Published online Jan 14, 2014. doi: 10.3748/wjg.v20.i2.532
Revised: September 30, 2013
Accepted: October 17, 2013
Published online: January 14, 2014
Processing time: 162 Days and 3.9 Hours
AIM: To evaluate the changing trends and outcomes of colorectal cancer (CRC) surgery performed at a large single institution in Taiwan.
METHODS: This study retrospectively analyzed 778 patients who received colorectal cancer surgery at E-Da Hospital in Taiwan from 2004 to 2009. These patients were from health examination, inpatient or emergency settings. The following attributes were analyzed in patients who had undergone CRC surgical procedures: gender, age, source, surgical type, tumor number, tumor size, number of lymph node metastasis, pathologic differentiation, chemotherapy, distant metastases, tumor site, tumor stage, average hospitalization cost and average lengths of stay (ALOS). The odds ratio and 95% confidence intervals were calculated to assess the relative rate of change. Regression models were employed to predict average hospitalization cost and ALOS.
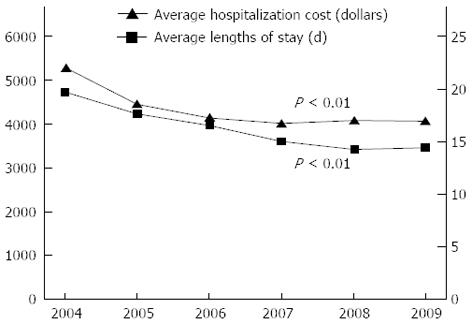
RESULTS: The study sample included 458 (58.87%) males and 320 (41.13%) females with a mean age of 64.53 years (standard deviation, 12.33 years; range, 28-86 years). The principal patient source came from inpatient and emergency room (96.02%). The principal tumor sites were noted at the sigmoid colon (35.73%) and rectum (30.46%). Most patients exhibited a tumor stage of 2 (37.28%) or 3 (34.19%). The number of new CRC surgeries performed per 100000 persons was 12.21 in 2004 and gradually increased to 17.89 in 2009, representing a change of 46.52%. During the same period, the average hospitalization cost and ALOS decreased from $5303 to $4062 and from 19.7 to 14.4 d, respectively. The following factors were associated with considerably decreased hospital resource utilization: age, source, surgical type, tumor size, tumor site, and tumor stage.
CONCLUSION: These results can be generalized to patient populations elsewhere in Taiwan and to other countries with similar patient profiles.
Core tip: We evaluated the trend of colorectal cancer surgery and compared hospitalization cost and length of stay with those in other countries. Age, source, surgical type, tumor size, tumor site, and tumor stage were associated with decreased hospital resource utilization. To efficiently allocate of medical resources, these factors must be managed.
- Citation: Perng DS, Lu IC, Shi HY, Lin CW, Liu KW, Su YF, Lee KT. Incidence trends and predictors for cost and average lengths of stay in colorectal cancer surgery. World J Gastroenterol 2014; 20(2): 532-538
- URL: https://www.wjgnet.com/1007-9327/full/v20/i2/532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i2.532
Colorectal cancer (CRC) is one of the leading causes of cancer-related death in the world[1]. In a recent data analysis from cancer registries participating in the Surveillance, Epidemiology, and End Results program, 148810 new cases of CRC with 49960 deaths were estimated for the United States in 2008[2]. In Europe, CRC was the second most common form of cancers and cause of death from cancer in 2006[3]. By contrast, incidence rates tend to be lower in Africa and Asia, which is attributed to differences in diet and lifestyle. Moreover, the medical expenses associated with CRC cannot be ignored[4,5]. The estimated medical expense for a patient with CRC in the United States of America per year is $38577, which is seven times higher than that for a patient without CRC ($5126)[6].
The effectiveness of CRC surgery in relieving pain and improving physical function has been well documented[7,8]. In addition to improved surgical techniques, the excellent performance of new materials and designs has substantially increased the demand for CRC surgery. The growing population of elderly patients is yet another factor[9]. Because the system provides insurance coverage for expensive and frequently used medical items, the financial burden of CRC surgery should not be overlooked.
Although the volume of CRC surgical procedures is increasing annually, the incidence rates and hospital resource utilization for these procedures have not been documented in a Taiwan study. Thus, this study explored the changing trends and risk factors of these outcomes for CRC surgery.
E-Da Hospital is a 1200-bed hospital, a large medical institution in Taiwan, and provides secondary and tertiary medical care for approximately one million people. A retrospective review of all patients who underwent CRC surgery from 2004 to 2009 was performed. If patients had intraoperative perforations, concurrent malignancy, or a psychological or linguistic impairment, they were excluded. All malignancies were confirmed upon histological evaluation. The study analyzed 778 CRC surgical procedures. The study protocol was approved by the Institutional Review Board of E-Da Hospital.
The following attributes were analyzed in patients who had undergone CRC surgical procedures in Taiwan: gender, age, source, surgical type, tumor number, tumor size, number of lymph node metastasis, pathologic differentiation, chemotherapy, distant metastases, tumor site, tumor stage, average hospitalization cost and average lengths of stay (ALOS). The age categories were ≤ 30, 31-40, 41-50, 51-60, 61-70, 71-80 and ≥ 81 years old. The patient source came from health examination, inpatient and emergency room settings. The surgical types were grouped as low anterior resection, high anterior resection, abdominoperineal resection, right hemicolectomy, left hemicolectomy, super low anterior resection and endoscopic polypectomy. Pathologic differentiation was classified as well, moderate or poor. The patients were grouped by tumor site as follows: ascending colon, transverse colon, descending colon, sigmoid colon and rectum. Tumor stage was categorized as 1, 2, 3 or 4. All colorectal cancers were staged according to the guidelines of the American Joint Committee of Cancer.
Continuous variables were tested for statistical significance by one-way analysis of variance (ANOVA), and categorical variables were tested by χ2 analysis. Temporal trends were assessed by the Cochrane-Armitage trend test. The study period was divided into three equal time intervals (P1: 2004-2005; P2: 2006-2007; and P3: 2008-2009). The odds ratio and 95% confidence interval were determined to assess the relative change for each factor when using P1 as the reference group when compared to P3. We define the incidence rate as the number of new cases of colorectal cancer surgery from health examination, inpatient or emergency room divided by the total number of cases from those settings[10].
Regarding treatment costs, the standard administrative claims data required by the Bureau of National Health Insurance (BNHI) included the following fees: operating room, radiology, physical therapy, hospital room, anesthetist, pharmacy, laboratory, special materials, surgeon, and others. To reflect changes in real dollar value, the cost data were adjusted by the consumer price index for each year from 2004-2009 (93.70, 95.86, 96.43, 98.17, 101.63 and 100.00). The hospital treatment costs were then converted from Taiwan dollars to United States dollars at an exchange rate of 31.5:1, which was the average exchange rate during 2004-2009. The hospital treatment costs at different hospital levels were also adjusted for differences in BNHI reimbursements. The multiple regression models used to predict average hospitalization cost and ALOS included both patient and clinical attributes.
Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, United States). All of the tests were two-sided, and P values less than 0.05 were considered statistically significant.
The study sample included 458 (58.87%) males and 320 (41.13%) females with a mean age of 64.53 years (standard deviation, 12.33 years; range, 28-86 years). The principal patient source came from inpatient and emergency rooms (96.02%). The principal surgical types for the study population were low anterior resection, right hemicolectomy, super low anterior resection, left hemicolectomy, high anterior resection, abdominoperineal resection, and endoscopic polypectomy at the following frequencies: 25.32%, 23.78%, 17.74%, 12.20%, 10.54%, 6.56%, and 3.86%, respectively. On average, the number of tumors, size of the tumor, and number of lymph node metastases were (mean ± SD) 1.05 ± 0.29, 5.06 ± 2.14, and 3.56 ± 5.15, respectively. Pathologic differentiation included the following classifications: moderate (82.01%), well (11.05%), and poor (6.94%). In the study, 52.70% cases received chemotherapy after colorectal cancer surgery, and distant metastases were not found in 93.44% cases when these patients were included in the study. The principal tumor site was described in the sigmoid colon (35.73%) and rectum (30.46%). Most patients exhibited a tumor stage of 2 (37.28%) or 3 (34.19%). Additionally, the average treatment costs were US$4285 ± 2845.4, and the ALOS was 15.40 ± 8.12 d. The detailed patient characteristics are shown in Table 1.
| Variable | n (%) |
| Gender | |
| Female | 320 (41.13) |
| Male | 458 (58.87) |
| Age (yr) | 64.53 ± 12.33 |
| ≤ 30 | 9 (1.16) |
| 31-40 | 23 (2.96) |
| 41-50 | 62 (7.97) |
| 51-60 | 187 (24.04) |
| 61-70 | 218 (28.02) |
| 71-80 | 219 (28.15) |
| ≥ 81 | 60 (7.71) |
| Source | |
| Health examination | 31 (3.98) |
| Inpatient or emergency room | 747 (96.02) |
| Surgical type | |
| Low anterior resection | 197 (25.32) |
| High anterior resection | 82 (10.54) |
| Abdominoperineal resection | 51 (6.56) |
| Right hemicolectomy | 185 (23.78) |
| Left hemicolectomy | 95 (12.2) |
| Super low anterior resection | 138 (17.74) |
| Endoscopic polypectomy | 30 (3.86) |
| Tumor number | 1.05 ± 0.29 |
| Tumor size | 5.06 ± 2.14 |
| No. of lymph node metastases | 3.56 ± 5.15 |
| Pathologic differentiation | |
| Well | 86 (11.05) |
| Moderate | 638 (82.01) |
| Poor | 54 (6.94) |
| Chemotherapy | |
| No | 368 (47.30) |
| Yes | 410 (52.70) |
| Distant metastasis | |
| No | 727 (93.44) |
| Yes | 51 (6.56) |
| Tumor site | |
| Ascending colon | 145 (18.64) |
| Transverse colon | 67 (8.61) |
| Descending colon | 51 (6.56) |
| Sigmoid colon | 278 (35.73) |
| Rectum | 237 (30.46) |
| Tumor stage | |
| 1 | 144 (18.51) |
| 2 | 290 (37.28) |
| 3 | 266 (34.19) |
| 4 | 78 (10.03) |
| Treatment cost (dollars) | 128543.94 ± 85360.59 |
| Average length of stay (d) | 15.40 ± 8.12 |
The incidence rate of CRC surgery in 2004 was 12.21 per 100000 persons, and the rate gradually increased to 17.89 in 2009, which represented a change of 46.52% (Table 2). A significant decreased trends analysis was also observed in average hospitalization cost and ALOS in CRC surgery patients during the study period (P < 0.01) (Figure 1).
| Year | Patients (n) | Surgeries (n) | Incidence rate (1/105) |
| 2004 | 245734 | 30 | 12.21 |
| 2005 | 621243 | 99 | 15.94 |
| 2006 | 727286 | 158 | 21.72 |
| 2007 | 782659 | 136 | 17.38 |
| 2008 | 825907 | 199 | 24.09 |
| 2009 | 877791 | 157 | 17.89 |
Table 3 shows the increasing volume of CRC surgical procedures and the changes in patient demographic and clinical characteristics. Approximately 40% of all CRC surgery patients treated from P1 to P3 were female, and the number of female patients significantly increased between P1 and P3 (OR = 1.13, 95%CI: 1.01-1.25). Conversely, the number of male patients significantly decreased. The number of CRC surgery patients younger than 30 years old significantly decreased between P1 and P3 (OR = 0.46, 95%CI: 0.33-0.60). The number of CRC surgery patients aged 51 to 60 years old significantly increased from P1 to P3 (OR = 1.78, 95%CI: 1.58-1.97) but the number of CRC surgery patients aged 61 to 70 years old significantly decreased (OR = 0.71, 95%CI: 0.56-0.87). The number of CRC surgery patients older than 81 years old significantly increased between P1 and P3 (OR = 1.22, 95%CI: 1.06-1.37). The number of CRC surgery patients from health examinations significantly increased (OR = 5.78, 95%CI: 5.48-6.08). In terms of surgical type, the data revealed a statistically significant decrease in the number of patients with low anterior resection and super low anterior resection between P1 and P3 (OR = 0.02, 95%CI: 0.00-0.04 and OR = 0.57, 95%CI: 0.38-0.76, respectively) but a statistically significant increase in the number of patients with high anterior resection, abdominoperineal resection and right hemicolectomy between P1 and P3 (OR = 5.49, 95%CI: 5.19-5.82; OR = 3.76, 95%CI: 3.54-3.99; and OR = 1.16, 95%CI: 1.00-1.36, respectively). The number of CRC surgery patients who exhibited well-differentiated tumors significantly decreased (OR = 0.75, 95%CI: 0.51-0.92). The number of CRC surgery patients without distant metastases significantly increased from P1 to P3 (OR = 1.16, 95%CI: 1.05-1.31) while the number of CRC surgery patients with distant metastases significantly decreased (OR = 0.20, 95%CI: 0.02-0.40). The number of CRC surgery patients with tumors at the sigmoid colon significantly increased from P1 to P3 (OR = 1.75, 95%CI: 1.43-2.23) while the number of CRC surgery patients with tumors at the rectum significantly decreased (OR = 0.64, 95%CI: 0.42-0.93). The number of CRC surgery patients with stage 1 tumors significantly increased from P1 to P3 (OR = 1.39, 95%CI: 1.12-1.74).
| Variables | P1 (n = 118) | P2 (n = 273) | P3 (n = 387) | OR (95%CI) | |
| Gender | Female | 35.59 | 44.69 | 40.31 | 1.13 (1.01-1.25) |
| Male | 64.41 | 55.31 | 59.69 | 0.93 (0.83-1.06) | |
| Age (yr) | ≤ 30 | 1.69 | 1.47 | 0.78 | 0.46 (0.33-0.60) |
| 31-40 | 2.54 | 3.30 | 2.84 | 1.12 (0.96-1.28) | |
| 41-50 | 5.93 | 10.62 | 6.72 | 1.13 (0.99-1.27) | |
| 51-60 | 16.10 | 20.88 | 28.68 | 1.78 (1.58-1.97) | |
| 61-70 | 35.59 | 28.94 | 25.06 | 0.71 (0.56-0.87) | |
| 71-80 | 31.36 | 27.47 | 27.65 | 0.88 (0.73-1.03) | |
| ≥ 81 | 6.78 | 7.33 | 8.27 | 1.22 (1.06-1.37) | |
| Source | Health examination | 0.85 | 4.03 | 4.91 | 5.78 (5.48-6.08) |
| Inpatient or emergency room | 99.15 | 95.97 | 95.09 | 0.96 (0.74-1.18) | |
| Surgical type | Low anterior resection | 35.59 | 25.64 | 0.62 | 0.02 (0.00-0.04) |
| High anterior resection | 3.39 | 2.2 | 18.60 | 5.49 (5.19-5.82) | |
| Abdominoperineal resection | 2.54 | 4.03 | 9.56 | 3.76 (3.54-3.99) | |
| Right hemicolectomy | 20.34 | 25.64 | 23.51 | 1.16 (1.00-1.36) | |
| Left hemicolectomy | 14.41 | 12.09 | 11.63 | 0.81 (0.61-1.05) | |
| Super low anterior resection | 21.19 | 24.18 | 12.14 | 0.57 (0.38-0.76) | |
| Endoscopic polypectomy | 2.54 | 6.23 | 2.58 | 0.31 (0.05-1.77) | |
| Pathologic differentiation | Well | 14.41 | 9.89 | 10.85 | 0.75 (0.51-0.92) |
| Moderate | 77.97 | 82.78 | 82.69 | 1.06 (0.82-1.27) | |
| Poor | 7.63 | 7.33 | 6.46 | 0.85 (0.59-1.05) | |
| Chemotherapy | No | 52.54 | 38.1 | 52.20 | 0.99 (0.70-1.37) |
| Yes | 47.46 | 61.9 | 47.80 | 1.01 (0.80-1.28) | |
| Distant metastasis | No | 83.05 | 93.41 | 96.64 | 1.16 (1.05-1.31) |
| Yes | 16.95 | 6.59 | 3.36 | 0.20 (0.02-0.40) | |
| Tumor site | Ascending colon | 18.64 | 19.05 | 18.35 | 0.98 (0.75-1.19) |
| Transverse colon | 10.17 | 7.69 | 8.79 | 0.86 (0.70-1.06) | |
| Descending colon | 6.78 | 6.96 | 6.20 | 0.91 (0.79-1.14) | |
| Sigmoid colon | 22.88 | 35.16 | 40.05 | 1.75 (1.43-2.23) | |
| Rectum | 41.53 | 31.14 | 26.61 | 0.64 (0.42-0.93) | |
| Tumor stage | 1 | 15.25 | 16.12 | 21.19 | 1.39 (1.12-1.74) |
| 2 | 38.98 | 35.16 | 38.24 | 0.98 (0.78-1.19) | |
| 3 | 38.98 | 32.60 | 33.85 | 0.87 (0.56-1.14) | |
| 4 | 6.78 | 6.12 | 6.72 | 0.99 (0.80-1.29) |
Table 4 shows the multiple regression models used to evaluate the predictors for hospital resource utilization. After controlling for time, the statistically significant predictors for hospital resource utilization among CRC surgery patients were age, source, surgical type, tumor size, tumor site, and tumor stage (P < 0.05).
| Variables | Average hospitalization cost2 | Average lengths of stay3 | |||||
| Coefficient | Standard coefficient | P value | Coefficient | Standard coefficient | P value | ||
| Gender | Female | 0.01 | 0.02 | 0.344 | 0.02 | 0.05 | 0.140 |
| Age | 0.01 | 0.08 | < 0.001 | 0.01 | 0.10 | 0.010 | |
| Source | Inpatient or emergency room | -0.17 | -0.11 | < 0.001 | 0.10 | 0.10 | 0.010 |
| Surgical type | High anterior resection | -0.03 | -0.03 | 0.168 | -0.06 | -0.11 | 0.009 |
| Abdominoperineal resection | 0.03 | 0.03 | 0.213 | 0.05 | 0.07 | 0.090 | |
| Right hemicolectomy | -0.01 | -0.01 | 0.891 | 0.01 | 0.02 | 0.816 | |
| Left hemicolectomy | -0.01 | -0.01 | 0.625 | -0.01 | -0.02 | 0.707 | |
| Super low anterior resection | 0.02 | 0.02 | 0.478 | 0.01 | 0.02 | 0.687 | |
| Endoscopic polypectomy | -1.16 | -0.75 | < 0.001 | -0.69 | -0.14 | < 0.001 | |
| Tumor number | 0.03 | 0.03 | 0.110 | -0.01 | -0.01 | 0.805 | |
| Tumor size | 0.01 | 0.06 | 0.010 | 0.01 | 0.07 | 0.062 | |
| No. of lymph node metastases | 0.01 | -0.01 | 0.568 | 0.01 | 0.01 | 0.997 | |
| Pathologic differentiation | Moderate | 0.04 | 0.05 | 0.104 | 0.04 | 0.07 | 0.126 |
| Poor | 0.05 | 0.04 | 0.139 | 0.04 | 0.05 | 0.289 | |
| Chemotherapy | Yes | -0.01 | 0.02 | 0.345 | -0.02 | -0.05 | 0.239 |
| Distant metastasis | Yes | 0.01 | 0.01 | 0.903 | 0.04 | 0.06 | 0.117 |
| Tumor site | Transverse colon | 0.01 | 0.01 | 0.726 | 0.09 | 0.14 | 0.003 |
| Descending colon | 0.02 | 0.02 | 0.557 | 0.04 | 0.06 | 0.287 | |
| Sigmoid colon | 0.01 | 0.02 | 0.730 | 0.06 | 0.15 | 0.149 | |
| Rectum | 0.03 | 0.04 | 0.514 | 0.04 | 0.11 | 0.314 | |
| Tumor stage | 2 | 0.04 | 0.06 | 0.076 | 0.03 | 0.07 | 0.198 |
| 3 | 0.05 | 0.08 | 0.019 | 0.03 | 0.08 | 0.177 | |
| 4 | 0.17 | 0.17 | < 0.001 | 0.09 | 0.15 | 0.002 | |
This large survey study is the first to examine how patient and clinical attributes reflect changing trends in the incidence of CRC surgery and the first to identify factors that predict average hospitalization costs and ALOS for the procedure. This study showed a gradual increase in the incidence of new CRC surgeries during the study period and, during the same period, a demographic decrease in hospital resource utilization, which is consistent with other series studies. The following factors were associated with the considerably decreased hospital resource utilization of CRC surgery: age, source, surgical type, tumor size, tumor site, distant metastasis, and tumor stage.
Old age is a risk factor for the occurrence of CRC[2,11,12]. However, according to the results of this study, we found a trend of increasing incidence of CRC in patients aged 51-60 years old from the first to third time period. In the first time period, the percentage of 51-60 years old patients with CRC is only 16.10%, but this incidence increases to 20.88% and 28.68% in the second and third time periods. The increasing incidence of CRC is obvious and substantial in early old age from 51-60 years of age. From the first to third time period, the other difference is the increased rate of CRC cases from health examinations. In the first time period, the percentage of CRC cases from health examinations is less then 1%, but this incidence increases to nearly 5% in the third time period. We also noted that the percentage of state 1 tumor cases increases from the first to third time period. The incidence of colorectal cancer from 2004 to 2009 is between 12.21 per 100000 per year and 24.09 per 100000 per year in this study. In another study, the median unadjusted incidence of colorectal cancer from 1989 to 2008 was 6.17 per 100000 per year in South Asians compared with 71.70 per 100000 per year in non-South Asians (77.79% white British)[13].
In the results from this study, we note that CRC cases at an older age and with stage 4 tumors increase the average hospitalization cost and average lengths of stay. However, if the CRC cases receive endoscopic polypectomy, the average hospitalization cost and average lengths of stay decrease. Most of the CRC cases who receive endoscopic polypectomy are from the health examinations, demonstrating both cost and health benefits for the patients who receive health examinations. However, in our study, surgical types including high anterior resection, abdominoperineal resection, right hemicolectomy, left hemicolectomy and super low anterior resection were done by open approach. Other studies show have documented that the laparoscopic surgery is safe, feasible and less traumatic, with an oncological adequacy comparable to the open approach, a shorter compromise of immunological homeostasis and faster recovery[14-18]. Furthermore, another meta-analysis study showed the robotic surgery for rectal cancer is superior to laparoscopic surgery for treatment of rectal cancer[19,20].
A previous study showed that colorectal cancer that originates from a different site may provide additional prognostic information[21]. In our study, the trend analysis showed an increasing percentage of sigmoid colon cancer, from 22.88% at time period 1 to 40.05% at time period 3, and the multiple regression mode showed that tumors at transverse colon were a significant factor leading to increased ALOS.
According to the result of this study, we note that CRC patients with or without distal metastases have no statistically significant difference between the average hospitalization cost and ALOS. An increasing tumor size elevates the average hospitalization cost but dose not significantly affect ALOS. These findings may be due to the limited case numbers, which affected out ability to demonstrate the effect of distal metastases and tumor size on ALOS for CRC patients.
A study in Brazil showed the increasing hospital admission rates and economic burden for colorectal cancer from 1996 to 2008[22]. Our results reveal an approximate four times greater average hospital cost for patients who received colorectal cancer surgery in Taiwan compared to Brazil, but the average hospitalization costs and length of stay decrease each year in Taiwan. The median length of stay was 14 d for elective admissions in Ireland from 2002 to 2008[23]. Our study shows a similar length of stay of 15.40 d. Compared to the conditions in the United States and Europe (between March and November 2003 in the United Kingdom, France, Germany, Italy, and Spain, and between September 2003 and October 2004 in the United States), the ALOS for a colonic operation is shortest in the United States at 7.8 d, while the ALOS for a colonic operation in countries in Europe, including France, Italy, United Kingdom, Spain and Germany, is 12.8-16.5 d[24]. The ALOS is similar between Taiwan and Europe. In Taiwan, the pre-operative preparation, such as radiology examination, biochemical laboratory examination, electrocardiogram and colon preparation, are arranged and performed after admission, so the average length of stay is longer in Taiwan than in the United States. Another reason that may contribute to the longer average length of stay in Taiwan is the health insurance. Public citizens in Taiwan have well-care health insurance, and they also have a higher frequency of out-patient department visits and a longer average length of stay. Furthermore, we used the regression model to observe trends and effective predictors for the average hospitalization costs and ALOS in patients who received colorectal cancer surgery.
The average hospitalization cost and ALOS decrease each year in the study. This finding may be related to the increase in stage 1 CRC that was found in a younger group of patients (51-60 years old) by general screening using a stool occult blood test and a colonoscopy at a health examination. Compared to patients with stage 4 CRC, the patients with stage 1 CRC not only have a decreased average hospitalization cost but also ALOS. The study indirectly show the benefit of the general screening using a stool occult blood test for the older patients and the value of colonoscopy at health examinations. However, the effectiveness of colonoscopy is diminished by operator-dependent factors. Therefore, quality assurance programs should be implemented in all colonoscopy practices[25].
Our results should be interpreted in the context of certain limitations. For example, the existing data set used for the study contained only 6 years of data from one institution. Thus, it is possible that some of the patients in our analysis who apparently did not undergo CRC surgery had, in fact, received CRC surgery in the 6 years prior to the study’s time frame. Second, our analysis was not able to capture data on tests performed outside of the study hospital if they were not paid for by the study hospital. This limitation may have resulted in an underestimation of the adequacy of average hospitalization cost. Another potential limitation of our analysis is its dependence on claims data and the lack of data for readmission. A different study previously showed that enhanced recovery after surgery protocols may decrease the length of stay in a hospital. However, that study also noted that a reduced length of stay at a hospital stay was associated with a high rate of readmission[26]. Finally, the analysis did not examine outcome data such as patient-reported quality of life and indirect costs incurred after discharge. However, given the robust magnitude of the effects and the statistical significance of the effects in this study, these limitations are unlikely to compromise the results.
In conclusion, this analysis of CRC data from a large scale survey in Taiwan evaluated changing trends and risk factors of hospital resource utilization. The data improve the understanding of medical resource allocation for CRC surgery and may help to formulate public health policies for optimizing hospital resource utilization for related diseases. Government officials and health care providers should recognize that hospital resource utilization of CRC surgery may depend on both patient and clinical attributes. These results can be generalized to CRC surgery patient populations elsewhere in Taiwan and to similar populations in other countries.
Colorectal cancer (CRC) is one of the leading causes of cancer-related death in the world. Although the volume of CRC surgical procedures is increasing annually, the incidence and hospital resource utilization for these procedures have not been documented in a Taiwan study. Thus, this study explored the changing trends and risk factors of these outcomes for CRC surgery.
These results can be generalized to patient populations elsewhere in Taiwan and to other countries with similar patient profiles.
This is a large retrospective study focused on the relationship between hospital costs, hospital stay and patients characteristics. It is beneficial to analyze changes in these factors over time.
To efficiently allocate of medical resources, these factors must be carefully managed. Moreover, government officials and health care providers should understand that these outcomes depend on both patient and hospital attributes.
This research is a large sample, retrospective study and presents an interesting investigation of trend analysis and predictors for costs and hospital stay lengths after CRC surgery in the Taiwan region. The results showed that age, source, surgical type, tumor size, tumor site, and tumor stage were associated with decreased hospital resource utilization, and these findings may help to formulate public health policies in optimizing hospital resource utilization.
| 1. | Zhang S, Cui Y, Weng Z, Gong X, Chen M, Zhong B. Changes on the disease pattern of primary colorectal cancers in Southern China: a retrospective study of 20 years. Int J Colorectal Dis. 2009;24:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8236] [Article Influence: 457.6] [Reference Citation Analysis (11)] |
| 3. | Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1761] [Cited by in RCA: 1706] [Article Influence: 89.8] [Reference Citation Analysis (4)] |
| 4. | van den Hout WB, van den Brink M, Stiggelbout AM, van de Velde CJ, Kievit J. Cost-effectiveness analysis of colorectal cancer treatments. Eur J Cancer. 2002;38:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Delcò F, Egger R, Bauerfeind P, Beglinger C. Hospital health care resource utilization and costs of colorectal cancer during the first 3-year period following diagnosis in Switzerland. Aliment Pharmacol Ther. 2005;21:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Wright GE, Barlow WE, Green P, Baldwin LM, Taplin SH. Differences among the elderly in the treatment costs of colorectal cancer: how important is race? Med Care. 2007;45:420-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 7. | Kim JC, Kim DD, Lee YM, Kim TW, Cho DH, Kim MB, Ro SG, Kim SY, Kim YS, Lee JS. Evaluation of novel histone deacetylase inhibitors as therapeutic agents for colorectal adenocarcinomas compared to established regimens with the histoculture drug response assay. Int J Colorectal Dis. 2009;24:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Fujii S, Ota M, Ichikawa Y, Yamagishi S, Watanabe K, Tatsumi K, Watanabe J, Suwa H, Oshima T, Kunisaki C. Comparison of short, long-term surgical outcomes and mid-term health-related quality of life after laparoscopic and open resection for colorectal cancer: a case-matched control study. Int J Colorectal Dis. 2010;25:1311-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Tan KK, Hong CC, Zhang J, Liu JZ, Sim R. Surgery for perforated colorectal malignancy in an Asian population: an institution’s experience over 5 years. Int J Colorectal Dis. 2010;25:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Klaver YL, Lemmens VE, Nienhuijs SW, Luyer MD, de Hingh IH. Peritoneal carcinomatosis of colorectal origin: Incidence, prognosis and treatment options. World J Gastroenterol. 2012;18:5489-5494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Devon KM, Vergara-Fernandez O, Victor JC, McLeod RS. Colorectal cancer surgery in elderly patients: presentation, treatment, and outcomes. Dis Colon Rectum. 2009;52:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Faiz O, Warusavitarne J, Bottle A, Tekkis PP, Clark SK, Darzi AW, Aylin P. Nonelective excisional colorectal surgery in English National Health Service Trusts: a study of outcomes from Hospital Episode Statistics Data between 1996 and 2007. J Am Coll Surg. 2010;210:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Hebbar S, Fuggle WJ, Nevill AM, Veitch AM. Colorectal cancer incidence and trend in UK South Asians: a 20-year study. Colorectal Dis. 2012;14:e319-e322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Gong J, Shi DB, Li XX, Cai SJ, Guan ZQ, Xu Y. Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol. 2012;18:7308-7313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ferri M, Rossi Del Monte S, Salerno G, Bocchetti T, Angeletti S, Malisan F, Cardelli P, Ziparo V, Torrisi MR, Visco V. Recovery of immunological homeostasis positively correlates both with early stages of right-colorectal cancer and laparoscopic surgery. PLoS One. 2013;8:e74455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Anania G, Santini M, Scagliarini L, Marzetti A, Vedana L, Marino S, Gregorio C, Resta G, Cavallesco G. A totally mini-invasive approach for colorectal laparoscopic surgery. World J Gastroenterol. 2012;18:3869-3874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kapritsou M, Korkolis DP, Konstantinou EA. Open or laparoscopic surgery for colorectal cancer: a retrospective comparative study. Gastroenterol Nurs. 2013;36:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Champagne BJ, Makhija R. Minimally invasive surgery for rectal cancer: are we there yet? World J Gastroenterol. 2011;17:862-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Lin S, Jiang HG, Chen ZH, Zhou SY, Liu XS, Yu JR. Meta-analysis of robotic and laparoscopic surgery for treatment of rectal cancer. World J Gastroenterol. 2011;17:5214-5220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Lai JH, Law WL. Laparoscopic surgery for colorectal cancer. Br Med Bull. 2012;104:61-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 21. | Benedix F, Schmidt U, Mroczkowski P, Gastinger I, Lippert H, Kube R. Colon carcinoma--classification into right and left sided cancer or according to colonic subsite?--Analysis of 29,568 patients. Eur J Surg Oncol. 2011;37:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Torres Udos S, Almeida TE, Netinho JG. Increasing hospital admission rates and economic burden for colorectal cancer in Brazil, 1996-2008. Rev Panam Salud Publica. 2010;28:244-248. [PubMed] |
| 23. | Kelly M, Sharp L, Dwane F, Kelleher T, Comber H. Factors predicting hospital length-of-stay and readmission after colorectal resection: a population-based study of elective and emergency admissions. BMC Health Serv Res. 2012;12:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Kehlet H, Büchler MW, Beart RW, Billingham RP, Williamson R. Care after colonic operation--is it evidence-based? Results from a multinational survey in Europe and the United States. J Am Coll Surg. 2006;202:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Macken E, Moreels T, Vannoote J, Siersema PD, Van Cutsem E. Quality assurance in colonoscopy for colorectal cancer diagnosis. Eur J Surg Oncol. 2011;37:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Ahmed J, Khan S, Lim M, Chandrasekaran TV, MacFie J. Enhanced recovery after surgery protocols - compliance and variations in practice during routine colorectal surgery. Colorectal Dis. 2012;14:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
P- Reviewers: Bujanda L, Chen JL, Tong WD S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN