Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7480
Revised: December 14, 2013
Accepted: January 8, 2014
Published online: June 21, 2014
Processing time: 304 Days and 5.7 Hours
AIM: To determine the prevalence and characteristics of additional primary malignancies in gastric cancer (GC) patients.
METHODS: GC patients (862 total; 570 men, 292 women; mean age 59.8 ± 12.8 years) diagnosed at the Department of Gastroenterology at Pomeranian Medical University over a period of 23 years were included in this retrospective analysis of a prospectively maintained database. Mean follow-up time was 31.3 ± 38.6 mo (range 1-241 mo). The following clinicopathological features of patients with synchronous tumors were compared to those with metachronous tumors: age, sex, symptom duration, family history of cancer, tumor site, stage (early vs advanced), histology, and blood group. GC patients with and without a second tumor were compared in terms of the same clinicopathological features.
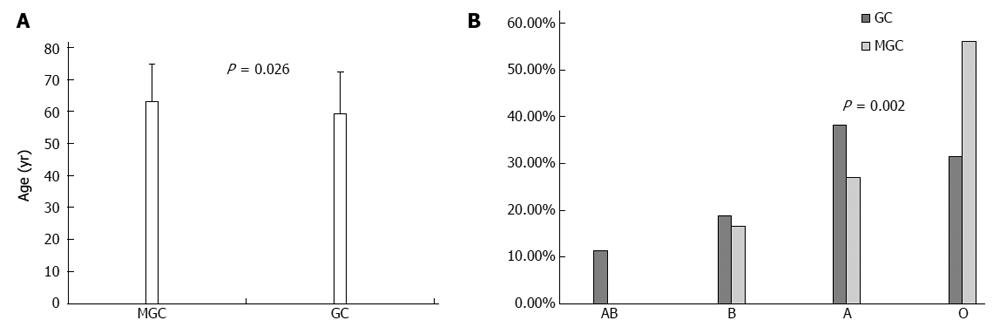
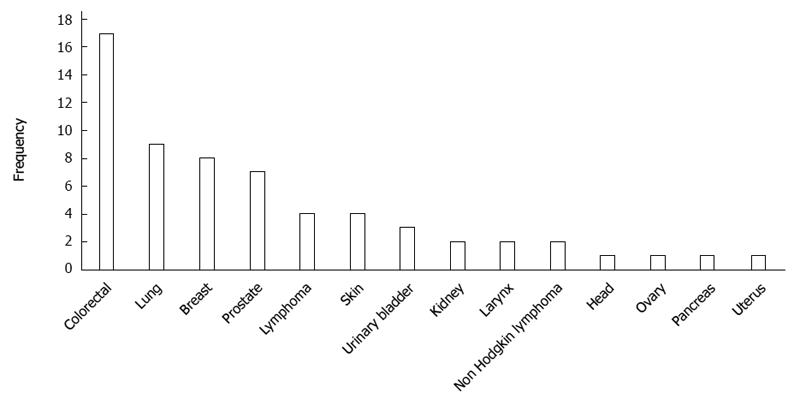
RESULTS: Of 862 GC patients, 58 (6.7%) developed a total of 62 multiple primary tumors, of which 39 (63%) were metachronous and 23 (37%) synchronous. Four (6.9%) of the 58 multiple GC patients developed two or more neoplasms. The predominant tumor type of the secondary neoplasms was colorectal (n = 17), followed by lung (n = 9), breast (n = 8), and prostate (n = 7). Age was the only clinicopathological feature that differed between GC patients with synchronous vs metachronous malignancies; GC patients with synchronous neoplasms were older than those with metachronous neoplasms (68.0 ± 10.3 years vs 59.9 ± 11.1 years, respectively, P = 0.008). Comparisons between patients with and without a second primary cancer revealed that the only statistically significant differences were in age and blood group. The mean age of the patients with multiple GC was higher than that of those without a second primary tumor (63.4 ± 11.4 years vs 59.5 ± 13.0 years, respectively, P = 0.026). GC patients with a second primary tumor were more commonly blood group O than those without (56.2% vs 31.6%, respectively, P = 0.002).
CONCLUSION: GC patients may develop other primary cancers; appropriate preoperative and postoperative diagnostic modalities are thus required, particularly if patients are older and blood group O.
Core tip: In our study, the incidence of second primary malignancies in gastric cancer (GC) patients was 6.7%. The predominant tumor type of the secondary neoplasms was colorectal cancer, followed by lung, breast, and prostate. GC patients with synchronous neoplasms were older than those with metachronous neoplasms. GC patients with second primary tumors were significantly more likely to be blood group O and older than those without. This suggests a need for additional procedures, such as colonoscopy, chest X-ray, mammography and computed tomography, particularly for those who are older and blood group O.
- Citation: Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Raszeja-Wyszomirska J, Białek A, Karpińska-Kaczmarczyk K, Starzyńska T. Synchronous and metachronous neoplasms in gastric cancer patients: A 23-year study. World J Gastroenterol 2014; 20(23): 7480-7487
- URL: https://www.wjgnet.com/1007-9327/full/v20/i23/7480.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i23.7480
The first systematic study of this of multiple malignancies phenomenon was published in the 1930s by Warren and Gates[1]. These authors proposed the first working definition of multiple primary neoplasms: (1) both tumors should be confirmed histologically as malignant; (2) each cancer must be anatomically separate and distinct; and (3) the second tumor must not be a recurrence or metastasis of the first cancer. Multiple tumors may develop synchronously or metachronously. The phenomenon of multiple primary neoplasms is increasingly being discussed in the literature due to the increased survival time of cancer patients after treatment and because of advances in diagnostic methods. It is estimated that multiple neoplasms affect about 10% of all cancer patients[2-4]. Estimates of the incidence of multiple primary neoplasms in patients with gastric cancer (GC) range from 1.7% to 8.0%[5-12]. A few reports suggest that the development of multiple primary neoplasms in patients with GC is even more frequent. Green et al[13] reported that this phenomenon affects approximately 8% of advanced GC patients and 32% of early GC patients. Studies in Japan and Italy estimated that 9%-11% of early GC patients develop other malignancies[14,15]. Kim et al[16] described 113 patients with multiple primary cancers at three or more sites; 41 (36.3%) of these patients had GC.
Most studies of multiple malignancies have found colorectal cancer as the second tumor in GC patients[6-8,10,16,17]. The other sites of second tumors include breast, lung, prostate, uterus, small intestine, liver, esophagus, and kidney[6,8-11,16,17]. Many studies of multiple malignancies have been conducted in Asia; only a few have been conducted in Europe. To the best of our knowledge, none have been conducted in Poland, where GC is diagnosed in > 5000 people every year. The purpose of the present study was to determine the prevalence and characteristics of additional primary malignancies in GC patients in a Polish population and to compare GC patients with and without second primary tumors.
Between January 1988 and December 2011, data were collected from 862 patients with histopathologically confirmed GC who were diagnosed at the Department of Gastroenterology at the Pomeranian Medical University in Szczecin, Poland. The following clinical and pathological data were collected: patient sex, age, family history of cancer, duration of symptoms, tumor site, stage (early vs advanced), histology, blood group, and previously or subsequently histologically verified second primary malignancy other than GC. In the current study, we retrospectively analyzed a prospective maintained database.
The stage and histological type of GC were assessed by routine histopathological examination. Histological types were classified according to the Lauren classification[18]. Early GC was defined as invasive cancer that invades no more deeply than the submucosa, irrespective of lymph node metastasis. In patients who did not undergo surgery and distal metastases or tumor infiltration were confirmed by diagnostic procedures (e.g., computed tomography, ultrasonography, biopsy) the stage was classified as advanced. The criteria of Warren et al[1] were used to classify synchronous and metachronous tumors. If the time interval between the appearance of the two neoplasms did not exceed 6 mo, they were defined as synchronous, and if the interval time was longer than 6 mo, they were classified as metachronous. Tumor location was classified as proximal (cardiac region) or other (truncus, antrum, entire stomach, or anastomosis).
The mean follow-up time for the GC patients was 31.3 ± 38.6 mo (range 1-241 mo). The group of metachronous GC patients (n = 34) was compared to the synchronous GC patient group (n = 22). Two patients were excluded from this comparison because they developed both metachronous and synchronous cancers. The group with multiple GC (n = 58) was compared to the group without a second cancer (n = 804).
For statistical analysis, we used χ² or Fisher’s exact tests for categorical variables, and Student’s t-test and Mann-Whitney U-test for continuous variables. P < 0.05 was considered to be statistically significant. All statistical analyses were performed with the statistical software STATISTICA 10.
The baseline characteristics of the GC patients included in the study are shown in Table 1. The median age of all 862 patients (570 men and 292 women) was 59.8 ± 12.8 years (range 15-89 years). The mean duration of symptoms from the first alarming symptoms was 25.1 mo (range 0-480 mo). In 21 cases, there were no symptoms and gastric cancer was diagnosed during emergency endoscopy due to gastrointestinal bleeding. Among GC patients with a family history positive for cancer, 40.2% reported gastric cancer in first- or second-degree relatives, of whom 35.8% also had neoplasms other than gastric cancer. In 18% of patients with a family history positive for cancer, cancers of the gastrointestinal tract excluding the stomach (e.g., colon, pancreas, and esophagus) were noted. In addition, 41.8% of patients reported other cancers (e.g., leukemia, uterus, skin breast, lung, and larynx) in close family members. Twenty six (49%) of 53 multiple GC patients reported cancer in their first- or second-degree relatives; almost half (46.2%) of these relatives had GC.
| Characteristic | Total | Gastric cancer | Multiple gastric cancer | P value |
| Gender | 0.499 | |||
| Male | 570 (66.1) | 534 (66.4) | 36 (62.1) | |
| Female | 292 (33.9) | 270 (33.6) | 22 (37.9) | |
| Total | 862 | 804 | 58 | |
| Age1 (yr) | 59.8 ± 12.8 | 59.5 ± 13.0 | 63.4 ± 11.4 | 0.026 |
| DS (mo) | 25.1 ± 46.4 | 25.1 ± 46.5 | 26.3 ± 46.3 | 0.433 |
| FHC | 0.806 | |||
| No | 369 (52.6) | 342 (52.7) | 27 (50.9) | |
| Yes | 333 (47.4) | 307 (47.3) | 26 (49.1) | |
| Total | 702 | |||
| Location2 | 0.467 | |||
| Proximal | 181 (21) | 171 (21.3) | 10 (17.2) | |
| Other location | 681 (79) | 633 (78.7) | 48 (82.8) | |
| Total | 862 | |||
| Histology | 0.116 | |||
| Intestinal | 260 (43.4) | 234 (42.2) | 26 (57.8) | |
| Diffuse | 289 (48.3) | 272 (49.1) | 17 (37.8) | |
| Mixed | 50 (8.3) | 48 (8.7) | 2 (4.4) | |
| Total | 599 | |||
| Stage | 0.100 | |||
| Early | 119 (16.4) | 106 (15.7) | 13 (24.5) | |
| Advanced | 608 (83.6) | 568 (84.3) | 40 (75.5) | |
| Total | 727 | |||
| Blood group | 0.002 | |||
| A | 211 (37.2) | 198 (38.1) | 13 (27.1) | |
| B | 106 (18.7) | 98 (18.9) | 8 (16.7) | |
| O | 191 (33.7) | 164 (31.6) | 27 (56.2) | |
| AB | 59 (10.4) | 59 (11.4) | None | |
| Total | 567 |
GC surgery was performed on 598 patients; one underwent mucosectomy and the rest underwent exploratory surgery or were treated nonsurgically because of advanced GC or general contraindications. Of the total patients with GC and a known stage of disease (n = 727), 119 (16.4%) had early GC. Of the 119 patients with early GC, 13 (10.9%) had multiple GC tumors.
In 181 (21%) of the total 862 cases, the tumor site was the cardia and fundus or the cardia and the upper part of the truncus (classified as the proximal site). In the remaining patients, the tumor site was classified as other localization, as follows: 376 (43.6%) truncus, 253 (29.4%) antrum, 39 (4.5%) entire stomach. In 13 (1.5%) cases, the tumor was located in the anastomosis after a previous operation to treat ulcers.
The main histological type of gastric cancer was diffuse (48.3%), followed by intestinal (43.4%) and mixed (8.3%). The most common blood group in all GC cases was group A (37.2%), followed by group O (33.7%), group B (18.7%), and group AB (10.4%).
A comparison between the groups of patients with or without a second primary tumor indicated that the only significant differences occurred in age and blood group. The mean age of multiple GC patients was higher than that of those without a second primary tumor (63.4 ± 11.4 years vs 59.5 ± 13.0 years, respectively, P = 0.026) (Figure 1A).
GC patients with a second primary tumor were more commonly blood group O than those without a second primary tumor (56.2% vs 31.6%, respectively, P = 0.002) (Figure 1B). The following characteristics were not significantly different between the two groups: sex, duration of symptoms, family history of cancer (negative history vs positive history), disease stage (early vs advanced), site of GC (proximal vs other localization), and histology (Table 1).
The baseline characteristics of the patients with multiple GC who participated in the study are shown in Table 1. Fifty-eight (6.7%) of the 862 GC patients developed another primary malignancy. Of the 58 multiple GC patients, 36 (62.1%) were men and 22 (37.9%) were women. The median age at diagnosis for these GC patients was 63.4 years ± 11.4 years (range 33-89 years). Four (6.9%) of the 58 patients developed two or more additional neoplasms, yielding a total of 62 multiple primary tumors other than GC, including 39 (63%) metachronous and 23 (37%) synchronous neoplasms. Of the 58 cases, 22 (38%) had synchronous and 34 (58.6%) had metachronous malignancies, while two patients developed two metachronous neoplasms. Two patients (3.4%) were diagnosed with both metachronous and synchronous cancers. One was diagnosed with three tumors (synchronous skin cancer and metachronous colon cancer at two different sites), while the second patient developed synchronous lung cancer and metachronous prostate cancer.
Of the 39 diagnosed metachronous neoplasms, 26 (66.7%) malignances were diagnosed before GC and 13 (33.3%) were diagnosed after GC. Of the patients with metachronous tumors, 25% developed a stomach malignancy after receiving chemotherapy or radiotherapy to treat the first primary neoplasm. Seven patients (19%) developed metachronous neoplasms after chemotherapy to treat GC, and one patient developed metachronous neoplasms after radiotherapy alone.
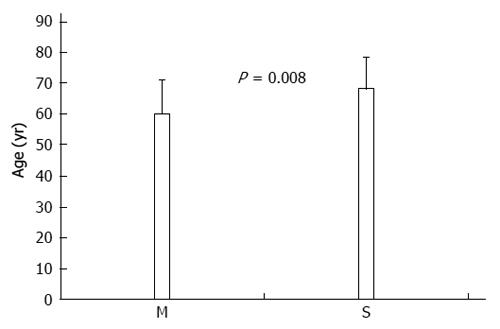
A comparison of the clinicopathological features of GC patients with synchronous and metachronous neoplasms yielded a statistically significant difference for patient age. GC patients with synchronous neoplasms were older than those with metachronous neoplasms (68.0 ± 10.3 years vs 59.9 ± 11.1 years, respectively, P = 0.008) (Figure 2). The following characteristics were not significantly different between the synchronous and metachronous neoplasm groups: sex, duration of symptoms, family history of cancer (negative history vs positive history), disease stage (early vs advanced), site of GC (proximal vs other localization), histology, and blood group (Table 2).
| Characteristic | Total | Metachronous | Synchronous | P value |
| Gender | 0.139 | |||
| Male | 34 | 18 (52.9) | 16 (72.7) | |
| Female | 22 | 16 (47.1) | 6 (27.3) | |
| Total1 | 56 | 34 | 22 | |
| Age2 (yr) | 63.1 ± 11.3 | 59.9 ± 11.1 | 68.0 ± 10.3 | 0.008 |
| DS (mo) | 27.3 ± 46.9 | 31.7 ± 44.0 | 22.1 ± 54.0 | 0.217 |
| FHC | 0.249 | |||
| No | 26 | 14 (53.8) | 12 (46.2) | |
| Yes | 25 | 18 (72.0) | 7 (28.0) | |
| Total | 51 | |||
| Location3 | 0.724 | |||
| Proximal | 10 | 7 (70.0) | 3 (30.0) | |
| Other location | 46 | 27 (58.7) | 19 (41.3) | |
| Total | 56 | |||
| Histology | 0.835 | |||
| Intestinal | 24 | 16 (66.7) | 8 (33.3) | |
| Diffuse | 17 | 12 (70.6) | 5 (29.4) | |
| Mixed | 2 | 1 (50.0) | 1 (50.0) | |
| Total | 43 | |||
| Stage | 0.553 | |||
| Early | 13 | 7 (53.8) | 6 (46.2) | |
| Advanced | 38 | 24 (63.2) | 14 (36.8) | |
| Total | 51 | |||
| Blood group | 0.675 | |||
| A | 13 | 9 (69.2) | 4 (30.8) | |
| B | 8 | 4 (50.0) | 4 (50.0) | |
| O | 25 | 15 (60.0) | 10 (40.0) | |
| AB | None | None | None | |
| Total | 46 |
An analysis of the site distribution in the 58 GC patients with multiple cancers (n = 62) showed that the most common site was colorectal (n = 17, 27.4%), followed by lung (n = 9, 14.5%), breast (n = 8, 12.9%), and prostate (n = 7, 11.3%) (Figure 3). In men, the most common site was colorectal (n = 10), lung (n = 8), and prostate (n = 7). In women, the most common site was breast (n = 8) and colorectal (n = 7). Among the 23 synchronous tumors, the most common primary sites were colorectal (n = 10, 43.5%), lung (n = 4, 17.4%), and prostate (n = 3, 13.1%). For the 39 metachronous cancers, the dominant types were colorectal cancer (n = 7, 17.9%) and breast cancer (n = 7, 17.9%), followed by lung cancer (n = 5, 12.8%) (Table 3).
| Site | No. tumors | Metachronous | Synchronous |
| n = 62 | n = 39 | n = 23 | |
| Colorectal | 17 (27.4) | 7 (17.9) | 10 (43.5) |
| Lung | 9 (14.5) | 5 (12.8) | 4 (17.4) |
| Breast | 8 (12.9) | 7 (17.9) | 1 (4.3) |
| Prostate | 7 (11.3) | 4 (10.3) | 3 (13.1) |
| Lymphoma | 4 (6.5) | 2 (5.1) | 2 (8.7) |
| Skin | 4 (6.5) | 2 (5.1) | 2 (8.7) |
| Urinary bladder | 3 (4.9) | 3 (7.7) | - |
| Kidney | 2 (3.2) | 1 (2.6) | 1 (4.3) |
| Larynx | 2 (3.2) | 2 (5.1) | - |
| Non Hodgkin Lymphoma | 2 (3.2) | 2 (5.1) | - |
| Head | 1 (1.6) | 1 (2.6) | - |
| Ovary | 1 (1.6) | 1 (2.6) | - |
| Pancreas | 1 (1.6) | 1 (2.6) | - |
| Uterus | 1 (1.6) | 1 (2.6) | - |
The main finding of the current study was a rate of 6.7% for the occurrence of multiple cancers in a group of GC patients in Poland. This rate is slightly higher than those reported by studies conducted in Italy, Portugal, and Sweden, in which the estimated rates were 1.9%-3.4%[9,10,19] in analyses of groups that included more than 34000 patients. In a study of more than 4500 patients in Korea who underwent surgery for GC, Eom et al[8] reported an incidence of multiple cancers of approximately 3.4%. Luciani et al[3] looked at 1503 consecutive GC patients and estimated that the overall prevalence of multiple malignances was 10% and that it was higher in patients ≥ 70 years old compared to younger patients (15% vs 6%, respectively). Our findings are similar to those of Kim et al[20], who analyzed 5778 patients with GC in Korea and found that 423 (7.3%) had been diagnosed with synchronous and metachronous double primary cancers.
A group of Japanese researchers[21] reported that about 5% of 1070 early GC patients developed multiple malignancies after surgical treatment; however, the authors described only metachronous tumors in their prospective study. Similarly, other Japanese researchers[22] reported ten (9.1%) patients who died due to primary cancers other than the original GC in a group of 109 early GC cases. We found that the incidence of multiple tumors in early GC cases was 10.9%, which remains consistent with data from Asia. Because early GC patients have a longer survival and thus are more likely to develop other neoplasms, it seems that multiple tumors would be expected to occur more frequently in early GC patients. The data from Green et al[13] confirms this. The authors estimate that this phenomenon may affect up to 32% of early GC patients, which seems to be a very high percentage considering that the authors analyzed only 28 patients.
Our data show that GC patients with synchronous neoplasms were older than those with metachronous neoplasms (68.0 ± 10.3 years vs 59.9 ± 11.1 years, respectively, P = 0.008). However, there were no statistically significant differences between these two groups in terms of sex, family history of cancer, duration of symptoms, tumor site in the stomach, histology, disease stage (early vs advanced), or blood group. This finding is consistent with the findings of Dinis-Ribeiro et al[10], who analyzed a group of 2668 GC patients and found 78 (3.4%) cases with primary tumors other than GC. They also found that patients with synchronous neoplasms were older than those with metachronous neoplasms. They, too, found no statistically significant differences with regard to sex, GC location, and TNM staging. Taken together, these results indicate that after a diagnosis of GC, older patients in particular should be investigated for second malignancies.
In our series, the most common types of synchronous and metachronous neoplasms were colorectal cancer, followed by cancers of the lung, breast, and prostate. Our data seems to be similar to the findings of others, who also reported that colorectal cancer is the most frequent neoplasm in GC patients with multiple malignancies[6,8,10,17,20,23], followed by lung, uterus, breast, and prostate cancers[6,8,10,11,17,24].
Our observations are somewhat surprising when one considers that in the Polish population, the most common type of cancer is lung cancer, while colon and rectal cancer are the fourth and seventh most common types (according to registration of new cases of cancer sites in men). However, in women, lung cancer is the second most common cancer after breast cancer, while colon cancer is the fifth and rectal is the eighth most common cancer (according to registration of new cases)[25]. The high incidence of colorectal cancer in GC patients may be due to the same environmental factors that affect the gastrointestinal tract; strikingly, however, in our study group, none of the GC patients had esophageal carcinoma. In contrast, other researchers have found that the esophagus is one of the more common sites for second primary tumors in GC patients[6,21,23]. It is difficult to interpret this difference in findings among studies, because carcinogenesis is such a complex and poorly understood process. Previous anticancer therapy is considered to predispose patients to developing additional malignancies. In the current study, 27.6% of the GC patients with multiple metachronous primary cancers had been treated with cytotoxic agents or radiation therapy for a first cancer regardless of whether GC was the first or second tumor.
A comparison between the groups of patients with or without a second primary tumor indicated that the only significant differences were in blood group and age. GC patients with second primary tumors were more commonly group O than those without (56.2% vs 31.6%, respectively, P = 0.002). However, out of our entire group of patients with GC, only 4.8% had both blood group O and multiple tumors. In GC patients with multiple malignancies, more than half were blood group O, 27% were blood group A, and almost 17% were blood group B. None of the patients were AB, although 40% of the Polish population are blood group A, 32% are O, 19% are B, and 9% are AB. In a previous study, we found that of 195 GC patients, 45% were blood group A, 27% were blood group O, 18% were blood group B, and 10% were blood group AB[26]. Previous studies reported that blood group A is associated with GC[27-29]. Furthermore, Wang et al[30] indicated a significantly higher risk of GC in individuals with blood group A; moreover, these patients were more likely to be infected with Helicobacter pylori than individuals with other ABO blood types. Other studies have found associations between pancreatic cancer and breast cancer and blood groups[31,32]; thus, this may not be a random association, and it may merit further investigation. To the best of our knowledge, our results represent the first investigation of these associations, and they indicate that patients with GC who have blood group O should be controlled for the development of second primary tumors.
The mean age of patients with multiple tumors was higher than that for patients without a second primary tumor (63.4 ± 11.4 years vs 59.5 ± 13.0 years, respectively, P = 0.026).
Ikeda et al[17] found that patients with a second tumor tended more frequently to be males and elderly than those without a second tumor. Eom et al[8] indicated that the mean age of patients and the proportion who had early GC were both higher in patients with a second cancer than in those without. Both studies were conducted in Asia and were carried out in larger groups.
Comparisons of clinicopathological features other than age and blood groups indicated that GC patients with and without a second cancer did not differ in terms of sex, tumor site, family history of cancer, stage, and histology. Despite the lack of statistically significant differences between GC patients with and without a second tumor in terms of family history of cancer, one observation is noteworthy: Almost half (49%) of the GC patients with multiple malignancies reported cancer in their first- or second-degree relatives; almost half (46.2%) of these relatives had GC. The only previous report of a similar finding came from Muela Molinero et al[19], who observed that 56% of GC patients diagnosed with multiple malignant primary neoplasms had a history of cancer in first-degree relatives. In our previous study of a group of 218 GC patients, a positive family history of cancer was noted in 36% of cases; of these cases, 46% had GC[26].
In conclusion, we confirmed the phenomenon of multiple malignancies in a group of GC patients in Poland. Specifically, we found that almost 7% of GC patients in our population had additional neoplasms. The most common additional malignancies were colorectal, breast, lung, and prostate cancers. Based on our findings, we believe that after a diagnosis of GC, the attending physician should be vigilant and perform additional procedures as appropriate, such as colonoscopy, mammography, chest X-ray, and abdominopelvic computed tomography. This may be particularly relevant for GC patients who are blood group O and are older.
The phenomenon of multiple primary neoplasms is increasingly being discussed due to the increased survival time of cancer patients after treatment and because of advances in diagnostic methods. It is estimated that multiple neoplasms affect about 10% of all cancer patients. In the study, 6.7% developed a total of 62 multiple primary tumors. The predominant tumor type of the secondary neoplasms was colorectal, followed by lung, breast, and prostate. The present paper is one of only a few European reports, it is the only study from Poland to explore this subject.
Gastric cancer patients may develop other primary cancers; appropriate preoperative and postoperative diagnostic modalities, such as colonoscopy, mammography, chest X-ray, and abdominopelvic computed tomography are thus required, particularly if patients are older and blood group O. It seems that future investigation will decide on their clinical suitability.
The current study shows not only the occurrence of multiple tumors in patients with gastric cancer, as has been reported by others, but also investigates associations between this phenomenon and the following clinical features: blood groups, duration of symptoms, and family history of cancer. The results represent the first investigation of these associations, and they indicate that patients with gastric cancer who have blood group O should be controlled for the development of second primary tumors.
Patients with gastric cancer patients may develop other primary tumors, particularly those who are blood group O and of advanced age. Appropriate additional preoperative and postoperative diagnostic modalities may be needed for patients with gastic cancer who have a higher likelihood of developing synchronous and metachronous tumors.
The authors presented the data of prevalence and clinical characteristics of gastric cancer patients with additional primary malignancies and found that patients with older age and blood O were more likely to develop other primary cancers beyond gastric cancers. Those findings were not reported in other similar articles. This is an interesting observations and study has been performed nicely. However, it would be more useful for clinical practice to find more clinical parameters valuable in identifying gastric cancer patients with multiple cancers.
| 1. | Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;16:1358-1414. |
| 3. | Luciani A, Ascione G, Marussi D, Oldani S, Caldiera S, Bozzoni S, Codecà C, Zonato S, Ferrari D, Foa P. Clinical analysis of multiple primary malignancies in the elderly. Med Oncol. 2009;26:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Frödin JE, Ericsson J, Barlow L. Multiple primary malignant tumors in a national cancer registry--reliability of reporting. Acta Oncol. 1997;36:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Hiyama T, Hanai A, Fujimoto I. Second primary cancer after diagnosis of stomach cancer in Osaka, Japan. Jpn J Cancer Res. 1991;82:762-770. [PubMed] |
| 6. | Lee JH, Bae JS, Ryu KW, Lee JS, Park SR, Kim CG, Kook MC, Choi IJ, Kim YW, Park JG. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol. 2006;12:2588-2592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Park YK, Kim DY, Joo JK, Kim JC, Koh YS, Ryu SY, Kim YJ, Kim SK. Clinicopathological features of gastric carcinoma patients with other primary carcinomas. Langenbecks Arch Surg. 2005;390:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, Lee KU. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008;98:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Lundegårdh G, Hansson LE, Nyrén O, Adami HO, Krusemo UB. The risk of gastrointestinal and other primary malignant diseases following gastric cancer. Acta Oncol. 1991;30:1-6. [PubMed] |
| 10. | Dinis-Ribeiro M, Lomba-Viana H, Silva R, Moreira-Dias L, Lomba-Viana R. Associated primary tumors in patients with gastric cancer. J Clin Gastroenterol. 2002;34:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Wu CW, Lo SS, Chen JH, Hsieh MC, Li AF, Lui WY. Multiple primary cancers in patients with gastric cancer. Hepatogastroenterology. 2006;53:463-467. [PubMed] |
| 12. | Buyukasik O, Hasdemir AO, Gulnerman Y, Col C, Ikiz O. Second primary cancers in patients with gastric cancer. Radiol Oncol. 2010;44:239-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Green PH, O’Toole KM, Weinberg LM, Goldfarb JP. Early gastric cancer. Gastroenterology. 1981;81:247-256. [PubMed] |
| 14. | Ikeguchi M, Ohfuji S, Oka A, Tsujitani S, Maeda M, Kaibara N. Synchronous and metachronous primary malignancies in organs other than the stomach in patients with early gastric cancer. Hepatogastroenterology. 1995;42:672-676. [PubMed] |
| 15. | Bozzetti F, Bonfanti G, Mariani L, Miceli R, Andreola S. Early gastric cancer: unrecognized indicator of multiple malignancies. World J Surg. 2000;24:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Kim JH, Rha SY, Kim C, Kim GM, Yoon SH, Kim KH, Kim MJ, Ahn JB, Chung HC, Roh JK. Clinicopathologic features of metachronous or synchronous gastric cancer patients with three or more primary sites. Cancer Res Treat. 2010;42:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Ikeda Y, Saku M, Kawanaka H, Nonaka M, Yoshida K. Features of second primary cancer in patients with gastric cancer. Oncology. 2003;65:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-colled intestinal type carcinoma. An attempt at histo-clinical classification. Acta Path et Microbiol Scandinav. 1965;64:31-49. |
| 19. | Muela Molinero A, Jorquera Plaza F, Ribas Ariño T, Malagón Rojo R, Espinel Diez V, Ballesteros del Río B, Olcoz Goñi JL, Santos Calderón JA. Multiple malignant primary neoplasms in patients with gatric neoplasms in the health district of León. Rev Esp Enferm Dig. 2006;98:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Kim JY, Jang WY, Heo MH, Lee KK, Do YR, Park KU, Song HS, Kim YN. Metachronous double primary cancer after diagnosis of gastric cancer. Cancer Res Treat. 2012;44:173-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Ikeda Y, Saku M, Kishihara F, Maehara Y. Effective follow-up for recurrence or a second primary cancer in patients with early gastric cancer. Br J Surg. 2005;92:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Itoh H, Oohata Y, Nakamura K, Nagata T, Mibu R, Nakayama F. Complete ten-year postgastrectomy follow-up of early gastric cancer. Am J Surg. 1989;158:14-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Furukawa H, Hiratsuka M, Iwanaga T, Imaoka S, Kabuto T, Ishikawa O, Sasaki Y, Kameyama M, Ohigashi H, Nakamori S. Treatments for second malignancies after gastrectomy for stomach cancer. Hepatogastroenterology. 1996;43:194-198. [PubMed] |
| 24. | Bae JS, Lee JH, Ryu KW, Kim YW, Bae JM. Characteristics of synchronous cancers in gastric cancer patients. Cancer Res Treat. 2006;38:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Ławniczak M, Starzyńska T. [Helicobacter pylori infection in gastric cancer patients]. Pol Merkur Lekarski. 2002;13:103-106. [PubMed] |
| 27. | AIRD I, BENTALL HH, ROBERTS JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1:799-801. [PubMed] |
| 28. | Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M, Nyrén O. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172:1280-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Gong Y, Yang YS, Zhang XM, Su M, Wang J, Han JD, Guo MZ. ABO blood type, diabetes and risk of gastrointestinal cancer in northern China. World J Gastroenterol. 2012;18:563-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Wang Z, Liu L, Ji J, Zhang J, Yan M, Zhang J, Liu B, Zhu Z, Yu Y. ABO Blood Group System and Gastric Cancer: A Case-Control Study and Meta-Analysis. Int J Mol Sci. 2012;13:13308-13321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL, Fuchs CS. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 286] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 32. | Stamatakos M, Kontzoglou K, Safioleas P, Safioleas C, Manti C, Safioleas M. Breast cancer incidence in Greek women in relation to ABO blood groups and Rh factor. Int Semin Surg Oncol. 2009;6:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
P- Reviewers: Ao R, Shinoto M, Singh SR, Yadav BS, Zhang KH S- Editor: Wen LL L- Editor: A E- Editor: Liu XM