Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6826
Revised: December 25, 2013
Accepted: February 20, 2014
Published online: June 14, 2014
Processing time: 261 Days and 21.6 Hours
Colorectal cancer (CRC) is a major health problem causing significant morbidity and mortality. Previous results from various studies indicate that CRC tumorigenicity encompasses tumor microenvironment, emphasizing the complex interacting network between cancer cells and nearby host cells, which triggers diverse signaling pathways to promote the growth and spread of cancer cells. The CCN family proteins share a uniform modular structure, mediating a variety of physiological functions, including proliferation, apoptosis, migration, adhesion, differentiation, and survival. Furthermore, CCN proteins are also involved in CRC initiation and development. Many studies have shown that CCN members, such as CCN1, CCN2, CCN3, Wnt-induced secreted protein (WISP)-1, WISP-2, and WISP-3, are dysregulated in CRC, which implies potential diagnostic markers or therapeutic targets clinically. In this review, we summarize the research findings on the role of CCN family proteins in CRC initiation, development, and progression, highlighting their potential for diagnosis, prognosis, and therapeutic application.
Core tip: Colorectal cancer (CRC) is a major health problem causing significant morbidity and mortality. Many studies have revealed that CCN members, such as CCN1, CCN2, CCN3, Wnt-induced secreted protein (WISP)-1, WISP-2, and WISP-3, are dysregulated in CRC, which implied potential diagnostic markers or therapeutic targets clinically. In this review, we summarize the research findings on the role of CCN family proteins in CRC initiation, development, and progression, highlighting their potential for diagnosis, prognosis, and therapeutic application, as well as discussing future perspectives.
- Citation: Chang CC, Lin BR, Wu TS, Jeng YM, Kuo ML. Input of microenvironmental regulation on colorectal cancer: Role of the CCN family. World J Gastroenterol 2014; 20(22): 6826-6831
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6826.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6826
Both mortality and morbidity associated with colorectal cancer (CRC) have been increasing exponentially over the past several decades, and this disease is a major health problem worldwide[1-3]. Although the regulatory events in CRC progression are diverse, microenvironment plays crucial roles in controlling CRC cell proliferation, apoptosis, replication, motility, angiogenesis, and metastasis[4-6]. Though our knowledge about the contents and interactions of the microenvironment in CRC is increasing, the cytokines within this compartment are still not well-understood. Chronic inflammation is also a key predisposing factor in CRC[7], especially inflammatory bowel disease (IBD)-related CRC. The inflammatory-related cytokines secreted from surrounding active stromal cells, immune cells, even tumor cells may stimulate the activation of more inflammation-associated molecules, such as transcription factors or microRNAs which could promote advanced colon carcinogenesis process[7,8]. Furthermore, many studies recently have shown that cancer and host cell-derived cytokines or chemokines drive the transition of tumor-associated macrophages (TAMs) towards an M2 phenotype from M1[9], which exert immnunosuppressive activity and induce cancer proliferation, apoptosis, autophagy, angiogenesis, and distal metastasis[10,11]. These signaling molecules also function to manipulate epigenetic modifications, such as DNA methylation and acetylation, which regulate post-transcriptional activities of possible downstream gene(s) in CRC initiation and progression[12]. The functions and underlying mechanisms of these cytokines are still to be clarified.
The effects of these cytokines in microenvironment are being studied including identification of the targeted and executioner cells of the cytokine, regulatory mechanism(s) of the secreted protein, and protein(s) that are involved in the process. As the understanding of the components and their interactions in microenvironment is important, we aimed to summarize the recent advances in the understanding of the molecular basis of CRC in this review.
The characters in microenvironment include cancer cells and host cells, for example, fibroblasts, TAMs, dendritic cells, lymphocytes, monocytes, endothelial and lymphatic cells. The interacting network between cancer cells and host cells is highly regulated, and is not completely defined. Receptors present within cells specifically respond to cytokines and act to form the unique microenvironment. Signaling pathways driven by growth factors, including epidermal growth factor, hepatocyte growth factor, or c-Met, and signaling proteins, such as transforming growth factor (TGF)-α, Wnt, sonic hedgehog, Notch, insulin, integrins, Src, and Ras, can significantly promote the transformation. The transformation process from single crypt lesions through colorectal adenomas to CRC can promote the spread of cancer cells to distal organs[13-20]. Moreover, signaling activates not only tumor cells, but also normal cells in the immediate environment such as TAMs, fibroblasts, and endothelial cells.
Since IBD is a paradigm of cancer-related inflammation, patients affected by IBD are at an increased risk of developing neoplasia. Transformed epithelial cells are able to secrete various inflammatory mediators, such as interleukin (IL)-1, IL-6, COX-2, and TNF-α, to affect proinflammatory leukocytes, endothelial cells, and fibroblasts to further establish a tumor-promoting microenvironment[11]. Therefore, some studies indicated that massive macrophage infiltration is correlated with CRC growth and progression. These TAMs resemble M2-polarized macrophages and have been shown to promote tissue remodeling and angiogenesis to secrete cytokines[21]. CRC cells create or modify a microenvironment that is conducive to metastasis colonization and angiogenesis, which provides a rationale for efforts to enhance cancer progression. Chemokines, cytokines, growth factors, and inflammatory mediators confer the infrastructures of CRC microenvironment, and their concentrations decide the cellular and molecular signaling transduction and functional outcome[22,23]. Thus, these small molecules orchestrate the responses to stimuli and help regulate this unique fine-tuned system.
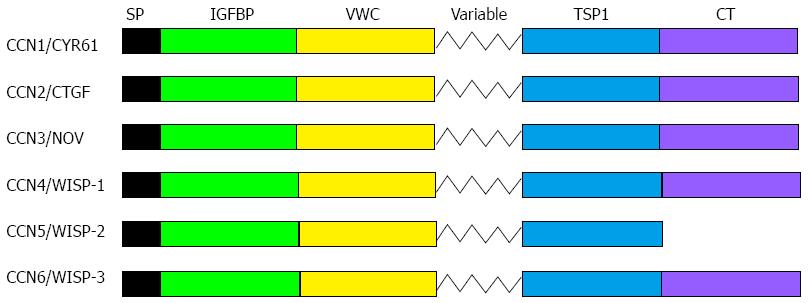
The CCN family was firstly described by P. Bork in 1993, and contains connective tissue growth factor/CCN2, cysteine-rich 61 (Cyr61/CCN1), and nephroblastoma overexpressed/CCN3, as well as Wisp-1/elm1 (CCN4), Wisp-2/rCop1 (CCN5), and Wisp-3 (CCN6). The CCN proteins all show a common multimodular organization, and contain an N-terminal signal peptide followed by four structural domains resembling insulin-like growth factor binding proteins, Von Willebrand factor, thrombospondin, and cysteine knot containing family of growth regulator-like module (CT) (Figure 1). They are involved in various physiological and pathological events, including proliferation, apoptosis, migration, adhesion, differentiation, and survival[24,25]. They also participate in the development of connective tissue such as cartilage and bone, nervous system, muscle, kidney, and bone marrow. Moreover, wound healing, bone fracture repair, pathological fibrosis, and tumorigenesis are all regulated by CCN proteins[26-28]. Recently, many studies have shown that these proteins play crucial roles in CRC progression, including cell migration, invasion, adhesion, and distal metastasis. In this paper we review the current knowledge regarding the implication of CCN proteins in CRC.
CCN proteins are believed to be multifunctional signaling molecules, and have been found to be involved in a variety of CRC initiation and development events. Experimental data indicated that CCN1 (also known as Cyr61) overexpression increased Matrigel invasion in vitro, which required integrin αvβ5, and promoted lung metastasis formation in vivo[29]. Moreover, local recurrence after radiotherapy in CRC often occurs within preirradiated stroma, and CCN1 has been found to be overexpressed in these areas and correlated with invasion and metastasis[29]. However, CCN1 is not highly expressed in advanced stages of CRC, and one may suggest that CCN1 may be crucial in the early stage of CRC development and play a role as an early prognostic biomarker[30,31]. CCN1 is an angiogenic factor, and may function through the ability of CCN1 to bind and activate cell surface integrins[32]. Using SW620, H460, and TE-7 cell lines and their isogenic variants with altered CCN1 expression, Jandova et al[33] in 2012 had proved that migration of CRC cells is CCN1- and αώβ5-dependent.
CCN2 (also named CTGF), a 36-38 kD cysteine-rich peptide containing 349 amino acids, is predominantly identified in fibroblasts, endothelial cells, smooth muscle cells, and cartilaginous cells[34]. TGF-β enhances CCN2 synthesis, and CCN2 is a typical downstream mediator of this major inflammatory mediator[34,35]. Although these two proteins share many functions in common, there are still many aspects of tumor regulation which are different. We have found that CCN2 inhibits CRC metastasis and acts as an independent prognostic marker. Mechanistically, CCN2 could inhibit the β-catenin/TCF signaling pathway and cause matrix metalloproteinase 7 down-regulation[36].
Clinically, peritoneal carcinomatosis (PC) has a very poor prognosis and is treated palliatively. CCN2 has been suggested to be a therapeutic agent, and can be used as a predictor of PC in CRC[37]. Low CCN2 expression in tumor samples was associated with an 8-fold increase in the peritoneal recurrence rate compared with tumors with high levels of CCN2 expression[37]. CCN2 alters cellular function, including adhesion which is the first and most crucial step of PC. CCN2 treatment could enhance cell adhesion in normal fibroblast 293T cells, but significantly decreased CRC adhesion ability[37] in vitro and in vivo. Although high expression of CCN2 is the hallmark of good prognosis of CRC, its roles in CRC cell differentiation and proliferation are still under investigation. In previous studies, Cunningham et al[38] showed that CCN2 expression was positively correlated with α-smooth muscle actin expression, which in turn indicated a potential role for CCN2 in myofibroblast-mediated fibrosis associated with ileal carcinoids. Moreover, Kaltsas et al[39] in 2010 demonstrated that CCN2 involved in the neoplastic transformation into ileal carcinoids is positively correlated with tumours larger than 1 cm. Jacobson et al[40] in 2012 also indicated that CCN2 expression is the hallmark of ileal carcinoids, and potentially is highly related to several functions including cell migration and anti-apoptosis, which proposes an oncogenic role of CCN2 in the progression of well-differentiated CRC and other tumors.
Wnt-induced secreted protein (WISP)-1 is the fourth member of CCN family, which was identified to be a Wnt-1- and β-catenin-regulated protein[41-44]. WISP-1 transcript was reported to be overexpressed in 80 % of human colon carcinomas[43], and immunohistochemistry studies of WISP-1 further supported this result. A comparative study of 47 CRCs exhibited positive interplays between Wnt-1, WISP-1, survivin, and cyclin-D1 proteins in CRC tumorigenicity[41]. Furthermore, WISP-1 may be used as a specific diagnostic and prognostic marker in CRC[42]. However, the precise underlying mechanism is still lacking and needs further investigations. WISP-2 and WISP-3 are parts of the CCN family, and have been showed to play crucial roles in angiogenesis and carcinogenesis[32]. The mRNA expression of WISP-2, but not WISP-1, was significantly decreased in CRC, compared to normal colonic mucosa[43]. Davies et al[44] in 2010 also showed that WISP-2 demonstrated the opposite pattern with lower levels of expression in CRC cancer cells compared to normal controls. The WISP-2 gene is considered a tumor suppressor gene, however, the molecular mechanism is not defined. The WISP-3 gene is located on 6q22-6q23, and its cellular function is linked to chondrocyte growth and cartilage integrity[45]. Previous studies showed that WISP-3 could be an oncogene in CRC[43,44], especially microsatellite instability subtype of CRC[25]. However, WISP-3 transcript levels showed no significant differences between cancer and normal groups[44]. According to the findings, WISPs may play crucial but contrasting roles in CRC, which demonstrated that WISP-1 could be an oncogene, but WISP-2 might tend to be a tumor suppressor gene and WISP-3 gene still needs further clarification (Table 1).
| Capital member | Model | Effects | Ref. |
| CCN1 | Periradiated stroma | Positively correlated with metastasis | [29] |
| CCN1 | Clinical sample | Positively correlated with early stage of tumor development | [30,31] |
| CCN1 | Cultured cancer cells | Promoting cancer cell migration | [33] |
| CCN2 | Clinical sample | Positively correlated with early stage of tumor development | [36,37,40] |
| Negatively correlated with prevalence of peritoneal carcinomatosis | |||
| Inhibiting invasion and metastasis | |||
| Negatively correlated with metastasis and patient survival | |||
| WISP-1 | Clinical sample | Promoting cell cycle checkpoint progression, accelerating cell growth and inhibiting apoptosis | [41-45] |
| Positively correlated with tumor grade | |||
| WISP-2 | Clinical sample | Negatively correlated with tumor grade | [43,44] |
| WISP-3 | Clinical sample | Positively correlated with tumor grade | [43,44] |
Microenvironmental regulation is crucial in cancer biology. In this compartment, many effecter host cells, immune cells, cytokines, chemokines, inflammatory proteins, besides intestinal microbiota (not discussed), are orchestrated to form the infrastructure of CRC. The interaction is complicated and but has potential therapeutic applications. In this review, we have extensively discussed the secreted proteins called CCN family, which function in many physiological and pathological processes, and showed their important regulatory roles in CRC microenvironment. Although genetic and epigenetic alternations drive the transformation of normal enterocytes into neoplasia, CCN family proteins mediate significant communications between CRC and host cells.
Understanding the detailed mechanisms involved in CCN-mediated regulation will provide further insight into the progression and metastasis of CRC. Furthermore, the utility of CCN family proteins to regulate metastasis and invasion/angiogenesis suggests that these growth factors may be relevant candidates or targets for CRC treatment.
| 1. | Boyle P. Progress in preventing death from colorectal cancer. Br J Cancer. 1995;72:528-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Tchekmedyian A, Messuti A, Richelli R, Stein S, Silveira A, Iade B, Cohen H. Colorectal cancer prevention. Am J Gastroenterol. 2009;104:S551-S576. [DOI] [Full Text] |
| 3. | Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 518] [Article Influence: 30.5] [Reference Citation Analysis (4)] |
| 4. | van der Bij GJ, Bögels M, Oosterling SJ, Kroon J, Schuckmann DT, de Vries HE, Meijer S, Beelen RH, van Egmond M. Tumor infiltrating macrophages reduce development of peritoneal colorectal carcinoma metastases. Cancer Lett. 2008;262:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Schwitalla S, Ziegler PK, Horst D, Becker V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer R, Slotta-Huspenina J. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Sund M, Zeisberg M, Kalluri R. Endogenous stimulators and inhibitors of angiogenesis in gastrointestinal cancers: basic science to clinical application. Gastroenterology. 2005;129:2076-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Schottelius AJ, Dinter H. Cytokines, NF-kappaB, microenvironment, intestinal inflammation and cancer. Cancer Treat Res. 2006;130:67-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Okayama H, Schetter AJ, Harris CC. MicroRNAs and inflammation in the pathogenesis and progression of colon cancer. Dig Dis. 2012;30 Suppl 2:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 12. | Lee JH, Kang MJ, Han HY, Lee MG, Jeong SI, Ryu BK, Ha TK, Her NG, Han J, Park SJ. Epigenetic alteration of PRKCDBP in colorectal cancers and its implication in tumor cell resistance to TNFα-induced apoptosis. Clin Cancer Res. 2011;17:7551-7562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Espinoza LA, Tone LG, Neto JB, Costa RS, Wang QJ, Ballejo G. Enhanced TGFalpha-EGFR expression and P53 gene alterations contributes to gastric tumors aggressiveness. Cancer Lett. 2004;212:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Xie Q, Liu KD, Hu MY, Zhou K. SF/HGF-c-Met autocrine and paracrine promote metastasis of hepatocellular carcinoma. World J Gastroenterol. 2001;7:816-820. [PubMed] |
| 15. | Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2790] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, Li CW, Hsu JL, Miller SA, Wang X. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 17. | Zheng Y, de la Cruz CC, Sayles LC, Alleyne-Chin C, Vaka D, Knaak TD, Bigos M, Xu Y, Hoang CD, Shrager JB. A rare population of CD24(+)ITGB4(+)Notch(hi) cells drives tumor propagation in NSCLC and requires Notch3 for self-renewal. Cancer Cell. 2013;24:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Thomopoulos P, Roth J, Lovelace E, Pastan I. Insulin receptors in normal and transformed fibroblasts: relationship to growth and transformation. Cell. 1976;8:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 464] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Chiariello M, Marinissen MJ, Gutkind JS. Regulation of c-myc expression by PDGF through Rho GTPases. Nat Cell Biol. 2001;3:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Huang D, Du X. Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World J Gastroenterol. 2008;14:1823-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (10)] |
| 22. | Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1031] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 23. | Royston D, Jackson DG. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J Pathol. 2009;217:608-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Chang CC, Yang MH, Lin BR, Chen ST, Pan SH, Hsiao M, Lai TC, Lin SK, Jeng YM, Chu CY. CCN2 inhibits lung cancer metastasis through promoting DAPK-dependent anoikis and inducing EGFR degradation. Cell Death Differ. 2013;20:443-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Thorstensen L, Diep CB, Meling GI, Aagesen TH, Ahrens CH, Rognum TO, Lothe RA. WNT1 inducible signaling pathway protein 3, WISP-3, a novel target gene in colorectal carcinomas with microsatellite instability. Gastroenterology. 2001;121:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 27. | French DM, Kaul RJ, D’Souza AL, Crowley CW, Bao M, Frantz GD, Filvaroff EH, Desnoyers L. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004;165:855-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Tsai MS, Bogart DF, Castañeda JM, Li P, Lupu R. Cyr61 promotes breast tumorigenesis and cancer progression. Oncogene. 2002;21:8178-8185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Monnier Y, Farmer P, Bieler G, Imaizumi N, Sengstag T, Alghisi GC, Stehle JC, Ciarloni L, Andrejevic-Blant S, Moeckli R. CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma. Cancer Res. 2008;68:7323-7331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Ladwa R, Pringle H, Kumar R, West K. Expression of CTGF and Cyr61 in colorectal cancer. J Clin Pathol. 2011;64:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 31. | Hong Y, Ho KS, Eu KW, Cheah PY. A susceptibility gene set for early onset colorectal cancer that integrates diverse signaling pathways: implication for tumorigenesis. Clin Cancer Res. 2007;13:1107-1114. [PubMed] |
| 32. | Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 410] [Article Influence: 17.8] [Reference Citation Analysis (3)] |
| 33. | Jandova J, Beyer TE, Meuillet EJ, Watts GS. The matrix protein CCN1/CYR61 is required for α(V)β5-mediated cancer cell migration. Cell Biochem Funct. 2012;30:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 34. | Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA. 1999;96:1603-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 614] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 35. | Gradl D, Kühl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576-5587. [PubMed] |
| 36. | Lin BR, Chang CC, Che TF, Chen ST, Chen RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005;128:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Lin BR, Chang CC, Chen RJ, Jeng YM, Liang JT, Lee PH, Chang KJ, Kuo ML. Connective tissue growth factor acts as a therapeutic agent and predictor for peritoneal carcinomatosis of colorectal cancer. Clin Cancer Res. 2011;17:3077-3088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Cunningham JL, Tsolakis AV, Jacobson A, Janson ET. Connective tissue growth factor expression in endocrine tumors is associated with high stromal expression of alpha-smooth muscle actin. Eur J Endocrinol. 2010;163:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Kaltsas GA, Cunningham JL, Falkmer SE, Grimelius L, Tsolakis AV. Expression of connective tissue growth factor and IGF1 in normal and neoplastic gastrointestinal neuroendocrine cells and their clinico-pathological significance. Endocr Relat Cancer. 2011;18:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Jacobson A, Cunningham JL. Connective tissue growth factor in tumor pathogenesis. Fibrogenesis Tissue Repair. 2012;5:S8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Khor TO, Gul YA, Ithnin H, Seow HF. A comparative study of the expression of Wnt-1, WISP-1, survivin and cyclin-D1 in colorectal carcinoma. Int J Colorectal Dis. 2006;21:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Tian C, Zhou ZG, Meng WJ, Sun XF, Yu YY, Li L, Luo HZ, Yang L, Zhou B, Gu J. Overexpression of connective tissue growth factor WISP-1 in Chinese primary rectal cancer patients. World J Gastroenterol. 2007;13:3878-3882. [PubMed] |
| 43. | Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717-14722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 413] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 44. | Davies SR, Davies ML, Sanders A, Parr C, Torkington J, Jiang WG. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010;36:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Davis L, Chen Y, Sen M. WISP-3 functions as a ligand and promotes superoxide dismutase activity. Biochem Biophys Res Commun. 2006;342:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
P- Reviewers: Bocci G, Deutsch JC, de la Cadena MP, Kir G S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Zhang DN