Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6906
Revised: January 21, 2014
Accepted: March 7, 2014
Published online: June 14, 2014
Processing time: 208 Days and 16.9 Hours
AIM: To investigate the expression of microRNA-218 (miR-218) in serum from gastric cancer patients and its relationship with clinicopathological characteristics.
METHODS: A total of 68 patients with pathologically diagnosed gastric cancer and 56 healthy individuals were recruited to this study. The expression of miR-218 was detected in the serum of gastric cancer patients and healthy individuals by quantitative real-time polymerase chain reaction. The clinical data were collected and analyzed by statistical software.
RESULTS: miR-218 was reduced significantly in the serum of gastric cancer patients compared to healthy individuals (1.15 ± 0.08 vs 0.37 ± 0.023; P = 0.026). In the gastric cancer group, serum expression of miR-218 was lower in patients with metastasis and poorly differentiated cancer compared with non-metastatic and well-differentiated cancer (0.19 ± 0.011 vs 0.45 ± 0.021, P = 0.031 and 0.21 ± 0.019 vs 0.49 ± 0.021, P = 0.025). Serum miR-218 was found to be significantly associated with gastric cancer metastasis (P = 0.003), tumor T stage (P = 0.018) and tumor grade (P = 0.012). Low serum expression of miR-218 was related to an increase in the stage of gastric cancer. The expression level of miR-218 in the serum was correlated with the 3-year survival. Ninety-seven percent of patients with a high level of miR-218 expression survived for 3 years, while only 54% of those with low miR-218 expression survived.
CONCLUSION: miR-218 is deregulated in gastric cancer patients and is strongly correlated with tumor stage, grade and metastasis. Serum expression of miR-218 may be a prognostic marker.
Core tip: microRNA-218 (miR-218) has been shown to be a tumor-suppressor miRNA in several cancers. In this study, we investigated the expression of miR-218 in the serum of gastric cancer patients and its relationship with clinicopathological characteristics. miR-218 was deregulated in gastric cancer patients and associated with tumor invasion and prognosis.
- Citation: Xin SY, Feng XS, Zhou LQ, Sun JJ, Gao XL, Yao GL. Reduced expression of circulating microRNA-218 in gastric cancer and correlation with tumor invasion and prognosis. World J Gastroenterol 2014; 20(22): 6906-6911
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6906
Gastric cancer is the second most common cancer worldwide and causes nearly 1 million deaths annually[1]. However, to date, our knowledge about gastric cancer invasion and metastasis is limited. Therefore, further screening and investigation of some new predictive and prognostic markers are warranted, which could be helpful in finding new therapeutic targets and strategies and improving prognosis. Recent studies about microRNAs (miRNAs) might shed light on this problem[2].
Two decades ago, miRNAs were discovered as a novel class of evolutionarily conserved, small (18-24 nucleotides), noncoding RNA molecules that are important regulators of gene expression[3,4]. By targeting the 3’ untranslated region (UTR) of mRNA transcripts, miRNAs influence RNA stability and translational efficiency via degradation or protein translation inhibition, respectively[3,5-8]. miRNAs are involved in cellular proliferation, differentiation, apoptosis, angiogenesis, invasion and migration[9,10]. Thus, alterations in miRNA expression can affect crucial biological processes in cancer development and progression, such as proliferation, differentiation and apoptosis[11,12]. As reported previously, some miRNAs are reduced in malignancies and function as tumor suppressors[13,14]. microRNA (miR)-218 is downregulated in glioma, bladder cancer, lung cancer and oral cancer[13,15-17]. In vitro assays have shown that restored expression of miR-218 enhances tumor growth and invasion, but reduces apoptosis[17-19]. Therefore, we investigated the expression of miR-218 in the serum of gastric cancer patients using quantitative real-time polymerase chain reaction (qRT-PCR), and analyzed the relationship between miR-218 levels and clinicopathological characteristics.
A total of 68 patients with pathologically diagnosed gastric cancer were recruited from January 2009 to June 2010 at the First Affiliated Hospital of Henan University of Science and Technology (Henan Province, China). Blood samples were obtained before any treatment and immediately centrifuged; sera and other components were stored at -80 °C. Blood samples from 56 healthy people were used as controls. The control group was defined as healthy individuals who visited hospital for routine check-up, and they did not have any gastric lesions or a history of malignancy. Permission was obtained from the hospital Ethical Committee, and written informed consent was provided by all patients.
TRIzol reagent (CWbio Co. Ltd., Beijing, China) was used to isolate total RNA from the snap-frozen tissues. The isolated RNA was treated with DNase I (Invitrogen, Carlsbad, CA, United States). The RNA concentration and purity were determined using NanoDrop ND-1000. The ratio of 28S/18S was analyzed by Glyko Bandscan 5.0. RNA quality and quantity were determined by spectrophotometer (Wilmington, DE, United States) at 260 and 280 nm. Reverse transcription of RNA was performed using the NCode miRNA First-Strand cDNA Synthesis Kit (Invitrogen, United States). qRT-PCR was performed with the Light Cycler 2.0 Real-Time PCR System (Roche, Germany) in a total volume of 20 μL in glass capillaries containing 2 μL cDNA, 0.8 μL each primer, and 10 μL Light Cycler TaqMan Master Mix (Invitrogen). The PCR for the miR-21 gene was initiated using a 10-min denaturation step at 95 °C followed by termination with a 30-s cooling step at 40 °C. The cycling protocol consisted of denaturation at 95 °C for 15 s and annealing at 60 °C for 60 s; this cycle was performed 40 times. Fluorescence detection was performed at the end of each extension step. The PCR products were confirmed by melting curve analysis. For data analysis, we used the cel-miR-39 as an endogenous control. All procedures were repeated three times. The relative expression of miR-218 for gastric cancer and normal controls was calculated by the 2-ΔΔCT method. The mean relative expression of miR-218 in the serum of healthy people was set as N, and all relative expressions of miR-218 in samples of gastric cancer were compared with it. Based on the ratios, we determined low miR-218 expression (T/N < 1.2); medium expression (T/N = 1.2-10); and high expression (T/N > 10).
Differences of miR-218 expression in the two groups were assessed by one-way analysis of variance. The Mann-Whitney U test was used to determine the associations of miR-218 expression and Gastric Cancer clinicopathological features. Survival functions and differences were calculated by the Kaplan-Meier method and assessed using the log-rank statistic. Multivariable survival analyses were performed using the Cox proportional hazards regression model. SPSS version 17.0 was used for statistical analysis. Statistically significant level was defined as P < 0.05.
Demographic and clinicopathological variables for the cohort are summarized in Table 1. Sixty-eight patients with gastric cancer were included in this study (46 male and 22 female) aged 35-82 years (mean = 63 years). Pathological classification of tumor grading resulted in 18 poorly differentiated, 24 moderately differentiated and 26 well differentiated tumors, and T staging resulted in 19 as T1, 17 as T2, 15 as T3, and 17 as T4. There were 28 cases of metastatic gastric cancer and 40 non-metastatic cases. Follow-up for the entire cohort ranged from 18 to 36 mo (mean = 29.6 mo).
| Parameters | n | microRNA-218 expression | P value | ||
| Low | Middle | High | |||
| Age | 0.0816 | ||||
| ≤ Mean | 22 | 11 | 8 | 3 | |
| > Mean | 46 | 24 | 16 | 6 | |
| Gender | 0.0866 | ||||
| Male | 49 | 24 | 19 | 6 | |
| Female | 19 | 11 | 4 | 4 | |
| Location | 0.916 | ||||
| Upper | 19 | 9 | 8 | 2 | |
| Middle | 12 | 6 | 5 | 1 | |
| Lower | 37 | 20 | 10 | 7 | |
| T-stage1 | 0.018 | ||||
| T1 | 19 | 4 | 12 | 3 | |
| T2 | 17 | 9 | 6 | 2 | |
| T3 | 15 | 10 | 3 | 2 | |
| T4a | 10 | 6 | 3 | 1 | |
| T4b | 7 | 4 | 3 | 0 | |
| N- stage1 | 0.021 | ||||
| N0 | 16 | 3 | 6 | 7 | |
| N1 | 11 | 6 | 4 | 1 | |
| N2 | 10 | 7 | 2 | 1 | |
| N3a | 2 | 2 | 0 | 0 | |
| Nx | 29 | 16 | 10 | 3 | |
| Metastasis1 | 0.003 | ||||
| Metastasis | 28 | 20 | 6 | 2 | |
| Non-metastasis | 40 | 11 | 19 | 10 | |
| Grade2 | 0.012 | ||||
| Well differentiated | 26 | 10 | 13 | 3 | |
| Moderately differentiated | 24 | 12 | 9 | 3 | |
| Poorly differentiated | 18 | 15 | 1 | 2 | |
| Resection | 0.0662 | ||||
| Resections | 39 | 18 | 12 | 9 | |
| No-resections | 29 | 16 | 10 | 3 | |
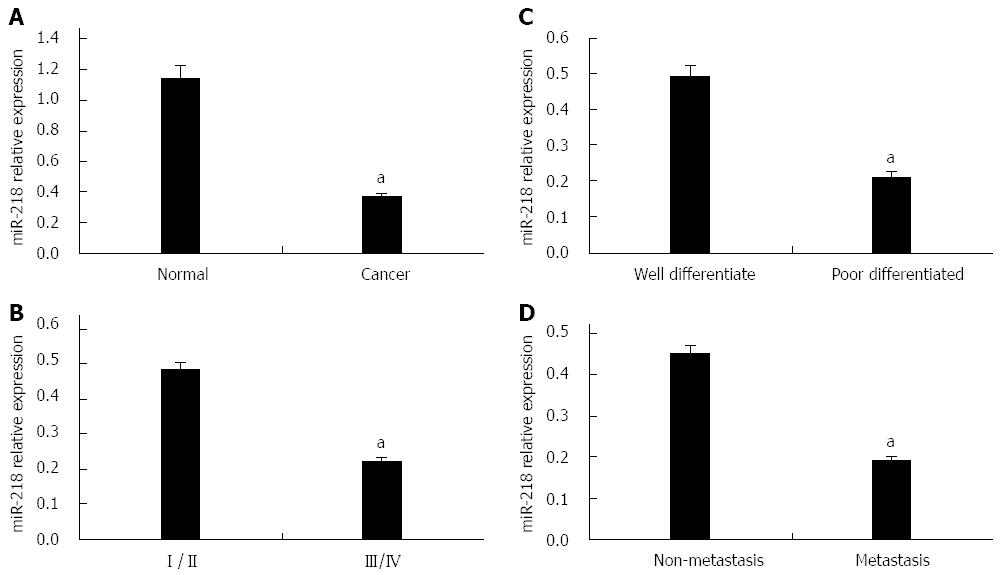
qRT-PCR analysis was performed to demonstrate the differences in miR-218 expression in gastric cancer and normal serum. Expression of miR-218 was significantly decreased in gastric cancer compared with normal serum (1.15 ± 0.08 vs 0.37 ± 0.023, P = 0.026, Figure 1A). Furthermore, compared with the earlier stages (stage I and II) and non-metastasis, the later stages (stage III and IV) (0.48 ± 0.023 vs 0.22 ± 0.011, P = 0.023 and 0.45 ± 0.021 vs 0.19 ± 0.011, P = 0.031; Figure 1B and D) and metastasis had lower serum expression of miR-218. There was a significant difference between the well-differentiated and poorly differentiated tumors in serum expression of miR-218 (0.49 ± 0.021 vs 0.21 ± 0.019, P = 0.025, Figure 1C). Moreover, there was a significant decrease in miR-218 expression in the serum of gastric cancer patients compared to the healthy individuals (P = 0.018, Table 2).
| Variable | n | microRNA-218 expression | P value | ||
| Low | Medium | High | |||
| GC | 68 | 36 | 24 | 8 | |
| NS | 56 | 7 | 32 | 17 | 0.018 |
The relationships between miR-218 expression and clinicopathological variables of gastric cancer are summarized in Table 1. There was no significant relationship between miR-218 expression and patient age (P = 0.816), sex (P = 0.866), and tumor location (P = 0.916). However, miR-218 was found to be significantly associated with tumor metastasis (P = 0.003), tumor T stage (P = 0.018), and tumor grade (P = 0.012). Taken together, these findings strongly imply that miR-218 expression was negatively correlated with tumor differentiation, invasion, and metastasis, which are associated with tumorigenesis and tumor progression.
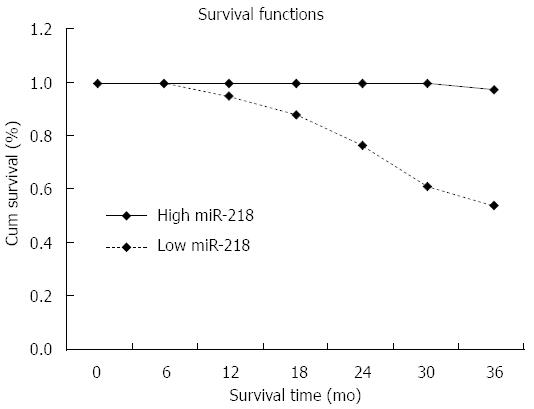
The expression level of miR-218 correlated with 3-year survival and tumor stage. Associations between the miR-218 expression parameters and overall survival were evaluated by Kaplan-Meier survival analysis with log-rank statistics for determining significance (Figure 2). The mean overall survival was significantly decreased in the low miR-218 group compared to the high miR-218 group (low miR-218: mean overall survival 23.6 mo, high miR-218: mean overall survival 35.6 mo; P = 0.008). For patients with high miR-218, 97% survived for 3 years after surgery, while for those with low miR-218 expression, only 54% survived. In patients with low, medium and high miR-218 expression, the percentage of those in stage I decreased by 87%, 31.5% and 17.5%, respectively. This implies that increased expression of miR-21 correlates with increased gastric cancer stage.
In a multivariate analysis based on the Cox proportional hazards regression model, we tested the independent predictive value for miR-218 expression as well as relevant clinical and pathological parameters including sex, age, tumor location, tumor size, metastasis or not, T stage and grade. As shown in Table 3, decreased miR-218 expression proved to be an independent prognostic marker for overall survival (P = 0.035), in addition to the presence of tumor metastasis (P = 0.005) and high tumor grade (P = 0.000).
| Variable | B | SE | Wald | df | P value | HR | 95%CI |
| miR-218 expression (high vs low) | 1.161 | 0.8556 | 4.284 | 1 | 0.035 | 3.162 | 1.062-9.401 |
| T-status (T1-2vs T3-4) | 1.713 | 0.4126 | 5.536 | 1 | 0.027 | 3.347 | 1.193-31.643 |
| N-status (N0-1vs N2-3) | 2.026 | 0.583 | 8.746 | 1 | 0.007 | 9.321 | 2.438-37.465 |
| Metastasis (non-M vs M) | -1.463 | 0.522 | 7.698 | 1 | 0.005 | 0.231 | 0.091-0.653 |
| Grade1 | 1.162 | 0.484 | 5.782 | 1 | 0.016 | 3.212 | 1.230-8.265 |
| Grade2 | 2.312 | 0.664 | 12.136 | 1 | 0.000 | 10.061 | 2.753-36.857 |
To the best of our knowledge, this is the first study of the expression of miR-218 in the serum of gastric cancer patients. We detected miR-218 in the serum of 68 patients with gastric cancer and in 56 healthy individuals using qRT-PCR. We also examined the relationships between expression of miR-218 and clinicopathological characteristics. The results showed that miR-218 expression in the serum of gastric cancer patients was lower than that in the serum of healthy individuals, indicating that miR-218 may be associated with carcinogenesis and cancer development.
In gastric cancer, patients with regional or distant metastasis usually have poor outcomes. Unfortunately, the exact mechanisms of invasion and metastasis are still unknown. In recent decades, studies on miRNAs have shown their potential advantage in uncovering the mechanisms of tumor invasion and metastasis[2]. In the latest studies, several tumor suppressor miRNAs were reduced in human malignancies[14]. miR-218 is one of those that could suppress tumor progression and invasion. Although many miRs, such as miR-21, miR-27a and the miR-106b-25 cluster, are increased in gastric cancer, other miRs, especially miR-218, are reduced. Furthermore, Gao et al[20] used a stem-loop RT-PCR assay to detect miR-218 expression levels in 20 pairs of gastric tissue samples. They identified the specific downregulation of miR-218 in gastric cancer, and they observed that the forced expression of miR-218 decreased cell proliferation markedly and induced apoptosis in gastric cancer cell lines, consistent with previous microarray data[21,22]. However, in other malignancies, miR-218 reportedly was highly expressed; for example, in acute and chronic lymphocytic leukemia, miR-218 and miR-34a were considered to function as carcinogenic miRs[23,24]. These differences in miR expression may reflect the developmental lineage and/or the state of differentiation in the tumor. As the status of serum miR-218 in gastric cancer has not been well studied, we investigated the expression of miR-218 in the serum of gastric cancer patients and detected its relationships with clinical parameters. According to our results, miR-218 in the serum was decreased in gastric cancer, especially in the later stages, highlighting its roles in tumor development and progression. This phenomenon was consistent with results of previous studies[15,19]. In addition, the expression of miR-218 in the serum was lower in later stage gastric cancer. In the gastric cancer group, patients with lymphatic node metastasis showed lower expression of serum miR-218. Furthermore, our Kaplan-Meier survival analysis with log-rank statistics suggested that downregulation of miR-218 expression was positively correlated with tumor-related mortality. Meanwhile, our multivariate analysis suggested that downregulation of miR-218 expression had a prognostic effect independent of tumor metastasis, stage and grade. Our findings further implied that miR-218 might be an important factor in tumor invasion[25]. However, one must keep in mind that the findings from multivariate analyses in our study were based on a limited number of cases and clinicopathological parameters, and require confirmation from the analysis of larger cohorts.
Many studies have tried to identify pathways through which miR-218 can regulate tumor invasion, such as IKK-β, Slits-Robo, nuclear factor-κB, Survivin, and Rictor-AKT[13,17,18,20,26]. According to these publications, the loop of Slit-miR-218-Robo has been the most studied. In a study about cervical cancer, it was demonstrated that miR-218 could inhibit tumor invasion and metastasis by targeting the Robo1 receptor[19]. Moreover, interactions between miR-218 and the Slit-Robo pathway were also demonstrated in nasopharyngeal cancer and vascular formation[26,27].
To date, there are few gastric cancer-specific biomarkers that have been used clinically for diagnosis and prediction of prognosis. Although miR-218 seems not to be a specific marker for gastric cancer, it can probably benefit patients due to its significance in prediction of tumor prognosis. From the clinical viewpoint, low expression of miR-218 might be considered as a risk factor for tumor progression, and thereby a strict systemic therapeutic strategy after surgery, such as immunotherapy, angiogenesis inhibitor drugs, chemotherapy, and radiotherapy with regular investigation might improve prognosis. Li et al[27] found a safe and highly efficient method for gene delivery to solid tumors by self-assembled supramolecular nanovesicles that shows significant potential for use in practical gene therapy. As such, we may improve the prognosis of gastric cancer patients by changing the expression of miR-218 with these simply tailored non-cytotoxic nanovesicles in future.
In summary, we found that the expression of miR-218 in serum was frequently reduced in gastric cancer, and that this expression had a close relationship with later tumor stages, metastasis and differentiation, which confirms that miR-218 is a tumor suppressor miRNA in gastric cancer. We also found that miR-218 might be a prognostic marker, which needs to be verified in further follow-up studies.
Poor expression of miRNAs reportedly plays an important role in gastric carcinogenesis. microRNA-218 (miR-218) has been shown to be a tumor suppressor miRNA in several cancers. Furthermore, large microarray assays have indicated that there is significant downregulation of miR-218 in gastric cancer.
It was reported that miR-218 was downregulated in glioma, bladder cancer, lung cancer and oral cancer. In vitro assays showed that restored expression of miR-218 enhanced tumor growth and invasion, and reduced apoptosis. In the present study, the authors demonstrated expression of miR-218 in the serum of gastric cancer patients and its relationship with clinicopathological characteristics, tumor invasion and prognosis.
Large microarray assays have indicated that there is significant downregulation of miR-218 in gastric cancer. The status of miR-218 in the serum of gastric cancer patients has not been established. This is believed to be the first study about the expression of miR-218 in the serum of gastric cancer patients and its relationship with clinicopathological characteristics, tumor invasion and prognosis. Furthermore, this study suggests that miR-218 expression in serum is a prognostic marker in gastric cancer.
By understanding the expression of miR-218 in the serum of gastric cancer patients and its relationship with clinicopathological characteristics, tumor invasion and prognosis, this study may provide a future strategy for the treatment of patients with gastric cancer.
miRNAs were discovered as a novel class of evolutionarily conserved small (18-24 nucleotides) noncoding RNA molecules that are important regulators of gene expression. miRNAs are involved in cellular proliferation, differentiation, apoptosis, angiogenesis, invasion and migration. Several tumor suppressor miRNAs are reduced in human malignancies. miR-218 is one of these, and in vitro assays showed that restored expression of miR-218 enhances tumor growth and invasion, and reduces apoptosis.
This study was interesting, with a novel approach to the molecular biology of gastric cancer. The assay method was of good quality. The number of patients and controls was adequate. This was a carefully performed study and the findings are of considerable interest. This is perhaps the first report about the expression of miR-218 in serum of gastric cancer patients, which may be important for the diagnosis, treatment and prognosis of gastric cancer.
| 1. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 2. | Davis-Dusenbery BN, Hata A. MicroRNA in Cancer: The Involvement of Aberrant MicroRNA Biogenesis Regulatory Pathways. Genes Cancer. 2010;1:1100-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 28209] [Article Influence: 1282.2] [Reference Citation Analysis (0)] |
| 4. | Ambros V. The functions of animal microRNAs. Nature. 2004;431:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7919] [Cited by in RCA: 8672] [Article Influence: 394.2] [Reference Citation Analysis (3)] |
| 5. | Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2105] [Cited by in RCA: 2391] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 6. | Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1293] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 7. | He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4964] [Cited by in RCA: 5380] [Article Influence: 244.5] [Reference Citation Analysis (0)] |
| 8. | Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1293] [Cited by in RCA: 1360] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 9. | Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1641] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 10. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7723] [Cited by in RCA: 7424] [Article Influence: 353.5] [Reference Citation Analysis (5)] |
| 11. | Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 12. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 5645] [Article Influence: 282.3] [Reference Citation Analysis (0)] |
| 13. | Uesugi A, Kozaki K, Tsuruta T, Furuta M, Morita K, Imoto I, Omura K, Inazawa J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011;71:5765-5778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 1996] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 15. | Tatarano S, Chiyomaru T, Kawakami K, Enokida H, Yoshino H, Hidaka H, Yamasaki T, Kawahara K, Nishiyama K, Seki N. miR-218 on the genomic loss region of chromosome 4p15.31 functions as a tumor suppressor in bladder cancer. Int J Oncol. 2011;39:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Wu DW, Cheng YW, Wang J, Chen CY, Lee H. Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting. Cancer Res. 2010;70:10392-10401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Song L, Huang Q, Chen K, Liu L, Lin C, Dai T, Yu C, Wu Z, Li J. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-β. Biochem Biophys Res Commun. 2010;402:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Alajez NM, Lenarduzzi M, Ito E, Hui AB, Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011;71:2381-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 19. | Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6:e1000879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 20. | Gao C, Zhang Z, Liu W, Xiao S, Gu W, Lu H. Reduced microRNA-218 expression is associated with high nuclear factor kappa B activation in gastric cancer. Cancer. 2010;116:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 22. | Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4471] [Cited by in RCA: 4560] [Article Influence: 228.0] [Reference Citation Analysis (0)] |
| 23. | Zanette DL, Rivadavia F, Molfetta GA, Barbuzano FG, Proto-Siqueira R, Silva-Jr WA, Falcão RP, Zago MA. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res. 2007;40:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 25. | Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol. 2010;116:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ Res. 2010;107:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Li W, Li H, Li J, Wang H, Zhao H, Zhang L, Xia Y, Ye Z, Gao J, Dai J. Self-assembled supramolecular nano vesicles for safe and highly efficient gene delivery to solid tumors. Int J Nanomedicine. 2012;7:4661-4677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
P- Reviewers: Li W, Huang PT, Nomura S, Szczepanik AM, Tong QS S- Editor: Qi Y L- Editor: A E- Editor: Ma S