodermatitis | 1 (0.6) |
Differential diagnosis of Crohn’s disease was considered in 79.2% of children, while sarcoidosis and tuberculosis were investigated in 20.8% and 14.4% of cases, respectively. Crohn’s disease was diagnosed in 70/173 (40.4%) children with OFG, either at time of presentation of OFG, or during the following months or years (mean time: 13.1 ± 11.6 mo, range: 3-36 mo). In contrast, only three children (1.7%) were diagnosed with tuberculosis[33-35], two (1.1%) with sarcoidosis[16], and 19 (10.9%) with allergy/atopy.
Overall, 21/173 (12.1%) of the children with OFG had perianal disease, while 11/173(6.4%) had a family history of Crohn’s disease. Both perianal disease and a family history of inflammatory bowel disease were significantly associated with an increased risk of Crohn’s disease in children with OFG [relative risk (RR) = 3.10, 95% confidence interval (CI): 2.46-3.90; RR = 2.74, 95%CI: 2.24-3.36, P < 0.0001 for both, Table 4].
| Disease | Value(n = 173) | RR (95%CI), P |
| Crohn’s disease | ||
| Total children | 70 (40.4) | |
| At presentation | 34 (19.6) | |
| During follow-up | 36 (20.8) | |
| Time from OFG diagnosis to Crohn’s diagnosis (mean ± SD, range) | 13.1 ± 11.6 (3-36) | |
| Presence of perianal disease | ||
| Total | 21/173 (12.1) | 3.10 (2.46-3.90), 0.0001 |
| In children with Crohn’s | 21/70 (30.0) | |
| In children without Crohn’s | 0/103 (0) | |
| Familiarity for inflammatory bowel diseases | ||
| Total | 11/173 (6.4) | 2.74 (2.24-3.36), 0.0001 |
| In children with Crohn’s | 11/70 (15.7) | |
| In children without Crohn’s | 0/103 (0) | |
| Allergy/atopy | ||
| Any allergy | 19 (10.9) | |
| Asthma | 7 (4.0) | |
| Atopy | 6 (3.5) | |
| Rhinitis/rhinoconjunctivitis | 6 (3.5) | |
| Eczema | 6 (3.5) | |
| Hives | 1 (0.6) | |
| Other diagnosis | ||
| Tuberculosis | 3 (1.7) | |
| Sarcoidosis | 2 (1.1) | |
| Other diseases | 10 (5.7) | |
| Erythema nodosum | 3 (1.7) | |
| Insulin dependent diabetes | 1 (0.6) | |
| Celiac disease | 1 (0.6) | |
| Alopecia | 1 (0.6) | |
| Low CD4/CD8 ratio | 1 (0.6) | |
| Epilepsy | 1 (0.6) |
There was heterogeneity in the reported incidence of Crohn’s disease and of perianal disease among different case series, with those reported by gastroenterologists[7,11], describing a significantly higher incidence of Crohn’s disease than other series. In Campbell et al[7], the largest OFG case series available including both adults and children (207 patients, of which 22% were children), the incidence of Crohn’s disease was similar in children and adults with OFG, but in children the occurrence of OFG anticipated the onset of Crohn’s disease significantly more often than in adults (84% vs 27%, P = 0.0003)[7]. Ulcers and raised C-reactive protein were more frequent in patients with OFG and Crohn’s disease than in those with OFG alone (respectively 49% vs 15%, P = 0.001 and 73% vs 49%, P = 0.01)[7]. Cases with intraoral involvement, perianal disease and intestinal Crohn’s disease were more likely to occur in those with childhood onset of OFG compared to those with adult onset (44% vs 24%, P = 0.09)[7].
When Crohn’s disease was diagnosed in children with OFG, oral and perioral lesions were not considered a metastatic manifestation of Crohn’s disease, but an expression of the disease in the digestive tract.
Although the duration of follow-up was short in most reports, overall, 26.4% and 17.6% of children received two and three or more treatment attempts, respectively (Table 5). A combination of two or more drugs in the same treatment attempt was reported in 53.7% of children. Steroids were prescribed in 70.8% of children. Other immunosuppressive or immunomodulatory drugs were administered to 42.7% of children, and among these, azathioprine/6-mercaptopurine (6MP; 12.3%), thalidomide (10.1%) and infliximab (9.1%) were the most frequently prescribed. Eight (9.1%) children underwent surgery.
| Treatment characteristics | Value(n = 104)1 |
| A treatment was prescribed2 | |
| Yes | 89 (96.7) |
| No | 3 (3.2) |
| Unspecified | 12 (11.5) |
| Number of treatment attempts reported2 | |
| One | 38 (55.8) |
| Two | 18 (26.4) |
| Three or more | 12 (17.6) |
| Unspecified | 21 (23.5) |
| More than one drug in the same treatment attempt2 | |
| Yes | 36 (53.7) |
| No | 31 (46.2) |
| Unspecified | 22 (24.7) |
| Type of treatments prescribed2 | |
| Antibiotics | 26 (29.2) |
| Anti-histaminic | 10 (11.2) |
| Steroids-total | 63 (70.8) |
| Topical | 27 (30.3) |
| Intralesional | 24 (27.0) |
| Oral | 39 (43.8) |
| Other immunosuppressive-total | 38 (42.7) |
| Azathioprine/6MP | 11 (12.3) |
| Thalidomide | 9 (10.1) |
| Infliximab | 8 (9.0) |
| Dapsone | 4 (4.5) |
| Tacrolimus (topic) | 4 (4.5) |
| Methotrexate | 3 (3.4) |
| Tacrolimus (systemic) | 3 (3.4) |
| Hydroxychloroquine/chloroquine | 2 (2.2) |
| Colchicine | 2 (2.2) |
| Other treatments | |
| 5ASA | 13 (14.6) |
| Chlorhexidine (topic) | 4 (4.5) |
| Enteral nutrition | 2 (2.2) |
| Fumaric acid esterase | 1 (1.1) |
| Surgery | 8 (9.0) |
This review of the pediatric literature highlights the fact that OFG is a disease that can occur during childhood and adolescence. The exact prevalence of the disease in children is unknown, and cannot be derived from this review. Reports from specialized centers suggest that the real prevalence of the disease may be higher than what has been reported so far in the literature[7]. Results from the present review also suggest that OFG in children is still not a well-known disease among physicians, and that there is uncertainty in respects to its diagnosis, with possible diagnostic delay.
This review highlights some important findings regarding Crohn’s disease and OFG. First, Crohn’s disease was diagnosed in 40.2% of children with OFG. This rate could be underestimated due to several factors: (1) Crohn’s disease was not systematically assessed in all children with OFG; (2) on average, children were followed up for a short time, while this review highlights that gastrointestinal signs and symptoms may appear at a later stage (on average, 13 mo later); (3) Crohn’s disease may be subclinical or silent, as observed in reports in adults, showing that a considerable percentage of patients with OFG without intestinal symptoms were diagnosed with Crohn’s disease after ileoscopy or radiolabeled white cell screening[58]; (4) patients with OFG were treated with steroids or other immunosuppressive drugs that could have silenced the intestinal symptoms of Crohn’s disease; (5) publication bias may have affected the characteristics of reported cases; and (6) there is some confusion between the diagnosis of OFG and oral Crohn’s disease[59], and this may have affected the number of cases reported as OFG. Although some of the above-mentioned factors may have affected the prevalence of Crohn’s disease in OFG by overestimating it, most factors may have led to an underestimation of the actual prevalence of Crohn’s disease in OFG.
Second, 6.4% of children with OGF also presented a family history of inflammatory bowel disease. Such a percentage is much closer to what is reported in the population with Crohn’s disease, than in those not affected by inflammatory bowel disease. Perianal disease, which is already a recognized subset of Crohn’s disease, was detected in 12.1% of children with OFG overall. Intraoral lesions and perianal disease often occur together in Crohn’s disease[60], and cohort studies have showed that both manifestations are important predictors of Crohn’s disease severity[59,60].
Third, this review highlights other similarities between Crohn’s disease and OFG: both OFG and Crohn’s disease[58-60] are more prevalent in boys; both diseases have a long-term course; and treatments used for OFG resemble those used for Crohn’s disease. Even the list of other diseases diagnosed in association with OFG (i.e., erythema nodosum, and alopecia), although the number of cases was limited, resembles the list of immunological diseases usually associated with Crohn’s disease.
All the above findings suggest that OFG and Crohn’s disease may be two variants, if not just two different localizations, of the same chronic inflammatory disease. Other authors have proposed this hypothesis, based on the high (20%-50%) reported prevalence of Crohn’s disease in adults with OFG[5,6].
To the best of our knowledge no systematic review of adult cases of OFG has been carried out so far, and cases reported in adults suffer from the same risk of bias as cases reported in children (e.g., short follow-up time). More studies in an adequate sample of patients with OFG (both children and adults) with a systematic evaluation for Crohn’s disease and long follow-up time are needed to explore further the hypothesis that OFG is a subtype of Crohn’s disease or even one of its manifestations.
So far, based on existing literature, Crohn’s disease should be considered in the differential diagnosis of every child with signs of OFG; in particular, if other signs of systemic, gastrointestinal or perianal involvement are present. If Crohn’s disease is not diagnosed at the time of OFG presentation, patients with OFG should be closely followed up for any sign of intestinal Crohn’s disease, including perianal disease.
If OFG and Crohn’s disease are two different clinical entities, more precise diagnostic criteria should be developed to differentiate between the two diseases.
In conclusion, OFG has been considered a different entity from Crohn’s disease due to its presentation (lip and buccal involvement) and to the absence of systemic signs and symptoms[1-5]. The findings of our systematic review on children with OFG suggest that OFG may be a subtype of Crohn’s disease. Mores studies with a systematic evaluation of patients and with adequate follow-up are needed to confirm this hypothesis. Based on the existing evidence, Crohn’s disease should be considered in the differential diagnosis of children with unexplained OGF. These children should be also followed up in the long term, because intestinal Crohn’s disease may develop after several years.
We would like to thank Genevieve Becker, for having revised the language of the manuscript.
The term orofacial granulomatosis (OFG) is conventionally used to describe patients with granulomatous lesions affecting the orofacial tissues. So far, OFG had been considered a different entity from Crohn’s disease, although the hypothesis that OFG may be a subtype of Crohn’s disease has been proposed.
To the best of our knowledge, no systematic review of pediatric cases of OFG has previously been published. The objective of this study was to review systematically all pediatric cases of OFG, report on the disease characteristics, and evaluate the association between OFG and Crohn’s disease.
Crohn’s disease was diagnosed in approximately 40% of children with OFG, either at time of presentation of OFG, or during the following months or year (mean time: 13.1 ± 11.6 mo, range: 3-36 mo). Such a high prevalence of Crohn’s disease in children with OFG, and other common features between the two diseases, suggest that OFG may be considered a subtype of Crohn’s disease.
Crohn’s disease should be considered in the differential diagnosis of children with OFG. Children with OFG should be also followed up in the long term, because intestinal Crohn’s disease may develop after several years.
OFG is a rare granulomatous disease that affects the orofacial tissues. Lip and facial swelling are the most common clinical signs, often presenting with a spectrum of other features. Over time the majority of patients tend to develop additional lesions, and the lip or facial swelling can become indurated, permanent, and significantly debilitating.
This is a well-documented review article regarding the association between OFG and Crohn’s disease in children.
| 1. | Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis--a 20-year review. Oral Dis. 2009;15:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Tilakaratne WM, Freysdottir J, Fortune F. Orofacial granulomatosis: review on aetiology and pathogenesis. J Oral Pathol Med. 2008;37:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Al Johani K, Moles DR, Hodgson T, Porter SR, Fedele S. Onset and progression of clinical manifestations of orofacial granulomatosis. Oral Dis. 2009;15:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Al Johani KA, Moles DR, Hodgson TA, Porter SR, Fedele S. Orofacial granulomatosis: clinical features and long-term outcome of therapy. J Am Acad Dermatol. 2010;62:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Sanderson J, Nunes C, Escudier M, Barnard K, Shirlaw P, Odell E, Chinyama C, Challacombe S. Oro-facial granulomatosis: Crohn’s disease or a new inflammatory bowel disease? Inflamm Bowel Dis. 2005;11:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Prisma statement. Accessed on Sep 10, 2013. Available from: http://www.prisma-statement.org/. |
| 7. | Campbell H, Escudier M, Patel P, Nunes C, Elliott TR, Barnard K, Shirlaw P, Poate T, Cook R, Milligan P. Distinguishing orofacial granulomatosis from crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Saalman R, Mattsson U, Jontell M. Orofacial granulomatosis in childhood-a clinical entity that may indicate Crohn’s disease as well as food allergy. Acta Paediatr. 2009;98:1162-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kolho KL, Heiskanen K, Verkasalo M, Pitkäranta A. Orofacial granulomatosis in children--a challenge for diagnosis and treatment. Int J Pediatr Otorhinolaryngol. 2011;75:864-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Khouri JM, Bohane TD, Day AS. Is orofacial granulomatosis in children a feature of Crohn’s disease? Acta Paediatr. 2005;94:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Sainsbury CP, Dodge JA, Walker DM, Aldred MJ. Orofacial granulomatosis in childhood. Br Dent J. 1987;163:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Hegarty A, Hodgson T, Porter S. Thalidomide for the treatment of recalcitrant oral Crohn’s disease and orofacial granulomatosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Clayden AM, Bleys CM, Jones SF, Savage NW, Aldred MJ. Orofacial granulomatosis: a diagnostic problem for the unwary and a management dilemma. Case reports. Aust Dent J. 1997;42:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Ahmad I, Owens D. Granulomatous cheilitis and Crohn’s disease. Can J Gastroenterol. 2001;15:273-275. [PubMed] |
| 16. | Bourgeois-Droin C, Havard S, Granier F, Vesse M, Salomon JL, Furioli J, Grossin M. Granulomatous cheilitis in two children with sarcoidosis. J Am Acad Dermatol. 1993;29:822-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | El-Hakim M, Chauvin P. Orofacial granulomatosis presenting as persistent lip swelling: review of 6 new cases. J Oral Maxillofac Surg. 2004;62:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Lynde CB, Bruce AJ, Orvidas LJ, Rogers RS, Depry JL. Cheilitis granulomatosa treated with intralesional corticosteroids and anti-inflammatory agents. J Am Acad Dermatol. 2011;65:e101-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Smith VM, Murphy R. Orofacial granulomatosis: three case reports illustrating the spectrum of disease and overlap with Crohn’s disease. Clin Exp Dermatol. 2013;38:33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Galbraith SS, Drolet BA, Kugathasan S, Paller AS, Esterly NB. Asymptomatic inflammatory bowel disease presenting with mucocutaneous findings. Pediatrics. 2005;116:e439-e444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Morton ME, Ead RD. Granulomatous cheilitis. A report of three cases. Br Dent J. 1984;156:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Sakuntabhai A, MacLeod RI, Lawrence CM. Intralesional steroid injection after nerve block anesthesia in the treatment of orofacial granulomatosis. Arch Dermatol. 1993;129:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 23. | Sussman GL, Yang WH, Steinberg S. Melkersson-Rosenthal syndrome: clinical, pathologic, and therapeutic considerations. Ann Allergy. 1992;69:187-194. [PubMed] |
| 24. | Kugathasan S, Miranda A, Nocton J, Drolet BA, Raasch C, Binion DG. Dermatologic manifestations of Crohn disease in children: response to infliximab. J Pediatr Gastroenterol Nutr. 2003;37:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Camacho-Alonso F, Bermejo-Fenoll A, López-Jornet P. Miescher’s cheilitis granulomatosa. A presentation of five cases. Med Oral Patol Oral Cir Bucal. 2004;9:427-429; 425-427. [PubMed] |
| 26. | Halevy S, Shalom G, Trattner A, Bodner L. Melkersson-Rosenthal syndrome: a possible association with psoriasis. J Am Acad Dermatol. 2012;67:795-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Kruse-Lösler B, Presser D, Metze D, Joos U. Surgical treatment of persistent macrocheilia in patients with Melkersson-Rosenthal syndrome and cheilitis granulomatosa. Arch Dermatol. 2005;141:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Ellitsgaard N, Andersson AP, Worsaae N, Medgyesi S. Long-term results after surgical reduction cheiloplasty in patients with Melkersson-Rosenthal syndrome and cheilitis granulomatosa. Ann Plast Surg. 1993;31:413-420. [PubMed] |
| 29. | Vijayalakshmi AM, Jayavardhana A. Persistent swelling of lip. Indian Pediatr. 2009;46:907-908. [PubMed] |
| 30. | Oliver DW, Scott MJ. Lip reduction cheiloplasty for Miescher’s granulomatous macrocheilitis (Cheilitis granulomatosa) in childhood. Clin Exp Dermatol. 2002;27:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Olivier V, Lacour JP, Castanet J, Perrin C, Ortonne JP. [Cheilitis granulomatosa in a child]. Arch Pediatr. 2000;7:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Bourrat E, Faure C, Vignon-Pennamen MD, Rybojad M, Morel P, Navarro J. [Anitis, vulvar edema and macrocheilitis disclosing Crohn disease in a child: value of metronidazole]. Ann Dermatol Venereol. 1997;124:626-628. [PubMed] |
| 33. | Ramesh V. Orofacial Granulomatosis due to Tuberculosis. Pediatr Dermatol. 2009;26:108-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Bhattacharya M, Rajeshwari K, Sardana K, Gupta P. Granulomatous cheilitis secondary to tuberculosis in a child. J Postgrad Med. 2009;55:190-192. [PubMed] |
| 35. | Kruschinski C, Welkoborsky HJ. Tuberculosis of the larynx associated with orofacial granulomatosis in childhood. Otolaryngol Head Neck Surg. 2005;132:967-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Dodi I, Verri R, Brevi B, Bonetti L, Balestrier A, Saracino A, Akamin R, Izzi GC, Vanelli M, Sesenna E. A monosymptomatic Melkersson-Rosenthal syndrome in an 8-year old boy. Acta Biomed. 2006;77:20-23. [PubMed] |
| 37. | Dummer W, Lurz C, Jeschke R, Meissner N, Rose C, Bröcker EB. Granulomatous cheilitis and Crohn’s disease in a 3-year-old boy. Pediatr Dermatol. 1999;16:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Bogenrieder T, Rogler G, Vogt T, Landthaler M, Stolz W. Orofacial granulomatosis as the initial presentation of Crohn’s disease in an adolescent. Dermatology. 2003;206:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Nabatian AS, Shah KN, Iofel E, Rosenberg S, Javidian P, Pappert A, Milgraum SS. Asymptomatic granulomatous vulvitis and granulomatous cheilitis in childhood: the need for Crohn disease workup. J Pediatr Gastroenterol Nutr. 2011;53:100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Rana AP. Orofacial granulomatosis: A case report with review of literature. J Indian Soc Periodontol. 2012;16:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Freeman GL. Complement abnormality in Melkersson-Rosenthal. Allergy. 2004;59:362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Somech R, Harel A, Rotshtein MS, Brazowski E, Reif S. Granulomatosis cheilitis and Crohn disease. J Pediatr Gastroenterol Nutr. 2001;32:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L. The multiform and variable patterns of onset of orofacial granulomatosis. J Oral Pathol Med. 2003;32:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Kleine R, Bröhl L, Amon U. [Treatment of granulomatous cheilitis with fumaric acid esters in a young woman]. Hautarzt. 2011;62:940-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Ishiguro E, Hatamochi A, Hamasaki Y, Ishikawa S, Yamazaki S. Successful treatment of granulomatous cheilitis with roxithromycin. J Dermatol. 2008;35:598-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | van der Waal RI, Schulten EA, van der Meij EH, van de Scheur MR, Starink TM, van der Waal I. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up--results of management. Int J Dermatol. 2002;41:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Pryce DW, King CM. Orofacial granulomatosis associated with delayed hypersensitivity to cobalt. Clin Exp Dermatol. 1990;15:384-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Macaigne G, Harnois F, Boivin JF, Dikov D, Ridoux G, Cheaib S, Chayette C. Crohn’s disease revealed by a cheilitis granulomatosa with favorable evolution by perfusions of infliximab: report of a case and review of the literature. Clin Res Hepatol Gastroenterol. 2011;35:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Ridder GJ, Fradis M, Löhle E. Cheilitis granulomatosa Miescher: treatment with clofazimine and review of the literature. Ann Otol Rhinol Laryngol. 2001;110:964-967. [PubMed] |
| 50. | Diamond T, Patterson PG, Emerson TG. Oral Crohn’s disease: the distinction from the Melkersson-Rosenthal syndrome. Ulster Med J. 1990;59:223-224, 186. [PubMed] |
| 51. | Gall Y, Bureau B, Stalder JF, Litoux P. [Miescher’s granulomatous macrocheilitis. Treatment with clofazimine]. Ann Dermatol Venereol. 1989;116:241-244. [PubMed] |
| 52. | Wadlington WB, Riley HD, Lowbeer L. The Melkersson-Rosenthal syndrome. Pediatrics. 1984;73:502-506. [PubMed] |
| 53. | Henry CH. Orofacial granulomatosis: report of a case with decreased CD4/CD8 ratio. J Oral Maxillofac Surg. 1994;52:317-322. [PubMed] |
| 54. | William T, Marsch WC, Schmidt F, Kreft B. Early oral presentation of Crohn’s disease. J Dtsch Dermatol Ges. 2007;5:678-679. [PubMed] |
| 55. | Rajah K, Oliver MR, McLeod L, Orchard D, Leal M. Unusual manifestations of a common gastrointestinal disorder. J Paediatr Child Health. 2014;50:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Burke KR, Green BP. An 11-year-old male with a swollen lip. Clin Pediatr (Phila). 2013;52:678-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Howell JL, Bussell RM, Hegarty AM, Zaitoun H. Service evaluation of patients with orofacial granulomatosis and patients with oral Crohn’s disease attending a paediatric oral medicine clinic. Eur Arch Paediatr Dent. 2012;13:191-196. [PubMed] |
| 58. | Rowland M, Fleming P, Bourke B. Looking in the mouth for Crohn’s disease. Inflamm Bowel Dis. 2010;16:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Wolters FL, Russel MG, Sijbrandij J, Schouten LJ, Odes S, Riis L, Munkholm P, Langholz E, Bodini P, O’Morain C. Disease outcome of inflammatory bowel disease patients: general outline of a Europe-wide population-based 10-year clinical follow-up study. Scand J Gastroenterol Suppl. 2006;46-54. [PubMed] |
| 60. | Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008;103:3082-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
P- Reviewers: Gaya DR, Ishida M, Korelitz BI S- Editor: Gou SX L- Editor: Kerr C E- Editor: Zhang DN