Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4811
Revised: November 21, 2013
Accepted: January 6, 2014
Published online: April 28, 2014
Processing time: 221 Days and 9.7 Hours
The association of primary sclerosing cholangitis (PSC) and autoimmune hepatitis (AIH) is known as an overlap syndrome (OS). OS can also be described in the setting of concomitant presence of AIH and PSC. These diseases can in some cases be associated with ulcerative colitis. In this case report we describe, to our knowledge, the first case in the literature of a young Caucasian male suffering from ulcerative colitis and an overlap syndrome consisting of an association between PSC-AIH, with the concomitant presence of a membranous glomerulonephritis.
Core tip: This case reports highlights a particular kind of hepatic overlap syndrome. To our knowledge it would be the first case to be described in the literature concerning 4 different diagnoses; all being autoimmune related. These autoimmune diseases were never described together, yet their association in this case is probably not a sheer coincidence. Auto-immune diseases will play an ever more import role in the future; yet the current knowledge and understanding concerning these pathologies is certainly only a fraction of what is necessary to offer the best treatment to our patients.
- Citation: Warling O, Bovy C, Coïmbra C, Noterdaeme T, Delwaide J, Louis E. Overlap syndrome consisting of PSC-AIH with concomitant presence of a membranous glomerulonephritis and ulcerative colitis. World J Gastroenterol 2014; 20(16): 4811-4816
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4811
The term “primary sclerosing cholangitis-autoimmune hepatitis (PSC-AIH) overlap syndrome” is used to describe different varieties of AIH and/or PSC in the setting where an unspecific number of characteristics of the other disease are present[1].
Herein we report a particular kind of hepatic overlap syndrome. To our knowledge it would be the first case to be described in the literature concerning a patient with ulcerative colitis, PSC, auto-immune hepatitis, and extra-membranous glomerulonephritis. These autoimmune diseases were never described together, yet their association in this case is probably not a sheer coincidence.
We report the case of a 29-year-old Caucasian male suffering from ulcerative colitis (UC) since 2000 and associated with PSC since 2003. The initial diagnosis of UC was based on concordant endoscopic and histological findings, and PSC diagnosis was established by means of a liver biopsy. The patient was treated using 1 g/d of ursodeoxycholic acid.
In September 2005, the patient was hospitalized for jaundice and fatigue without any indication of pain. Besides a lean physique and conjunctival jaundice, the clinical examination was inconspicuous.
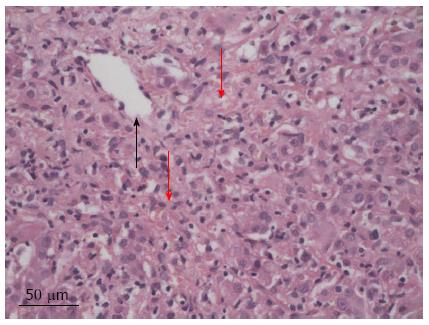
Initial blood analysis showed impaired liver function characterized essentially by the presence of cholestasis and cytolysis (Table 1). Further investigation showed increased IgG [25.2 (normal: 6.9-14) g/L) and IgM [2.36 (normal: 0.3-2.1) g/L] titers. Subanalysis for IgG4 and anti-nuclear antibodies remained negative. Anti-mitochondrial antibodies type 2, anti-myeloperoxidase, as well as anti-LKM were also negative. However, anti-neutrophil cytoplasmic antibodies (determined by indirect immunofluorescence assay) and anti-proteinase 3 antibodies turned out to be positive. Serotype analysis confirmed the presence of HLA-DR 3 in our patient. Further analyses concerning tumor markers (alpha fetoprotein, carcinoembryonic antigen, and CA19-9) as well as hepatitis serology (A, B, and C) were negative. Urinary copper levels were normal and no Keiser Fleischer rings were noted at clinical examination. Abdominal ultrasound only demonstrated the presence of dilated intrahepatic bile ducts. This picture was confirmed by endoscopic retrograde cholangiopancreatography (ERCP), which showed some minor irregularities of the intrahepatic portion. Cholangio-magnetic resonance imaging (MRI) proved to be non-contributive and only confirmed the results obtained by the ERCP. The overall appearance was compatible, but not specific for sclerosing cholangitis. A liver biopsy performed shortly afterwards showed the presence of chronic hepatitis with predominantly centrilobular necrosis (Figure 1), evoking a toxic origin or possibly AIH. Owing to the increased IgGs with concomitant cytolysis and the exclusion of any other causes (i.e., toxics) the diagnosis of AIH associated with PSC was retained, delimitating the picture of an overlap syndrome. The patient was promptly started on 32 mg/d of methyl-prednisolone. Seven days after the initiation of the treatment, there was a marked improvement in the patient’s overall condition. Total bilirubin dropped to 53 mg/L. Alanine aminotransferase and IgGs were significantly decreased to 228 U/L and 15.4 g/L, respectively. However, the IgGs titers fluctuated for the next four years before reaching a normal level again. Two months later, corticosteroids were reduced by half (16 mg/d). Nonetheless we were forced to start the patient on 50 mg/d of azathioprine shortly after this step, as liver function degraded. This medication was so poorly tolerated by the patient that he was switched to 6-mercaptopurine (6-MP). The dosage was steadily increased to 75 mg/d. TPMT gene variant genotyping didn’t show any abnormal activity, therefore excluding a genetic predisposition to bone marrow toxicity. Further withdrawal of corticosteroids proved to be difficult as clinical jaundice reoccurred as soon as the dosage was reduced 8 mg/d or less. As liver function [total bilirubin: 61 mg/L (range < 4 mg/L), TGP 72 UI/L (range: 3-36 UI/L)] as well as clinical evolution of the patient stagnated, we temporarily interrupted the administration of ursodeoxycholic acid to exclude any possible drug interaction. The titers for 6-MP were in normal range and another cholangio-MRI proved to be non-contributive. We therefore suspected a new episode of AIH, which led us to increase corticosteroids to 12 mg/d. This decision promoted into an excellent clinical and biological evolution with almost a complete normalization of liver function. Following this good evolution, corticosteroids were decreased progressively to 8 mg/d, which would be the minimum dose required for the next several years.
| Value | Range | |
| Hemoglobin (g/dL) | 12.1 | 14.0-17.4 |
| Gamma glutamyltransferase (UI/L) | 157 | 8-61 |
| C-reactive protein (mg/dL) | 11.5 | < 10 |
| GOT (AST, UI/L) | 920 | 0-35 |
| TGP (ALT, UI/L) | 701 | 3-36 |
| Total bilirubin (mg/dL) | 123.7 | < 15 |
| Direct bilirubin (mg/dL) | 93.8 | < 4 |
| Amylase (UI/L) | 94 | < 160 |
| Lipase (UI/L) | 77 | < 140 |
| Alkaline phosphatase (UI/L) | 208 | 35-100 |
| Total low density lipoprotein (UI/L) | 429 | 95-195 |
| Albumin (g/dL) | 33.4 | 35-50 |
In October 2009, four years after the initial diagnosis of overlap syndrome, the patient presented himself to the emergency department with severe edema of the lower extremities.
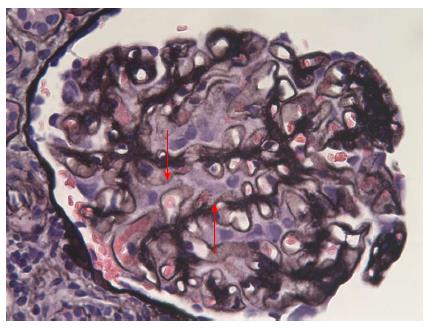
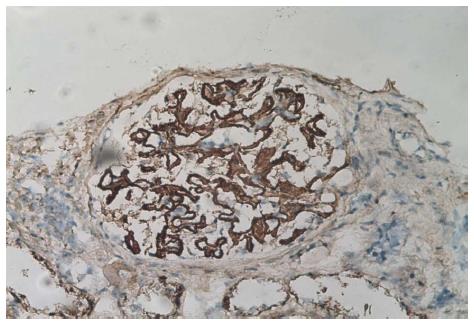
Besides a raised blood pressure of 155/105 mmHg, clinical examination was unremarkable. At that moment the patient treatment consisted of 75 mg/d of 6-mercaptopurine, 8 mg/d of methyl-prednisolone, 1 g/d of ursodeoxycholic acid, and 2 g/d of mesalazine. Blood work performed at the emergency department showed hypoalbuminemia [17 (range: 35-50) g/L] with normal creatinine level [1.15 (range: 0.6-1.3) mg/dL]. A 24 h urine sample confirmed the presence of severe nephrotic syndrome with a proteinuria of 23400 mg/L, or 10400 mg/g of creatinine with a total of 46.3% of albumin. The protein fractions were normal. An abdominal ultrasound was unremarkable. On the next day kidney biopsies (Figures 2 and 3) were performed showing the presence of an extra-membranous glomerulonephritis. Immunohistology showed the presence of subepithelial deposits of IgG, C3d, and C4d. C1q, IgA, and IgMs were not detected. Despite the absence of renal failure, the presence of massive proteinuria pushed us to increase the corticosteroids to 64 mg/d. Cyclophosphamide, as well as calcineurin inhibitors, were not available as alternatives due to the patient receiving 6-MP at that time. This treatment was supplemented with bumetanide 0.5-1 mg/d, lisinopril 20 mg/d, CaCO3 1250 mg/d, and omeprazole 20 mg/d. One month after initiation of treatment, the nephrotic syndrome went into complete remission. Diuretics were stopped and corticosteroids were progressively reduced. Unfortunately, the nephrotic syndrome reemerged as soon as corticosteroids were weaned, and after two consecutive episodes we decided to convert the treatment regimen to cyclophosphamide 2 mg/kg per day. 6-MP was stopped during the next 6 mo. The patient responded well to this alteration so that corticosteroids could again be reduced to 16 mg/d. Liver function remained stable.
In August 2011, the clinical and biological evolution stabilized so that the patient was finally weaned from systemic corticosteroids. His final treatment consisted of 5 mg/d of beclomethasone, 75 mg/d of 6-MP, 2 g/d of mesalazine, and 1 g/d of ursodeoxycholic acid. The administration of cyclophosphamide was stopped indefinitely.
The term “PSC-AIH overlap syndrome” is used to describe different varieties of AIH autoimmune and/or PSC in a setting where an unspecific number of characteristics of one disease are present[1].
Other forms of overlap syndromes have been described as combining the simultaneous presence of different diseases like AIH and primary biliary cirrhosis. A precise clinical and pathological definition is still missing. Concerning PSC-AIH overlap syndrome, Czaja[2] suggested that it is possibly an atypical manifestation of a classic disease, rather than a completely new entity. However, the International Auto-Immune Hepatitis Group (IAIHG) takes no clear position on the matter, leaving the door open to a wide variety of possibilities: consecutive presentation, concomitant presentation, existence of a continuum between the two diseases, or presence of a disease that also has one or more other features of the other[3]. The PSC-AIH overlap syndrome is most often described in children, adolescents, or young adults[1,3-5]. Before confirming the diagnosis of an overlap syndrome it is of the upmost importance to exclude liver toxicity of the pre-existing treatment.
Most large series published in the literature[5-7] concerning overlap syndrome present patients with an initial diagnosis of PSC to whom the diagnostic criteria for auto-immune hepatitis were applied to diagnose or postulate the presence of an overlap syndrome. This situation is comprehensible in the light that well-defined inclusion criteria, although needed, are still absent. The IAIHG recalls, however, that clear and strict criteria are not intended to diagnose an overlap syndrome[3].
A study published in 2005[5] estimated that 17% of patients with PSC have an overlap syndrome (PSC + AIH according to the criteria of IAIHG 1999[8]). On behalf of the IAIHG[8], Boberg estimated a prevalence of 1.8% of AIH (according to the diagnostic criteria of the IAIHG) and 8.8% of probable AIH (according to the criteria of the IAIHG) in a sample of 114 patients suffering from PSC. If one would consider that the diagnosis of overlap syndrome doesn’t necessitate a complete coverage of both diagnostic criteria of each disease, these figures would be much higher.
The patient mentioned in this case report meets both diagnostic criteria for PSC[9,10], as well as for AIH, according to the IAIHG criteria[8], giving us the confidence to diagnose our patient with overlap syndrome. It is important to note that centrilobular necrosis has been described in cases of AIH, particularly in young patients as well as in hepatic transplants[11,12]. No antibody were detected in our patient, but this is the case in almost 10% of AIH[3].
A prospective study[5] of 41 patients comparing those with PSC (n = 37) alone vs those associated with autoimmune hepatitis (n = 7) showed that patients in the latter group were generally younger, had higher IgGs, and higher levels of transaminases. The authors believe that there is a benefit of treating such patients with immunosuppressive and ursodeoxycholic acid. Patients with overlap seemed to have a better survival than those with PSC alone[5]. However, this study didn’t take into account the possibility that the difference in survival may be due to the fact that patients in the overlap group generally presented with less severe forms of PSC. These findings are nonetheless in sharp disagreement with the fact that patients with overlap syndrome have a lower response rate than those with PSC. Statistical analysis methods are not detailed in this study, therefore rendering correct interpretation of results difficult.
Given the lack of specific diagnostic criteria, it is difficult to establish a line of scientifically proven conduct. The guidelines published by the European Association for the Study of the Liver (EASL) recommend treating patients with AIH-PSC overlap by ursodeoxycholic acid and immunosuppressive agents, even though this is not “evidence-based”per se[9]. Note that the American Association for the Study of Liver Diseases also recommends the use of corticosteroids and immunosuppressive agents in these patients[3]. This is in agreement with the usual treatment of patients with PSC associated with ulcerative colitis, since it appears that the treatment of PSC with ursodeoxycholic acid reduces not only the risk of dysplasia[4], but also the prevalence of colorectal carcinomas[4,13], and should be given to every patient with ulcerative colitis and PSC. However, the EASL puts forward that no study could demonstrate any positive effects on the prognosis of patients with PSC, and that the treatment is therefore not “evidence-based”[9]. Culver and Chapman published a review in 2011[4] on the management of the treatment of sclerosing cholangitis and its variants, and concluded that many studies included patients with advanced stages of disease, and that the impact of ursodeoxycholic acid may be different if used much earlier. However, this statement has not been fully evaluated[4]. The notion of overlap syndrome is important because it has been shown[2] that in cases of PSC-AIH overlap there is a weakened response to corticosteroids. It has been equally shown[2,3] that patients, like the one presented in this case report, with AIH, PSC, and ulcerative colitis, have a reduced probability of remission and higher treatment failure rates than patients with normal cholangiography.
The IAIHG noted several working groups that mention a benefit of steroids, but also mentions another group who found a reduced response in these particular patients[8]. Several reviews[4,14] also suggest that liver transplantation is indicated for advanced stages of this disease.
Extra-intestinal manifestations of inflammatory bowel disease, like glomerulonephritis (GN), are considered rare[15] in cases of overlap syndrome. In such instances, GN can have a wide spectrum from minimal change to rapidly progressive (crescent) GN, and may also be accompanied by active tubular-interstitial nephritis[15].
However, we only found 6 cases of membranous glomerulonephritis associated with ulcerative colitis[16-21] and only one published case of PSC associated with membranous glomerulonephritis by Verresen et al[22] in 1988. Furthermore, there are 3 described cases in which an extra-membranous glomerulonephritis is associated with auto-immune hepatitis[23-25].
Lastly, in our review of the literature we found one case similar to the one mentioned in here (i.e., suffering from UC alongside with PSC and membranous glomerulonephritis)[26]. To our knowledge, this case report seems to be the only one presenting a patient with UC, an overlap syndrome involving PSC and AIH, and membranous glomerulonephritis.
Glomerulonephritis caused by aminosalicylates, cyclosporine[15,27-29], and tumor necrosis factor-α inhibitor[27,28] are well known, and described even with a delayed presentation after several years of continuous treatment[30]. 6-mercaptopurine shows no significant direct nephrotoxicity[28]. In the group of six patients described with membranous glomerulonephritis associated with ulcerative colitis, 4 patients[17,18,20,21] did not receive potentially nephrotoxic treatment before the onset of nephrotic syndrome. So it seems that the treatment may not be incriminated in the association of these diseases blindly.
Currently, the research concerning the pathophysiology of glomerulonephritis is in full progress. The recent discovery of the phospholipase A2 receptor as a target antigen in 70% of membranous glomerulonephritis[31] validates these efforts. Nonetheless, until now no study has been able to link certain forms of membranous glomerulonephritis and ulcerative colitis, even though the autoimmune context seems inevitable.
Jaundice, fatigue, and edema in a patient with ulcerative colitis and primary sclerosing cholangitis.
Overlap syndrome [autoimmune hepatitis (AIH) and primary sclerosing cholangitis (PSC)] and membranous glomerulonephritis in a patient with ulcerative colitis.
PSC exacerbation, primary biliary cirrhosis, Wilson disease, and neoplasia are all valid differential diagnoses for hepatic alteration should nephritic syndrome due to treatment toxicity be excluded.
Elevation of liver enzymes and IgG for the diagnosis of AIH. Proteinuria and hypoproteinemia for the membranous nephritis.
For the liver, magnetic resonance imaging and computer tomography were not contributive; the diagnosis is based on histological findings. Such methods are nonetheless necessary to exclude other causes (i.e., neoplastic). For the kidney, the diagnosis is made by means of a urine analysis and kidney biopsy.
Ulcerative colitis was diagnosed by endoscopy and histology, primary sclerosing cholangitis by magnetic resonance imaging and histology, auto-immune hepatitis by histology, and the criteria of the International Auto-Immune Hepatitis Group. Extra-membranous glomerulonephritis was diagnosed by histology (with Jones’ staining).
Corticosteroids are used in treatment in cases of auto-immune exacerbation. Maintenance treatment could be azathioprine, ursodeoxycholic acid for auto-immune hepatitis and primary sclerosing cholangitis, or cyclophosphamide for the treatment of membranous nephritis.
The term “PSC-AIH overlap syndrome” is used to describe different varieties of autoimmune hepatitis autoimmune and/or primary sclerosing cholangitis in a setting where an unspecific number of characteristics of the other disease are present. The term overlap just designs an association of disease. It’s not very clear if it’s just a concomitant presence of the disease, the potential, or if the overlap design are a variant of the diseases.
It’s very important not to forget that clear diagnostic criteria are sometimes more of a theoretical concept and rarely apply to the circumstances found in a specific patient. Therefore a correct knowledge and interpretation of the different sub-characteristics of each disease is the key to allowing tailored therapeutic approaches.
This paper reports associated “autoimmune” diseases in a single patient. The addition of membranous glomerulonephritis to ulcerative colitis and a PSC-AIH overlap is new information. A short note on this new association could be of interest and useful for the future.
| 1. | Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14:3368-3373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Czaja AJ. Difficult treatment decisions in autoimmune hepatitis. World J Gastroenterol. 2010;16:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 3. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 4. | Culver EL, Chapman RW. Systematic review: management options for primary sclerosing cholangitis and its variant forms - IgG4-associated cholangitis and overlap with autoimmune hepatitis. Aliment Pharmacol Ther. 2011;33:1273-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 5. | Floreani A, Rizzotto ER, Ferrara F, Carderi I, Caroli D, Blasone L, Baldo V. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Kaya M, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary sclerosing cholangitis: an evaluation of a modified scoring system. J Hepatol. 2000;33:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Boberg KM, Fausa O, Haaland T, Holter E, Mellbye OJ, Spurkland A, Schrumpf E. Features of autoimmune hepatitis in primary sclerosing cholangitis: an evaluation of 114 primary sclerosing cholangitis patients according to a scoring system for the diagnosis of autoimmune hepatitis. Hepatology. 1996;23:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 108] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 2015] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1227] [Article Influence: 72.2] [Reference Citation Analysis (1)] |
| 10. | Navaneethan U, Venkatesh PG, Lashner BA, Shen B, Kiran RP. The Impact of ulcerative colitis on the long-term outcome of patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Pongpaibul A, Venick RS, McDiarmid SV, Lassman CR. Histopathology of de novo autoimmune hepatitis. Liver Transpl. 2012;18:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Abraham SC, Freese DK, Ishitani MB, Krasinskas AM, Wu TT. Significance of central perivenulitis in pediatric liver transplantation. Am J Surg Pathol. 2008;32:1479-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Saich R, Chapman R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J Gastroenterol. 2008;14:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 14. | Beuers U, Rust C. Overlap syndromes. Semin Liver Dis. 2005;25:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 16. | Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300-2307. [PubMed] |
| 17. | Casella G, Perego D, Baldini V, Monti C, Crippa S, Buda CA. A rare association between ulcerative colitis (UC), celiac disease (CD), membranous glomerulonephritis, leg venous thrombosis, and heterozygosity for factor V Leiden. J Gastroenterol. 2002;37:761-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Makhlough A, Fakheri H. Membranous glomerulonephritis associated with ulcerative colitis. Iran J Kidney Dis. 2008;2:102-104. [PubMed] |
| 19. | Dhiman RK, Poddar U, Sharma BC, Arora P, Saraswat VA, Pandey R, Naik SR. Membranous glomerulonephritis in association with ulcerative colitis. Indian J Gastroenterol. 1998;17:62. [PubMed] |
| 20. | Ridder RM, Kreth HW, Kiss E, Gröne HJ, Gordjani N. Membranous nephropathy associated with familial chronic ulcerative colitis in a 12-year-old girl. Pediatr Nephrol. 2005;20:1349-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Regéczy N, Lakos G, Balogh I, Kappelmayer J, Kiss E. Membranous glomerulonephritis in a patient with inherited activated protein C resistance. Clin Nephrol. 2000;53:390-393. [PubMed] |
| 22. | Verresen L, Waer M, Verberckmoes R, Morias P, Michielsen P. Primary sclerosing cholangitis associated with membranous nephropathy. Ann Intern Med. 1988;108:909-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Efe C, Wahlin S, Ozaslan E, Berlot AH, Purnak T, Muratori L, Quarneti C, Yüksel O, Thiéfin G, Muratori P. Autoimmune hepatitis/primary biliary cirrhosis overlap syndrome and associated extrahepatic autoimmune diseases. Eur J Gastroenterol Hepatol. 2012;24:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Stefanidis I, Giannopoulou M, Liakopoulos V, Dovas S, Karasavvidou F, Zachou K, Koukoulis GK, Dalekos GN. A case of membranous nephropathy associated with Sjögren syndrome, polymyositis and autoimmune hepatitis. Clin Nephrol. 2008;70:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 25. | Takahashi K, Takasaki S, Morita C, Hayashida K, Matsui M, Tamechika Y, Ishibashi H. Autoimmune hepatitis with membranous glomerulonephritis. J Gastroenterol Hepatol. 2001;16:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Wilcox GM, Aretz HT, Roy MA, Roche JK. Glomerulonephritis associated with inflammatory bowel disease. Report of a patient with chronic ulcerative colitis, sclerosing cholangitis, and acute glomerulonephritis. Gastroenterology. 1990;98:786-791. [PubMed] |
| 27. | Oikonomou K, Kapsoritakis A, Eleftheriadis T, Stefanidis I, Potamianos S. Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1034-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Oikonomou KA, Kapsoritakis AN, Stefanidis I, Potamianos SP. Drug-induced nephrotoxicity in inflammatory bowel disease. Nephron Clin Pract. 2011;119:c89-94; discussion c96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Firwana BM, Hasan R, Chalhoub W, Ferwana M, Kang JY, Aron J, Lieber J. Nephrotic syndrome after treatment of Crohn’s disease with mesalamine: Case report and literature review. Avicenna J Med. 2012;2:9-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2007;13:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Hofstra JM, Beck LH, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
P- Reviewers: Efe C, Lindgren S, Tebo AE S- Editor: Zhai HH L- Editor: Rutherford A E- Editor: Zhang DN