Published online Jan 14, 2014. doi: 10.3748/wjg.v20.i2.509
Revised: September 29, 2013
Accepted: October 19, 2013
Published online: January 14, 2014
Processing time: 168 Days and 6.3 Hours
AIM: To clarify the impact of cytomegalovirus (CMV) activation and antiviral therapy based on CMV antigen status on the long-term clinical course of ulcerative colitis (UC) patients.
METHODS: UC patients with flare-up were divided into CMV-positive and -negative groups according to the CMV antigenemia assay. The main treatment strategy provided for the patients in the CMV-positive group comprised a dose reduction of corticosteroids and administration of ganciclovir.
RESULTS: The median number of days to initial remission was significantly greater for the patients in the CMV-positive group (21 d vs 16 d, P = 0.009). However, the relapse rate after remission and colectomy rate during more than 30 mo of observation did not differ between the two groups. Multivariate analysis revealed that administration of ganciclovir was the only independent factor for avoiding colectomy in patients of the CMV-positive group.
CONCLUSION: CMV antigen status did not significantly affect the long-term prognosis in UC patients under treatment with appropriate antiviral therapy.
Core tip: Cytomegalovirus (CMV) reactivation has a deleterious effect in patients with ulcerative colitis (UC). Although antiviral therapy for CMV antigen-positive UC patients may be effective in the short-term, the long-term prognosis of UC patients with CMV treated by antiviral agents remains unknown. Our study revealed that positive CMV antigen status is likely to prolong time to remission in the treatment of flare-up of UC; however, long-term prognosis, including colectomy rate, was not affected by CMV antigen status treated with antiviral agents. Ganciclovir use is an independent factor for avoidance of colectomy in CMV antigen-positive UC patients.
- Citation: Inokuchi T, Kato J, Hiraoka S, Suzuki H, Nakarai A, Hirakawa T, Akita M, Takahashi S, Harada K, Okada H, Yamamoto K. Long-term follow-up of ulcerative colitis patients treated on the basis of their cytomegalovirus antigen status. World J Gastroenterol 2014; 20(2): 509-517
- URL: https://www.wjgnet.com/1007-9327/full/v20/i2/509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i2.509
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by periods of remission and clinical relapses, the affected mucosa spreading from the rectum to the proximal large intestine, diffusely, circumferentially and continuously. The symptoms include bloody diarrhea, fever, tachycardia, anemia, and colicky abdominal pain[1]. In this decade, anti-tumor necrosis factor-α (TNF-α) antibodies, calcineurin inhibitors and apheresis, in addition to amino-salicylates and corticosteroids, have been used widely for the treatment of UC with relapse. The clinical course of UC patients has improved, but 20%-30% of patients with total colitis still come to colectomy[2-4]. Although the mortality of UC patients is not significantly higher than standardized mortality ratios of non-IBD populations, the cause of UC remains uncertain and there is still room for further research[5-7].
Cytomegalovirus (CMV) reactivation is sometimes associated with relapse of UC. A number of reports have debated the pathogenicity of CMV in UC patients and there are contrasting hypotheses that this is a pathogenic virus exacerbating the clinical course of IBD patients and that it is “an innocent bystander” and does not contribute to increased morbidity and mortality of UC patients[5-14]. As the influence of CMV infection on the UC patient remains uncertain, the clinical management of UC patients with CMV reactivation has not yet been standardized. In this context, some studies reported that antiviral therapy decreased the colectomy rate and mortality of UC patients with CMV reactivation[8,9]. Meanwhile, another reported that there were no significant differences in the rates of remission and colectomy between UC patients with and without CMV[10]. Moreover, it is problematic that there are so few studies indicating the long-term prognosis of UC patients complicated with CMV.
Another important problem regarding CMV infection of UC patients is the diagnostic modality. There are several diagnostic techniques for CMV “infection”, including endoscopy, histology, serology, viral culture, CMV antigenemia assay and CMV DNA testing, which have varying sensitivity and specificity. At this point, immunohistochemistry (IHC) or CMV DNA testing of intestinal mucosa tissue is recommended for diagnosis of CMV “disease” in IBD patients, according to several guidelines[15-17]. However, these require colonoscopy and the former may have too low sensitivity and the latter too high sensitivity for the indication of antiviral therapy[8,16,18]. The CMV antigenemia assay has been widely used for pre-emptive therapy in hematopoietic stem cell transplantation[19-22]. In the field of UC, in contrast, relatively few studies evaluated the clinical significance of the CMV antigenemia assay, e.g., for decision of administration of antiviral therapy[8,10]. However, the CMV antigenemia assay has advantages in that it is relatively inexpensive and facilitates monitoring of CMV activity continuously without endoscopy. Moreover, the assay may have less sample bias than examinations using tissues from the colon.
We have examined the CMV status in relapse of UC patients using the CMV antigenemia assay and treatment strategies were determined based on the CMV antigen status. The mainstay of our strategy for the patients found to be CMV antigen positive comprised ganciclovir administration and dose reduction of corticosteroids. The patients treated according to the strategy were followed-up at our institute for a relatively long period and the disease course of those patients may reveal the impact of CMV reactivation and antiviral therapy on the long-term prognosis of UC patients with CMV.
In this study, therefore, we investigated the long-term disease course of UC patients diagnosed as CMV-positive and -negative using the CMV antigenemia assay. The aim of this study was to clarify the impact of CMV antigen and antiviral therapy on the UC patients’ prognosis: remission rate, relapse rate and colectomy rate.
We retrospectively analyzed UC patients who were treated for their first-attack or relapse of disease at Okayama University Hospital from April 2004 to November 2011. Inclusion criteria were disease activity of Lichtiger’s clinical activity index (CAI) 7 or more and having results of CMV antigen status evaluated by CMV antigenemia assay, and 121 patients met the criteria[23]. Of these patients, 3 who underwent emergency colectomy prior to the outcome of the CMV antigenemia assay were excluded. The remaining 118 patients were treated according to the strategy based on CMV antigen status, as shown below, and clinical, operative, pathological and treatment data were obtained from the patients’ medical records.
CMV antigen status was evaluated using the CMV-pp65 antigenemia assay (SRL, Tokyo, Japan). The test is based on immunocytochemical detection of CMV immediate early antigens in blood leukocytes. The results were expressed as the number of CMV-pp65-positive cells per 5 × 104 leukocytes and reported on the day following blood submission to the laboratory, although this took a few days at weekend. A positive result from the CMV antigenemia assay was defined as detection of one or more CMV-pp65-positive cells. Patients were divided into two groups according to CMV antigen status: CMV-positive group and CMV-negative group. CMV antigenemia assay was performed when treatment was started in relapse of UC patients and disease condition did not become better despite administration of immunosuppressive therapy. CMV antigenemia was usually measured once two weeks in patients with positive CMV antigen on the initial assay. Those of whom disease condition worsened after starting ganciclovir received the assay once a week.
If general condition permitted, we performed colonoscopy around the time of the CMV antigenemia assay and took biopsies for hematoxylin and eosin staining to search for CMV inclusion bodies and for IHC staining with a monoclonal antibody against CMV by means of an automated preparation and processing system, Ventana® (Roche Diagnostics, Tokyo, Japan).
In our hospital, UC patients were treated based on the guidelines published by the British Society of Gastroenterology, the European Crohn’s and Colitis Organization, and the American College of Gastroenterology[15,17,24]. In general, a sufficient dose of corticosteroids, 0.5-1 mg/kg per day of prednisolone for patients with moderate activity and 1-1.5 mg/kg per day for patients with severe activity, was administered with a target period set at two weeks and then gradually reduced by 5-10 mg/d at weekly intervals until a daily dose of 20 mg was reached. In patients with a steroid-refractory or -dependent course, another treatment, such as apheresis, a calcineurin inhibitor or anti-TNF-α antibody, was started according to their disease severity and treatment history. In this study, anti-TNF-α antibody was used less frequently because infliximab for UC was approved in Japan in June 2010. The effectiveness of additional treatments was also evaluated within about two weeks. Antiviral therapy with ganciclovir was not generally administered for patients in the CMV-negative group. However, antiviral therapy was administered for some patients who underwent acute exacerbation with endoscopic findings suggestive of CMV infection such as large, deep, or longitudinal ulcers[25].
When patients were found to be CMV antigen positive, ganciclovir administration with reduction of the dose of corticosteroids was considered to be the first choice. However, the antiviral agent was not provided for cases with mild disease activity or for cases for which conventional treatment without ganciclovir appeared to be effective. As the next step, steroid-refractory patients received apheresis, anti-TNF-α antibody, or a calcineurin inhibitor after diminishment of CMV antigen.
Remission, relapse, and colectomy rates were compared between the CMV-positive and -negative groups. The severity was expressed according to Truelove and Witt’s criteria[26]. State of relapse was defined as CAI score 7 or more[23]. The definition of remission meets the CAI score 4 or less without any abdominal pains and bloody stools. Short-term clinical courses were evaluated according to patient status within 8 wk of examination of CMV antigen status, while long-term clinical courses were determined at the last visit before March 2012 or the time of colectomy.
This retrospective analysis was approved by the institutional review board of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences and all the patients and subjects were included after obtaining their written informed consent. There were no conflicts of interest or sponsors of this study.
Patient characteristics were compared using the χ2 test, Fisher’s exact test and Mann-Whitney U test. Analyses of remission, relapse and colectomy rates were carried out using the method of Kaplan and Meier. Statistical comparison was carried out by Log-rank test. Univariate and multivariate analysis using a Cox proportional hazard model were also conducted. P value < 0.05 was considered statistically significant. All statistical analyses were performed using JMP ver.9 software (SAS Institute, Cary, NC, United States).
The clinical characteristics of analyzed patients are summarized in Table 1. A total of 118 UC patients with known CMV antigen status were treated during the study period; 40 were included in the CMV-positive group and 78 were in the CMV-negative group. CMV antigen was detected more frequently in male patients (P = 0.009). The dose of corticosteroids at the beginning of the treatment was significantly higher for the patients in the CMV-positive group than those in the CMV-negative group (35 mg/d of prednisolone vs 20 mg/d, P = 0.0003). CMV status of all the CMV-positive patients except those who underwent colectomy in a short term became negative, regardless of ganciclovir administration or not. The average period between the start of therapy and measurement of CMV antigenemia assay was 10.8 ± 13.4 d.
| Characteristics | CMV Ag+ (n = 40) | CMV Ag- (n = 78) | Pvalue |
| Gender | 0.009 | ||
| Male | 26 | 31 | |
| Female | 14 | 47 | |
| Age(yr), median (range) | 45 (14-79) | 36 (12-87) | 0.10 |
| Duration of disease (yr), median (range) | 1.5 (0.1-28) | 4.6 (0-38) | 0.23 |
| Disease activity | 0.191 | ||
| Mild | 2 | 8 | |
| Moderate | 15 | 35 | |
| Severe | 23 | 35 | |
| Lichtiger’s CAI, median (range) | 11 (7-19) | 13 (7-21) | 0.15 |
| Extent of disease | 0.31 | ||
| Total colitis | 33 | 58 | |
| Left-sided | 7 | 20 | |
| Dose of prednisolone (mg/d), median (range) | 35 (0-80) | 20 (0-100) | 0.0003 |
| Follow-up period (yr), median (range) | 3.2 (0.1-9.3) | 2.8 (0.1-9.7) | 0.66 |
The correlation between CMV antigen status and IHC for CMV in the colonic mucosa was examined using 49 patients who underwent colonoscopy around the time of the CMV antigenemia assay. Of the 23 patients in the CMV-positive group, 9 (39.1%) were positive for IHC. On the other hand, 25 (96.2%) of the 26 patients in the CMV-negative group were negative for IHC. The results of the CMV antigenemia assay were closely correlated with IHC of inflamed colon mucosa for CMV (P = 0.003, Fisher’s exact test). Taking IHC as the gold standard, positive CMV antigen status predicted positive IHC with 90% sensitivity and 64% specificity.
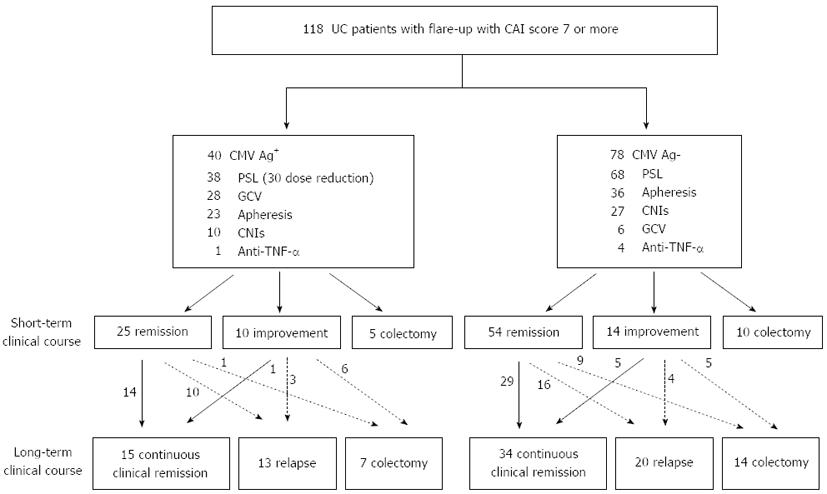
Figure 1 is a flow chart of the clinical courses of the 118 patients treated according to the strategy based on CMV antigen status. Of the 38 patients in the CMV-positive group who had received corticosteroids, 30 (78.9%) underwent dose reduction of corticosteroids. The remaining 8 patients did not undergo dose reduction of corticosteroids; 3 received colectomy in the early period and 5 showed a marked response to the corticosteroids. Twenty-eight (70%) patients in the CMV-positive group received ganciclovir infusion. On the other hand, 68 (87.2%) of the 78 patients in the CMV-negative group received corticosteroids without any dose reductions. Six (7.7%) patients in the CMV-negative group were administered ganciclovir infusion because CMV reactivation was suspected, based on specific endoscopic findings and clinical refractoriness to the first-line therapy with clinical symptoms worsening. In both groups, apheresis and calcineurin inhibitors were used relatively frequently.
In the CMV-positive group, 25 (62.5%) patients went into remission and 5 (12.5%) received colectomy during the short-term treatment. The remaining 10 (25%) patients improved, but did not fulfill the criteria of remission. Among the CMV-negative group, on the other hand, 54 (69.2%) patients entered remission successfully, 14 (17.9%) improved, and 10 (12.8%) underwent colectomy in the short-term (Figure 1, center part).
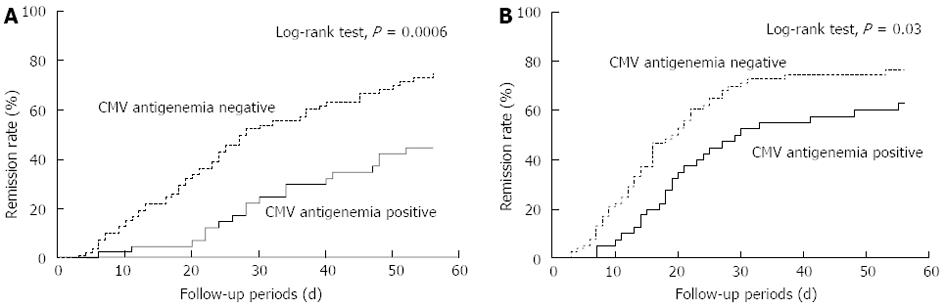
Two types of the Kaplan-Meier curves for the rate of remission induction are shown (Figure 2). Figure 2A indicates the remission rate from the starting day of the remission-induction therapy and Figure 2B shows from the day when the CMV antigen status was determined. Both curves show the better clinical course in the CMV-negative group (P = 0.0006 and P = 0.03, respectively, Log-rank test). The median number of days to remission from the start of the treatment with our strategy was significantly greater for the patients in the CMV-positive group than those in the CMV-negative group (21 d vs 16 d, P = 0.009, Mann-Whitney U test). In addition, we analyzed remission rate from the starting day of the remission-induction therapy between the two groups; one group was limited to CMV-positive patients administered ganciclovir and another was CMV-negative patients not administered ganciclovir. These curves showed the better clinical course in the CMV-negative group, too (P = 0.03, Log-rank test).
In the CMV-positive group, 14 (56%) of the 25 patients who had gone into remission within the short-term maintained that status. In contrast, only one (10%) of the 10 patients with improvement in the short-term entered remission afterwards. Meanwhile, in the CMV-negative group, 34 (50%) of 68 patients who avoided colectomy in the short-term could maintain continuous clinical remission (Figure 1, lower part).
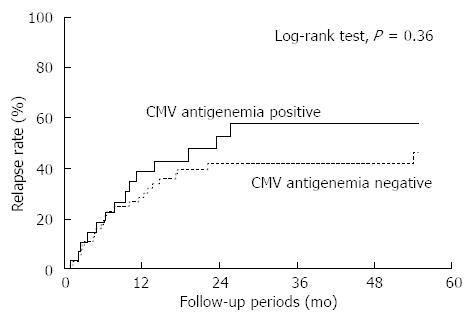
The rate of relapse after successful remission induction with the initial treatment did not differ between the patients in the CMV-positive and -negative groups (Figure 3; P = 0.36, Log-rank test). Among 20 patients in the CMV-positive group who underwent relapse or colectomy after the initial induction of remission, 2 became positive for CMV antigen again. These patients were intractable to any therapy, including anti-TNF-α antibodies, and calcineurin inhibitors, and continued to suffer from chronic symptoms during the study period. None of the 34 patients in the CMV-negative group who relapsed or underwent colectomy after the initial remission became positive for CMV antigen.
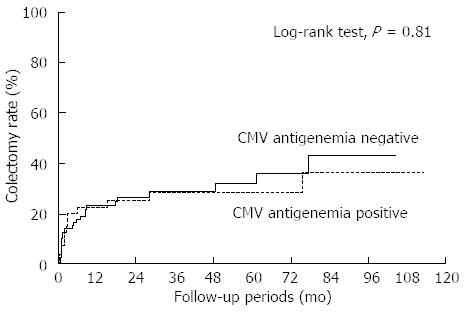
The cumulative colectomy rate according to CMV antigen status is shown in Figure 4. Twelve (30%) in the CMV-positive patients and 24 (30.8%) in the CMV-negative patients underwent colectomy during the observation period and there was no significant difference in the colectomy rate between the two groups (Figure 4; P = 0.81, Log-rank test, median observation period: 31 mo). No independent risk factors for colectomy could be identified among demographic, treatment and disease parameters including CMV antigen status in the analysis of all 118 patients with or without CMV antigen (Table 2). Whereas, for patients in the CMV-positive group, multivariate analysis using parameters including ganciclovir administration, dose reduction of corticosteroids and number of CMV antigen revealed that administration of ganciclovir at the start of therapy was the only factor correlated with avoidance of colectomy (Table 3; OR = 0.04; 95%CI: 0.01-0.50).
| Risk factor | Risk ratio | 95%CI | Pvalue | |
| Lower | Upper | |||
| Gender (male) | 0.97 | 0.59 | 1.59 | 0.89 |
| Age (> 40 yr) | 1.13 | 0.70 | 1.82 | 0.61 |
| Duration of disease (> 3 yr) | 0.99 | 0.61 | 1.61 | 0.97 |
| Severity (severe) | 1.24 | 0.74 | 2.08 | 0.42 |
| Extent of disease (total colitis) | 1.21 | 0.68 | 2.23 | 0.51 |
| CMV Ag (+) | 0.64 | 0.36 | 1.13 | 0.12 |
| Apheresis use | 0.71 | 0.44 | 1.17 | 0.18 |
| CNI use | 1.11 | 0.63 | 1.92 | 0.73 |
| Risk factor | Risk ratio | 95%CI | Pvalue | |
| Lower | Upper | |||
| Gender (male) | 0.38 | 0.03 | 4.24 | 0.42 |
| Age (> 45 yr) | 1.22 | 0.15 | 12 | 0.85 |
| Duration of disease (> 2 yr) | 0.98 | 0.09 | 9.18 | 0.98 |
| Severity (severe) | 8.37 | 0.46 | 751 | 0.17 |
| Extent of disease (total colitis) | 2.43 | 0.15 | 118 | 0.55 |
| Apheresis use | 0.87 | 0.08 | 9.59 | 0.91 |
| CNI use | 0.54 | 0.01 | 10.2 | 0.69 |
| Ganciclovir use | 0.04 | 0.01 | 0.5 | 0.01 |
| Dose reduction of corticosteroids | 0.76 | 0.03 | 13.1 | 0.85 |
| Number of CMV Ag1 (> 3) | 3.77 | 0.53 | 42.3 | 0.19 |
We analyzed data from UC patients with flare-up and who were treated according to a strategy based on the CMV antigen status and demonstrated the clinical courses of CMV antigen-positive and -negative patients. In UC patients with flare-up, CMV antigen positive status significantly prolonged the time to remission from the start of treatment but did not affect the relapse or colectomy rate. In addition, ganciclovir administration was a significant factor correlated with avoidance of colectomy in patients with positive CMV antigen status.
It has been suggested that CMV is pathogenic for UC patients and causes clinical condition of UC patients with relapse to become worse and complicate. In fact, several small scale studies indicated that UC patients with CMV infection had higher colectomy rates than those without CMV. Moreover, treatment of CMV infection by ganciclovir can improve the clinical outcome of those patients[8,27-29]. Although a few reports indicated the possibility of CMV being an innocent bystander in the exacerbation of UC[10], CMV can affect some of UC patients deleteriously. However, the long-term effect of CMV infection and antiviral therapy on the prognosis of UC patients has not been elucidated completely. In this context, our results, including the long-term disease course of UC patients with CMV infection, should have a great impact on the clinical practice of UC.
We showed that patients with CMV infection were less likely to enter remission in the short-term. This result is consistent with those of previous reports[9,27]. In contrast, however, no significant difference in colectomy rate was observed between patients with and without CMV, even in the short-term. In this context, the CMV antigenemia assay could reliably detect CMV “disease” which requires antiviral therapy. In our finding, use of ganciclovir is not always correlated with CMV negative conversion in short-term but a predictor of avoidance of colectomy in long-term. These suggest that use of ganciclovir exerts clinical effectiveness in long-term follow-up, e.g., avoiding exacerbation and/or relapse after remission, for a portion of patients with CMV.
In term of diagnosis regarding CMV infection in UC patients, IHC or CMV DNA testing in the intestinal mucosa tissue is recommended as a diagnosis of CMV “disease” in IBD patients according to several guidelines[15-17]. In particular, CMV DNA testing would be a very useful method if the correct cut-off level of CMV DNA load was established[30]. However, CMV DNA testing is expensive and needs colonoscopy with biopsy that has a risk of bleeding or perforation in severely ill UC patients. In addition, there is a sample bias in taking tissues from the mucosa. CMV DNA testing in blood sample may be convenient, but the method and cutoff values remain to be standardized. On the other hand, the CMV antigenemia assay is less expensive and facilitates monitoring of CMV activity continuously without endoscopy. Moreover, there is less sample bias than in taking from colon tissue. We confirmed that the results of the antigenemia assay were consistent with the results of IHC for CMV, although the specificity for IHC was relatively low. In fact, lower specificity may indicate overestimation of CMV disease by the antigenemia assay. Therefore, our strategy may provide unnecessary antiviral therapy for part of patients.
Reduction of the dose of corticosteroids is another aspect of our treatment strategy for UC patients with CMV. There has been no consensus regarding whether immunosuppressive therapies should be continued or discontinued, and whether the dose of corticosteroids should be increased or decreased for UC patients with CMV. Some have reported cases with continuation of the immunomodulating drugs[8,29], while others have discontinued the immunosuppressive therapies[27,28]. Interestingly, all of these studies showed good clinical courses whether immunosuppressive therapies were ongoing or not. In addition, our results indicated that reduction of the dose of corticosteroids and immunosuppressive therapies were not significant factors in multivariate analysis for avoiding colectomy both in the short and long-term. This suggests that status of immunosuppressive therapies, including the dose of corticosteroids, may not affect the clinical course of UC patients with CMV greatly, at least under antiviral therapy.
The highlight of this study is determination of the long-term prognosis of UC patients with CMV infection, including relapse rate and colectomy rate, which have rarely been reported. Unexpectedly, no significant difference was observed in overall long-term prognosis between patients with and without CMV. Kaplan-Meier curves for colectomy were almost parallel, both in the short-term and in the long-term, between those patients. These results suggest that UC patients with positive CMV antigen should be treated with antiviral therapy and that our treatment strategy may be appropriate for UC patients with CMV with regard to long-term prognosis.
However, there may be two types of rebuttal to our results. First, antiviral therapy may not always be required for UC patients with CMV. Although the efficacy of antiviral agents in CMV disease has been recognized widely[15,31,32], a previous report demonstrated that UC patients with CMV infection who were treated without the use of antiviral agents showed similar rates of remission and colectomy to UC patients without CMV[10]. However, the report consisted of 25 patients with CMV (4 underwent colectomy) and 23 patients without CMV (1 underwent colectomy) with an observation period of 8 wk, and such small number of patients with short-term observation may not be sufficient to verify the appropriateness of a treatment strategy without antiviral therapy. For accurate validation of the use of antiviral agents, long-term follow-up data of patients who were treated according to a strategy without antiviral therapy would be expected. However, such a treatment strategy may not be feasible under the guidelines which recommended antiviral therapy for at least a proportion of patients with CMV.
The second problem is the appropriateness of the antigenemia assay for detecting the presence of CMV infection and administration of ganciclovir. As described above, a positive result from the antigenemia assay may overestimate the necessity of administration of antiviral therapy, because specificity for IHC was relatively low. In this regard, Roblin et al[30] indicated 250 copies/mg of CMV DNA in the colonic tissue as the cutoff for antiviral therapy, based on 42 hospitalized UC patients. However, few studies indicated a correlation between CMV antigen status and CMV DNA in colonic tissues, perhaps because of sample bias and the lack of validation of the technology of DNA testing, and therefore it is not known what is best as the cutoff for antiviral therapy. Determination of the optimal test modality and cutoff values for CMV disease in UC which requires antiviral therapy should be the goal of future studies.
This study has a limitation mainly because of the retrospective design. First of all, the treatment strategy was not always followed. Not all of the CMV-positive patients were administered ganciclovir and some of the CMV-negative patients were administered ganciclovir. Dose reduction of corticosteroids was not carried out for CMV-positive patients if corticosteroids exerted marked efficacy. However, from the aspects of ethical and moral standpoints, it would be difficult not to administer antiviral agents to severe and refractory patients with CMV infection on the basis of the current evidence. In this regard, our retrospective analysis for the long-term prognosis of UC patients with CMV may be almost optimal for verifying the treatment strategy for those patients. There is also a limitation about patients’ selections; dose of corticosteroids at the beginning of the treatment was significantly lower in the CMV-negative group. The patients examined CMV status and enrolled in this study were suspected involvement with CMV for their refractoriness and endoscopic findings. In severer case which was suspected involvement with CMV reactivation, we would avoid using large dose of PSL not to reactivate CMV. As a result, we chose second-line therapy such as CNIs, apheresis and not dose up of corticosteroids, especially in CMV-negative group, CAI of which was severer than positive group. In addition, CMV antigen was detected more frequently in male. However, I tried to stratify the results by gender and dose of corticosteroids, respectively, and we confirm that each result did not show a significant difference.
In conclusion, our treatment strategy, which consisted of dose reduction of corticosteroids and antiviral therapy, appeared to be appropriate for the treatment of UC patients with CMV antigen in view of long-term prognosis. More work is needed for the standardization of appropriate test modalities and cutoff values for administration of antiviral therapy and the administration policy of immunosuppressive agents for UC patients with CMV.
Cytomegalovirus (CMV) activation is sometimes associated with exacerbation and refractoriness of ulcerative colitis (UC). While there are several diagnostic modalities for CMV disease, these cannot distinguish strictly CMV “infection”, meaning that only the symptoms of CMV “disease” should be treated. Although antiviral therapy has been shown to improve the short-term disease course, the long-term prognosis of UC patients with CMV has rarely been reported.
The CMV antigenemia assay has advantages in that it is relatively inexpensive and facilitates monitoring of CMV activity continuously, without endoscopy. However, the disease course of a UC patients treated according to CMV antigenemia assay has not been revealed. In this study, the authors clarified the impact of CMV antigen and antiviral therapy on the UC patients’ prognosis: remission rate, relapse rate and colectomy rate.
The CMV antigenemia assay has been widely used for pre-emptive therapy in hematopoietic stem cell transplantation. In the field of UC, however, relatively few studies have evaluated the clinical significance of the CMV antigenemia assay, e.g., for decision of administration of antiviral therapy. This is the first study to investigate the long-term prognosis of UC patients treated on the basis of cytomegalovirus antigen status.
The finding that ganciclovir use is a predictor of avoidance of colectomy suggests that ganciclovir use exerts clinical effectiveness, e.g., avoiding exacerbation and/or relapse after remission, for a subset of patients with CMV.
Cytomegalovirus antigenemia: The cytomegalovirus antigenemia assay is based on immunocytochemical detection of CMV immediate early antigens in blood leukocytes. The results were expressed as the number of CMV-pp65-positive cells per 5 × 104 leukocytes.
The authors examined the long-term prognosis of UC patients with CMV infection, including relapse rate and colectomy rate, which have rarely been reported. It revealed that positive CMV antigen status was likely to prolong time to remission and long-term prognosis. Colectomy rate was not affected by CMV antigen status under the treatment strategy with antiviral agents. In addition, ganciclovir use is an independent factor for avoidance of colectomy in UC patients positive for the CMV antigen. The results are interesting and may suggest that ganciclovir use exerts clinical effectiveness, e.g., avoiding exacerbation and/or relapse after remission, for a subset of patients with CMV.
P- Reviewers: Daniel F, Shi RH, Tsujikawa T S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3-11. [PubMed] |
| 2. | Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, Bernklev T, Henriksen M, Sauar J, Vatn MH. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 552] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 3. | Höie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, Odes S, Mouzas IA, Beltrami M, Langholz E. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102:1692-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Survival and cause-specific mortality in ulcerative colitis: follow-up of a population-based cohort in Copenhagen County. Gastroenterology. 2003;125:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004;28:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Begos DG, Rappaport R, Jain D. Cytomegalovirus infection masquerading as an ulcerative colitis flare-up: case report and review of the literature. Yale J Biol Med. 1996;69:323-328. [PubMed] |
| 7. | Al-Zafiri R, Gologan A, Galiatsatos P, Szilagyi A. Cytomegalovirus complicating inflammatory bowel disease: a 10-year experience in a community-based, university-affiliated hospital. Gastroenterol Hepatol (N Y). 2012;8:230-239. [PubMed] |
| 8. | Domènech E, Vega R, Ojanguren I, Hernández A, Garcia-Planella E, Bernal I, Rosinach M, Boix J, Cabré E, Gassull MA. Cytomegalovirus infection in ulcerative colitis: a prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008;14:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Kishore J, Ghoshal U, Ghoshal UC, Krishnani N, Kumar S, Singh M, Ayyagari A. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 10. | Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, Okamoto S, Morohoshi Y, Izumiya M, Ichikawa H. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Nguyen M, Bradford K, Zhang X, Shih DQ. Cytomegalovirus Reactivation in Ulcerative Colitis Patients. Ulcers. 2011;2011:pii: 282507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Hommes DW, Sterringa G, van Deventer SJ, Tytgat GN, Weel J. The pathogenicity of cytomegalovirus in inflammatory bowel disease: a systematic review and evidence-based recommendations for future research. Inflamm Bowel Dis. 2004;10:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Dimitroulia E, Spanakis N, Konstantinidou AE, Legakis NJ, Tsakris A. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:879-884. [PubMed] |
| 14. | Eyre-Brook IA, Dundas S. Incidence and clinical significance of colonic cytomegalovirus infection in idiopathic inflammatory bowel disease requiring colectomy. Gut. 1986;27:1419-1425. [PubMed] |
| 15. | Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 965] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 16. | Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 654] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 17. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-523; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 952] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 18. | Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16:1620-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Mori T, Mori S, Kanda Y, Yakushiji K, Mineishi S, Takaue Y, Gondo H, Harada M, Sakamaki H, Yajima T. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Nagata N, Kobayakawa M, Shimbo T, Hoshimoto K, Yada T, Gotoda T, Akiyama J, Oka S, Uemura N. Diagnostic value of antigenemia assay for cytomegalovirus gastrointestinal disease in immunocompromised patients. World J Gastroenterol. 2011;17:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063-4071. [PubMed] |
| 22. | Kanda Y, Yamashita T, Mori T, Ito T, Tajika K, Mori S, Sakura T, Hara M, Mitani K, Kurokawa M. A randomized controlled trial of plasma real-time PCR and antigenemia assay for monitoring CMV infection after unrelated BMT. Bone Marrow Transplant. 2010;45:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1189] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 24. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 708] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 25. | Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol. 2010;16:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | TRUELOVE SC, WITTS LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [PubMed] |
| 27. | Papadakis KA, Tung JK, Binder SW, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:2137-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Cottone M, Pietrosi G, Martorana G, Casà A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A, Pagliaro L. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol. 2001;96:773-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, Matsuura M, Ohmori K, Sakurai T, Nagayama S. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Roblin X, Pillet S, Oussalah A, Berthelot P, Del Tedesco E, Phelip JM, Chambonnière ML, Garraud O, Peyrin-Biroulet L, Pozzetto B. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Rahier JF, Ben-Horin S, Chowers Y, Conlon C, De Munter P, D’Haens G, Domènech E, Eliakim R, Eser A, Frater J. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 32. | Pillet S, Pozzetto B, Jarlot C, Paul S, Roblin X. Management of cytomegalovirus infection in inflammatory bowel diseases. Dig Liver Dis. 2012;44:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |