Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.7067
Revised: December 31, 2013
Accepted: February 26, 2014
Published online: June 14, 2014
Processing time: 237 Days and 0.5 Hours
Recently, there have been reports from liver biopsies that showed the progression of liver fibrosis in liver transplant patients after the cessation of immunosuppression. Herein, we focused on activated hepatic stellate cells expressing alpha smooth muscle actin (α-SMA) to understand the correlation between immunosuppressant medication and liver fibrosis. The study enrolled two pediatric patients who underwent living donor liver transplantation and ceased immunosuppressant therapy. The number of α-SMA-positive cells in the specimens obtained by liver biopsy from these two patients showed a three-fold increase compared with the number from four transplanted pediatric patients who were continuing immunosuppressant therapy. In addition, the α-SMA-positive area evaluated using the WinRooF image processing software program continued to increase over time in three adult transplanted patients with liver fibrosis, and the α-SMA-positive area was increasing even during the pre-fibrotic stage in these adult cases, according to a retrospective review. Therefore, α-SMA could be a useful marker for the detection of early stage fibrosis.
Core tip: The primary finding presented in this case report is that there is that the cessation of immunosuppressant therapy may promote liver fibrosis in patients after liver transplantation, even though normal liver function is maintained. In addition, the alpha smooth muscle actin (α-SMA)-positive area increased during the pre-fibrotic stage. Therefore, α-SMA may serve as a useful marker to detect early stage fibrosis.
- Citation: Hirabaru M, Mochizuki K, Takatsuki M, Soyama A, Kosaka T, Kuroki T, Shimokawa I, Eguchi S. Expression of alpha smooth muscle actin in living donor liver transplant recipients. World J Gastroenterol 2014; 20(22): 7067-7074
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/7067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.7067
Liver transplantation is an established treatment for hepatic failure. Recent developments in surgical techniques, anesthesia and perioperative management have contributed to a decrease in early mortality after liver transplantation. However, the mortality in patients with chronic hepatic failure has remained unchanged[1]. Some of the causes of the poor prognosis for these patients include renal disorders, vascular disorders, malignant tumors, and the use of immunosuppressant medication[2-4]. Therefore, a reduction in such medication may reduce the mortality rate[5].
Despite many reports describing patients who have acquired immune tolerance[6,7], the characteristics of patients with immune tolerance are still unknown[8]. Clinical immune tolerance refers to the state of maintaining normal organ graft function even after the cessation of immunosuppressant medication[9,10]. In practice, the cessation of immunosuppressant medication varies depending on each patient’s condition and must be individualized; although some patients have a favorable postoperative course and can successfully achieve a reduction of immunosuppressant medication, other patients have no choice but to stop the treatment, such as in the case of infection with the Epstein-Barr virus (EBV). The probability of adult patients acquiring immune tolerance has been reported to be 8%-33%[11-18], and this rate has been suggested to be much higher in pediatric patients[6,19,20].
However, liver transplant recipients with no abnormalities in hepatic function after the cessation of immunosuppressant medication have recently been reported to developed hepatic fibrosis, with the hepatic fibrosis improving after resumption of the medication[21]. Therefore, there is a need to understand the mechanism(s) of hepatic fibrosis induced by withdrawal of immunosuppression. We have herein focused on hepatic stellate cells (HSCs), which may be involved in hepatic fibrosis. HSCs constitute a large portion of the hepatic interstitium, representing 5%-8% of the total number of liver cells[22]. In the healthy liver, HSCs are quiescent, but can be activated by factors, including TGFβ1 and IFNγ, that are released by Kupffer cells (KC) and T cells after injury or stimulation[22,23]. The appearance of alpha smooth muscle actin (α-SMA) in the activated HSCs can be detected using α-SMA immunostaining[24]. Activated HSCs undergo apoptosis at sites of acute inflammation but induce sinusoidal sclerosis, leading to the development of sinusoidal portal hypertension at sites of chronic inflammation. The activated HSCs have also been suggested to be responsible for the expression of type I collagen and the progression of fibrosis[25]. We therefore predict that an immune response may cause fibrosis in patients who have discontinued immunosuppressant medication, however the mechanism underlying this response remains to be determined.
We performed immunohistological analysis to determine the mechanism underlying the fibrosis associated with immunosuppressant medication in two pediatric patients who were doing well with good graft function without immunosuppression for several years after receiving living donor liver transplantation (LDLT).
A total of 163 patients underwent LDLT in our department from August 1997 to May 2012. Among them, 12 were pediatric patients who were less than 18 years of age, and 2 of these pediatric patients had ceased immunosuppressant medication for a long period. One patient was an 18-year-old male who underwent LDLT for biliary atresia (BA) at 5-years of age. In this case, immunosuppression (IS) was stopped according to the weaning protocol because of his good condition 68 mo after the LDLT. Another patient was an 11-year-old female who underwent LDLT for BA at 11-mo of age. Her IS was stopped non-electively because of EBV infection 3 mo after the LDLT. A total of eight liver biopsies were performed in these two patients. As a control, this study also included four pediatric patients who did not have hepatic function abnormalities or fibrosis and continued their immunosuppressant medication (no-tolerance cases). To examine whether the findings in these pediatric cases were also relevant to adult patients with fibrosis, three randomly selected patients with liver fibrosis not due to hepatitis C were evaluated.
Specimens were collected by ultrasound-guided core needle biopsy. Each specimen was stained with hematoxylin eosin, and the severity of fibrosis was determined using Ishak’s modified staging system[26]. The evaluation of each specimen was conducted blindly by two pathologists.
Four-micrometer-thick sections, cut from formalin-fixed, paraffin-embedded tissues, were immunohistochemically stained for SMA, CD68, and CD79α. The following primary antibodies and a staining kit [MAX-PO (MULTI), Nichirei Corporation, Tokyo, Japan] containing peroxidase-labeled -secondary antibodies were used: anti-alpha-SMA (Nichirei; Code 412021), anti-CD68 (Dako, Tokyo, Japan; Code M0814), and anti-CD79α (Dako; Code N162830). The immunostaining was performed according to the manufacturer’s instructions.
A semiquantitative analysis was performed by light microscopy at × 100 magnification, and the number of positively immunostained cells was calculated in five arbitrarily selected fields of view.
The tissues in the α-SMA-stained area were subjected to objective semiquantitative analysis using the WinROOF image processing software program (MITANI Corporation, Tokyo, Japan). The ratio of the positive area in the specimen to the total area was calculated.
The number of cells in five randomly selected fields of view (Table 1) that were α-SMA-positive was 250.5 ± 102.8 (mean ± SD) in the two pediatric patients with tolerance, whereas the count was 69.6 ± 67.7 in the four pediatric cases without tolerance. The numbers of CD68/CD79α-positive cells in the cases with and without tolerance were 398.2 ± 121.6/14.8 ± 8.7 and 413.5 ± 164.2/10.3 ± 4.6, respectively.
| Tolerance cases | |||||||||
| Pediatric Case | Age at LDLT | Sex | Original disease | Duration of biopsy after cessation of IS | Fibrosis | Positive area of α-SMA (%) | Number of positive cells/5 fields | ||
| (F Stage) | α-SMA | CD68 | CD79α | ||||||
| 1 | 5 yr | M | BA | 2 yr | 0 | 2.10 | 377 | 395 | 19 |
| (Period of IS: 5 yr 8 mo) | 5 yr 4 mo | 0 | 2.03 | 250 | 194 | 3 | |||
| 6 yr 4 mo | 0 | 2.26 | 147 | 422 | 3 | ||||
| 7 yr 4 mo | 0 | 2.95 | 243 | 500 | 10 | ||||
| 2 | 11 mo | F | BA | 4 yr 9 mo | 0 | 1.53 | 91 | 264 | 20 |
| (Period of IS: 3 mo) | 7 yr 9 mo | 0 | 2.49 | 257 | 552 | 16 | |||
| 8 yr 9 mo | 1 | 2.69 | 398 | 490 | 27 | ||||
| 9 yr 9 mo | 1 | 2.76 | 241 | 369 | 21 | ||||
| 2.3 ± 0.46 | 250.5 ± 102.8 | 398.2 ± 121.6 | 14.8 ± 8.7 | ||||||
| No tolerance cases | |||||||||
| Pediatric Case | Age at LDLT | Sex | Original disease | Duration of biopsy after LDLT | Fibrosis | Positive area of α-SMA (%) | Number of positive cells/5 fields | ||
| (F Stage) | α-SMA | CD68 | CD79α | ||||||
| 3 | 11 yr | F | BA | 1 yr | 0 | 0.02 | 9 | 508 | 12 |
| 2 yr | 0 | 1.86 | 134 | 446 | 14 | ||||
| 4 | 10 mo | M | BA | 1 yr | 0 | 0.47 | 1 | 332 | 8 |
| 1 yr 6 mo | 0 | 0.32 | 2 | 424 | 10 | ||||
| 2 yr | 0 | 0.83 | 107 | 525 | 9 | ||||
| 5 | 7 yr | M | BA | 1 yr | 0 | 1.08 | 178 | 537 | 0 |
| 1 yr 6 mo | 0 | 0.63 | 153 | 637 | 7 | ||||
| 6 | 6 yr | F | BA | 2 yr | 0 | 0.38 | 15 | 92 | 12 |
| 8 yr | 0 | 0.70 | 37 | 202 | 16 | ||||
| 13 yr | 0 | 1.26 | 60 | 432 | 15 | ||||
| 0.75 ± 0.53 | 69.6 ± 67.7 | 413.5 ± 164.2 | 10.3 ± 4.6 | ||||||
| (mean ± SD) | |||||||||
In addition, the number of α-SMA-positive cells was 227.5 ± 99.0 in the tolerant patients with F0 stage fibrosis, which was higher than that in the patients without tolerance. The α-SMA-positive area ratio was also calculated using the WinROOF software program. The α-SMA-positive area ratio in the patients without tolerance with any fibrotic stage was 2.3% ± 0.46%; it was 2.2% ± 0.47% in cases with fibrotic stage F0 and 0.75% ± 0.53% in the no-tolerance patients (all patients with fibrotic stage F0). Accordingly, even among patients with no findings of fibrosis, the α-SMA area ratio was higher in patients with tolerance than in those without tolerance.
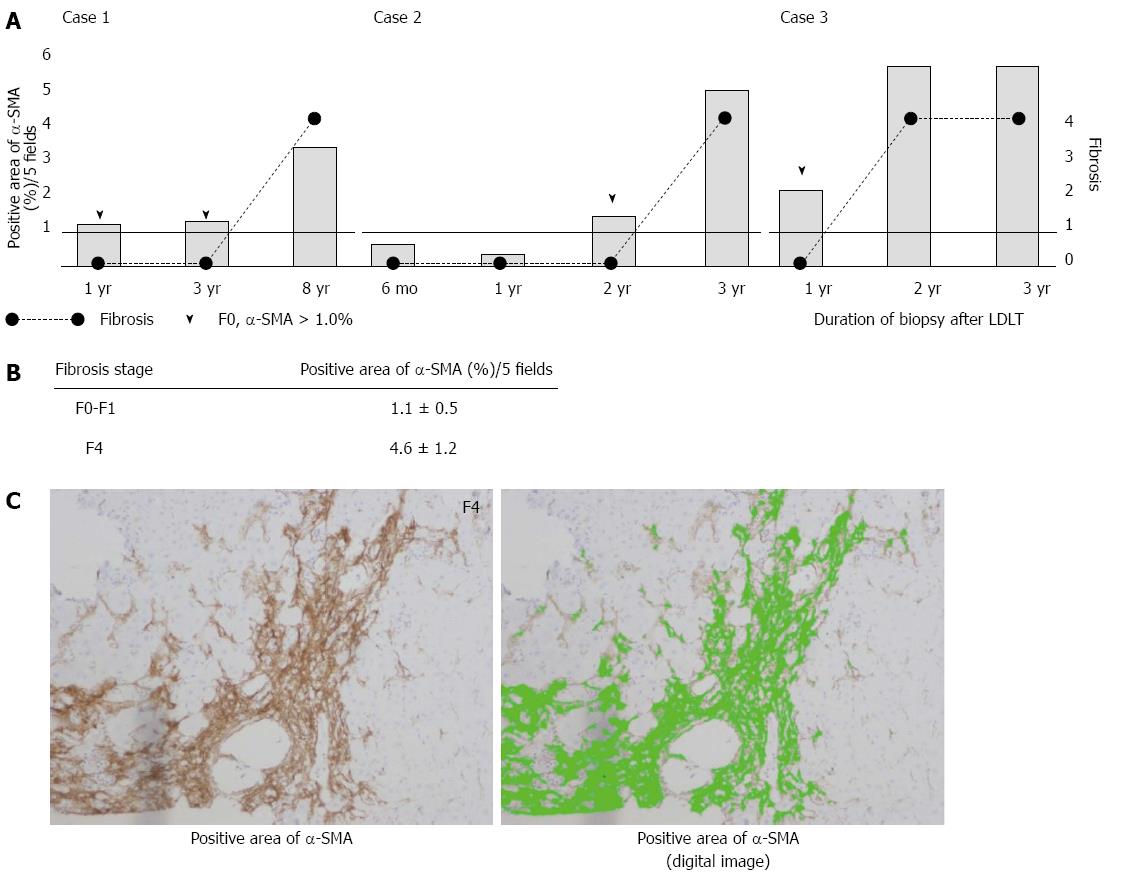
The α-SMA-positive area ratio was calculated for adult patients with fibrosis using the WinROOF software program. Liver specimens obtained from a total of 10 liver biopsies in fibrosis Cases 1 to 3 were subjected to the analysis (Figure 1). Figure 1A shows the timing of the biopsies, fibrosis grade, and α-SMA-positive area ratio. The α-SMA-positive area continued to increase over time in all patients, and the α-SMA-positive area also increased in all patients even when they were in the pre-fibrotic stage (arrowhead).
The α-SMA-positive area ratio in adult patients with fibrosis was also evaluated based on the fibrosis stage. The area ratio was 1.1% ± 0.5% in the F0-1 stage and 4.6% ± 1.2% in the F4 stage. The α-SMA area ratio was higher in the F0-1 stages than in the F4 stage (Figure 1B).
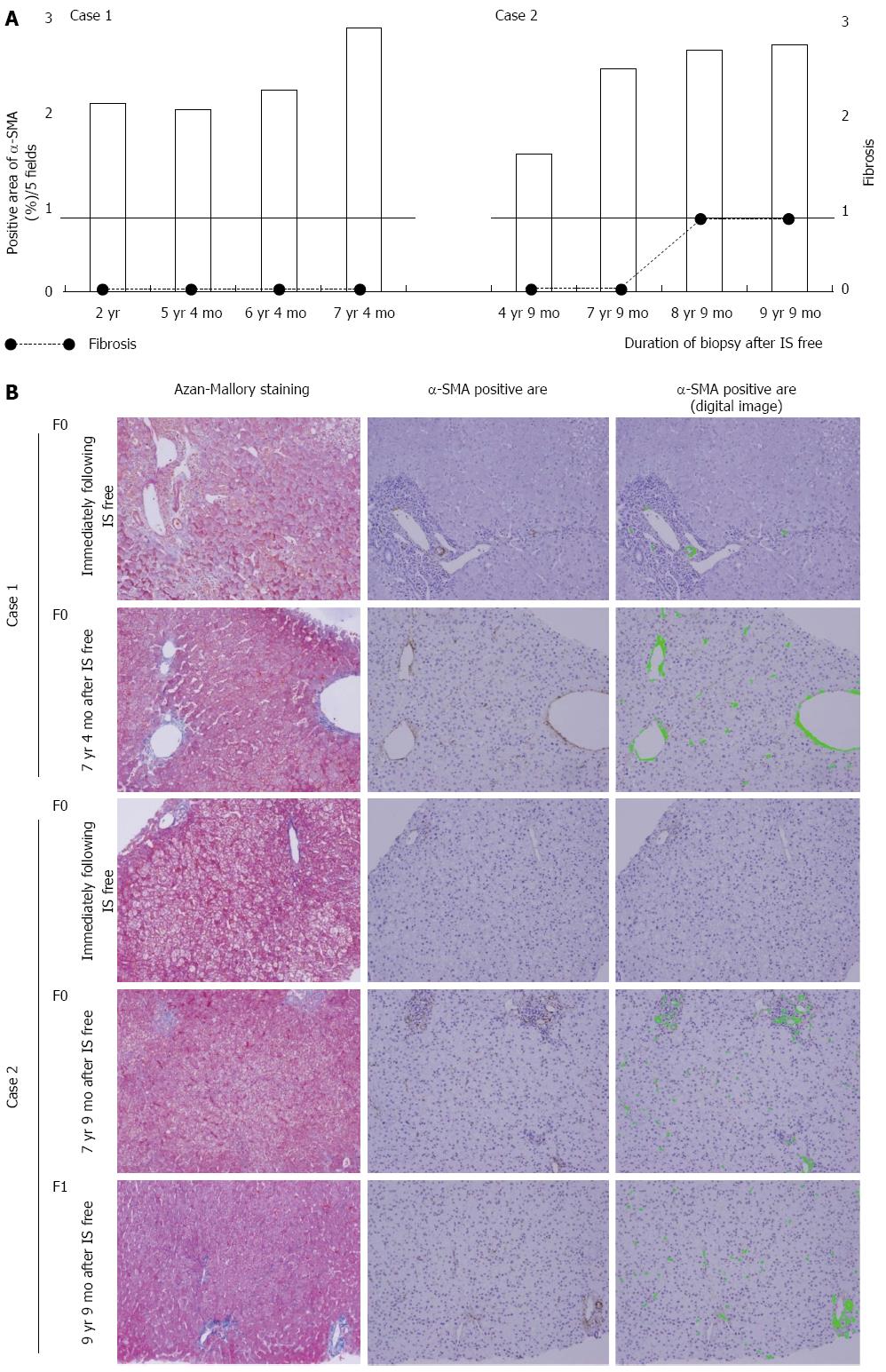
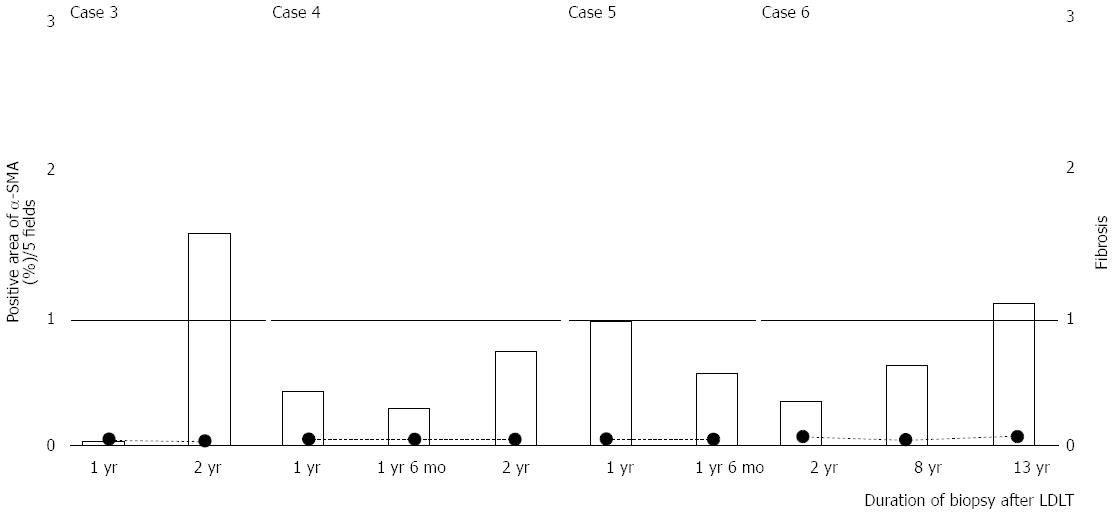
The α-SMA-positive area continued to increase over time in the pediatric patients with tolerance. Pediatric Case 1 showed F0 fibrosis in the liver at all time points, whereas pediatric Case 2 showed a slight progression of fibrosis (F1) eight years after the cessation of the immunosuppressant treatment (Figure 2). However, there were no significant increases in the α-SMA-positive area in the pediatric cases without tolerance (Figure 3).
Immune tolerance is the ultimate goal of transplantation, and many transplant patients have been reported to have ceased immunosuppressant medication for a long period while maintaining a favorable clinical course in clinical practice[6,7]. However, hepatic fibrosis has been reported to have developed in transplant patients who have ceased immunosuppressant medication[21], and there are some concerns over whether the cessation of immunosuppressive treatment leads to fibrosis. We performed a histological analysis in patients who had ceased immunosuppressant medication and examined the impact of the cessation on the liver graft.
As shown by the findings of this and previous studies[23], the expression of α-SMA increases with the progression of hepatic fibrosis because liver injury that is caused by hepatic viruses or medication allows T cells and Kupffer cells to release PDGF, IGF-I, TGF-β, activated oxygen, lipid peroxide, and α-SMA into the cytoplasm of HSCs. Subsequently, type I collagen is produced and fibrosis develops[24]. Therefore, it is supposed that the α-SMA level is increase in the pre-fibrotic stage, although there have been no reports on the correlation between the α-SMA expression level and fibrotic changes.
Here, we investigated the association between α-SMA expression and the fibrotic stage in patients with hepatic fibrosis. An increase in α-SMA over time was observed in all patients with fibrosis, suggesting that there is a correlation between the progression of fibrosis and the increase in α-SMA. Furthermore, all patients with fibrosis had increased α-SMA expression at the pre-fibrotic stage. In the current study, in two cases of tolerance, the progression of fibrosis remained low, although the ratio of α-SMA-positive areas remained high in both cases. However, Yoshitomi et al[21] reported that liver fibrosis progression in tolerant patients was higher than that in non-tolerant patients. Therefore, we propose that α-SMA may be a potential marker of the progression of liver fibrosis. Thus, we should continue to follow these two cases carefully to ensure that the progression of fibrosis is not missed. Because this study was a retrospective analysis and the time of biopsy varied for the individual patients, periodic follow-up examination will be required to evaluate and support this hypothesis. These findings suggest that a routine protocol biopsy could be an important tool to understand the dynamic state of α-SMA in detail.
These assumptions suggest that the possibility of developing fibrosis is higher in tolerant patients than in patients who continue immunosuppression, because α-SMA expression was consistently high in the tolerant patients in this study. In addition, the expression of α-SMA gradually increased in the tolerant patients, indicating that care should be taken in future follow-ups for these patients to ensure that their liver function does not deteriorate.
The liver is known to be the organ most susceptible to immune tolerance compared with other organs, and phenomena in different animal species have been observed that demonstrate that the major histocompatibility complex was successfully engrafted without immunosuppressant medication after an allogeneic liver transplant[27,28]. The effects of immunosuppressive factors produced in the liver and the correlations among antigen-presenting cells in the transplanted liver, including Kupffer cells, sinusoidal endothelial cells, and recipient-derived T cells, are believed to be involved in this immune tolerance. In addition, there have been some studies in mice showing that T cells became unresponsive to the antigen presented from sinusoidal endothelial cells of the specific donor type[29,30]. A treatment strategy leading to the acquisition of immune tolerance is considered to be important for human liver transplantation to prevent damage to hepatic sinusoidal endothelial cells.
CD68 (KCs) and CD79α (T cells) were immunostained to search for factors related to fibrosis in patients with and without immune tolerance, but there were no significant differences in either KCs or T cells. The major factor determining the progression of fibrosis in patients with immune tolerance still remains unknown, and predictors for the development of tolerance are also unknown. Therefore, we confirm that liver fibrosis staging assessed by biopsy is the main parameter influencing the treatment course.
A previous study indicated that calcineurin inhibitors (CNIs) may inhibit the activity of HSCs and the progression of fibrosis[21], but convincing evidence has not yet been provided. However, there is a good possibility that the cessation of immunosuppressant medication may cause a certain degree of rejection without abnormal hepatic function or histological rejection. There were also no differences in α-SMA expression between hepatitis C virus-infected patients with and without liver transplants[23]. In addition, CNIs administration may not always inhibit α-SMA expression if there is an infectious background. In addition, the infiltration of inflammatory cells stimulates the expression of pre-fibrotic growth factors[31], and inflammatory cells and activated HSCs are actually mixed in patients with chronic hepatic dysfunction[32]. Therefore, controlling inflammation is considered to inhibit the progression of fibrosis regardless of the use of immunosuppressant medication.
When deciding whether to resume immunosuppressant medication, it is important to determining whether the progression of fibrosis is due to an antigen response is important[33]. However, the factors associated with an increase in α-SMA were not determined in the present study in the two pediatric patients, who had most likely acquired immune tolerance. Immunosuppressant medication has not been resumed in these two pediatric patients because they have not shown clear abnormalities in liver function. However, we are performing a strict follow-up regime for both patients to determine whether they will continue to have a good long-term prognosis.
An 18-year-old male with a history of living donor liver transplantation (LDLT) for biliary atresia with no symptoms and an 11-year-old female with a history of LDLT for BA with no symptoms.
Immune tolerant state for a long period of time post-LDLT.
Progression of liver fibrosis.
The result of the liver function and all other tests were within normal limits.
In the imaging examinations, morbid findings were not detected.
In one patient, slight liver fibrosis was revealed by liver biopsy 9 years after the cessation of immunosuppressive therapy.
This case report suggested that alpha smooth muscle actin may be a predictor of liver fibrosis; however, this assumption needs further validation from additional cases.
This article provides possibility that the cessation of immunosuppressive therapy causes liver fibrosis.
| 1. | Adam R, McMaster P, O’Grady JG, Castaing D, Klempnauer JL, Jamieson N, Neuhaus P, Lerut J, Salizzoni M, Pollard S. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 415] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 2. | Mells G, Neuberger J. Reducing the risks of cardiovascular disease in liver allograft recipients. Transplantation. 2007;83:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1667] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 4. | Galve ML, Cuervas-Mons V, Figueras J, Herrero I, Mata M, Clemente G, Prieto M, Margarit C, Bernardos A, Casafont F. Incidence and outcome of de novo malignancies after liver transplantation. Transplant Proc. 1999;31:1275-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Mells G, Mann C, Hubscher S, Neuberger J. Late protocol liver biopsies in the liver allograft: a neglected investigation? Liver Transpl. 2009;15:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, Hayashi M, Kanematsu T, Tanaka K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 215] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Jonas S, Neuhaus R, Junge G, Klupp J, Theruvat T, Langrehr JM, Settmacher U, Neuhaus P. Primary immunosuppression with tacrolimus after liver transplantation: 12-years follow-up. Int Immunopharmacol. 2005;5:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Matthews JB, Ramos E, Bluestone JA. Clinical trials of transplant tolerance: slow but steady progress. Am J Transplant. 2003;3:794-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Tisone G, Orlando G, Angelico M. Operational tolerance in clinical liver transplantation: emerging developments. Transpl Immunol. 2007;17:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Assy N, Adams PC, Myers P, Simon V, Minuk GY, Wall W, Ghent CN. Randomized controlled trial of total immunosuppression withdrawal in liver transplant recipients: role of ursodeoxycholic acid. Transplantation. 2007;83:1571-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE. Tolerance: is it worth the risk? Transplantation. 2005;79:1157-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 292] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Pons JA, Yélamos J, Ramírez P, Oliver-Bonet M, Sánchez A, Rodríguez-Gago M, Navarro J, Bermejo J, Robles R, Parrilla P. Endothelial cell chimerism does not influence allograft tolerance in liver transplant patients after withdrawal of immunosuppression. Transplantation. 2003;75:1045-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Ramos HC, Reyes J, Abu-Elmagd K, Zeevi A, Reinsmoen N, Tzakis A, Demetris AJ, Fung JJ, Flynn B, McMichael J. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212-217. [PubMed] |
| 16. | Sandborn WJ, Hay JE, Porayko MK, Gores GJ, Steers JL, Krom RA, Wiesner RH. Cyclosporine withdrawal for nephrotoxicity in liver transplant recipients does not result in sustained improvement in kidney function and causes cellular and ductopenic rejection. Hepatology. 1994;19:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, Lionetti R, Anselmo A, Toti L, Angelico M. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol. 2006;44:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Tryphonopoulos P, Tzakis AG, Weppler D, Garcia-Morales R, Kato T, Madariaga JR, Levi DM, Nishida S, Moon J, Selvaggi G. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant. 2005;5:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Koshiba T, Li Y, Takemura M, Wu Y, Sakaguchi S, Minato N, Wood KJ, Haga H, Ueda M, Uemoto S. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol. 2007;17:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Mazariegos GV, Sindhi R, Thomson AW, Marcos A. Clinical tolerance following liver transplantation: long term results and future prospects. Transpl Immunol. 2007;17:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Yoshitomi M, Koshiba T, Haga H, Li Y, Zhao X, Cheng D, Miyagawa A, Sakashita H, Tsuruyama T, Ohe H. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Wu TJ, Wang YC, Wu TH, Lee CF, Chan KM, Lee WC. Inhibition of allogenic T-cell cytotoxicity by hepatic stellate cell via CD4+ CD25+ Foxp3+ regulatory T cells in vitro. Transplant Proc. 2012;44:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Cisneros L, Londoño MC, Blasco C, Bataller R, Miquel R, Bruguera M, Ginès P, Rimola A. Hepatic stellate cell activation in liver transplant patients with hepatitis C recurrence and in non-transplanted patients with chronic hepatitis C. Liver Transpl. 2007;13:1017-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Yu C, Wang F, Jin C, Huang X, Miller DL, Basilico C, McKeehan WL. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol. 2003;163:1653-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3839] [Article Influence: 123.8] [Reference Citation Analysis (2)] |
| 27. | Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 616] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Zimmermann FA, Davies HS, Knoll PP, Gokel JM, Schmidt T. Orthotopic liver allografts in the rat. The influence of strain combination on the fate of the graft. Transplantation. 1984;37:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Khanna A, Morelli AE, Zhong C, Takayama T, Lu L, Thomson AW. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol. 2000;164:1346-1354. [PubMed] |
| 31. | Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, Cosmi L, Romagnani P, Milani S, Failli P. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899-d1914. [PubMed] |
| 33. | Tullius SG, Tilney NL. Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation. 1995;59:313-318. [PubMed] |
P- Reviewers: Boucek C, Pompili M S- Editor: Ma YJ L- Editor: A E- Editor: Ma S