Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4797
Revised: February 24, 2014
Accepted: March 4, 2014
Published online: April 28, 2014
Processing time: 110 Days and 16.7 Hours
AIM: To investigate whether computed tomography with 3D imaging (3DCT) can reduce the risks associated with laparoscopic surgery.
METHODS: We performed a retrospective case-control study evaluating the efficacy of preoperative 3DCT of the splenic vascular anatomy on surgical outcomes in patients undergoing laparoscopic spleen-preserving splenic hilar lymph node (LN) dissection for upper- or middle-third gastric cancer. The clinical records of 312 patients with upper- or middle-third gastric cancer who underwent laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection in our hospital from January 2010 to June 2013 were collected, and the patients were divided into two groups (group 3DCT vs group NO-3DCT) depending on whether they underwent 3DCT or not. Clinicopathologic characteristics, operative and postoperative measures, the number of retrieved LNs, and complications were compared between these two groups. Patients were further compared regarding operative and postoperative measures, the number of retrieved LNs, and complications when subdivided by body mass index ( ≥ 23 and < 23 kg/m2) and the number of operations performed by their surgeon (≤ 40 vs > 40).
RESULTS: The mean numbers of retrieved splenic hilar LNs were similar in patients in group 3DCT and group NO-3DCT (2.85 ± 2.33 vs 2.48 ± 2.18, P > 0.05). The operation time and blood loss at the splenic hilum were lower in the patients in group 3DCT (P < 0.05 each). The postoperative recovery time and complication rates were similar between the two groups (P > 0.05 each). Subgroup analysis showed that the operation time at the splenic hilum in patients with a BMI ≥ 23 kg/m2 was significantly shorter in patients in group 3DCT than in group NO-3DCT (20.27 ± 5.84 min vs 26.17 ± 11.01 min, P = 0.003). In patients with a BMI < 23 kg/m2, the overall operation time (171.8 ± 26.32 min vs 188.09 ± 52.63 min, P = 0.028), operation time at the splenic hilum (19.39 ± 5.46 min vs 23.74 ± 9.56 min, P = 0.001), and blood loss at the splenic hilum (13.27 ± 4.96 mL vs 17.98 ± 8.12 mL, P = 0.000) were significantly lower in patients in group 3DCT than in group NO-3DCT. After 40 operations, the operation time (18.63 ± 4.40 min vs 23.85 ± 7.92 min, P = 0.000) and blood loss (13.10 ± 4.17 mL vs 15.10 ± 4.42 mL, P = 0.005) at the splenic hilum were significantly lower in patients who underwent 3DCT, but there were no significant between-group differences prior to 40 operations.
CONCLUSION: 3DCT is critical for surgical guidance to reduce the risks of splenic LN dissection. This method may be important in safely facilitating laparoscopic spleen-preserving splenic LN dissection.
Core tip: The JGCA guidelines recommend splenic hilar lymph node (LN) dissection in patients with upper- and middle-third advanced gastric cancer. However, the surgery is made more difficult by anatomic complications of the vessels around the stomach, particularly the splenic vessels, which are located in a narrow, deep space. The inability to intuitively judge the shape of the splenic vessels increases the likelihood of vascular injury. Preoperative assessment of the splenic vascular anatomy at the splenic hilum is important for the safe and rapid performance of laparoscopic spleen-preserving splenic hilar LN dissection. Computed tomography with 3D imaging can be used for surgical guidance to reduce the risks of splenic LN dissection.
- Citation: Wang JB, Huang CM, Zheng CH, Li P, Xie JW, Lin JX, Lu J. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol 2014; 20(16): 4797-4805
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4797.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4797
Since the first report of laparoscopic-assisted distal gastrectomy (LADG) for early gastric cancer[1], laparoscopic surgery for gastric cancer has been shown to be an effective modality, with significant advantages over open surgery, including a smaller surgical incision, reduced intraoperative bleeding, less postoperative pain, faster recovery of bowel function, a shorter hospital stay, faster return to daily activities, and improved quality of life[2-6]. The indications for laparoscopic gastrectomy have been extended to include patients with advanced gastric cancer (AGC). Laparoscopic surgery, however, still has limitations, including the lack of tactile sensation, a reduced view of the operative field, and the inability of the surgeon to directly manipulate organs and lesions during surgery. Laparoscopy-assisted total gastrectomy (LATG) is more difficult to perform than LADG due to the additional requirement for the dissection of lymph nodes (LNs) at the splenic hilum and the need for esophagojejunostomy. As laparoscopic techniques have improved, the number of patients undergoing LATG has increased annually[7]. LATG, which has been reported to be technically more feasible than LADG[2], is increasingly used in the treatment of upper- and middle-third gastric cancer. Japanese Gastric Cancer Association guidelines recommend splenic hilar LN dissection in patients with upper- and middle-third AGC who undergo LATG with D2 LN dissection[8]. With the regeneration of the surgical treatment concept and the development of laparoscopic surgery, laparoscopic spleen-preserving splenic hilar LN dissection has gradually been applied to upper- or middle-third gastric cancer patients.
Despite the increased use of laparoscopic spleen-preserving splenic hilar LN dissection in these patients, surgery is made more difficult by anatomic complications of the vessels around the stomach, particularly the splenic vessels, which are located in a narrow, deep space. The inability to intuitively judge the shape of the splenic vessels increases the likelihood of vascular injury and bleeding. Moreover, a lack of knowledge of splenic vascular anatomy, an inability to manipulate tissues, and a limited operative field of view may result in difficult and time-consuming dissections to search for blood vessels and anatomical landmarks, increasing the risk of iatrogenic vascular and visceral injuries.
The preoperative assessment of splenic vascular anatomy at the splenic hilum is important for the safe and rapid performance of laparoscopic spleen-preserving splenic hilar LN dissection. The vascular anatomy can be mapped preoperatively using computed tomography (CT) angiography, followed by processing of the images with rendering software to reconstruct 3D images of the splenic vessels. These models can be rotated and viewed from different angles to identify the course of each splenic vessel and its relationship to other anatomical structures. Determining vascular anatomy by CT with 3D imaging (3DCT) imaging is critical for reducing the risks associated with laparoscopic gastric cancer surgery[9]. We, therefore, performed a retrospective case-control study evaluating the efficacy of preoperative 3DCT of the splenic vascular anatomy on surgical outcomes in patients undergoing laparoscopic spleen-preserving splenic hilar LN dissection for upper- or middle-third gastric cancer.
Between January 2010 and June 2013, 312 patients with upper- or middle-third gastric cancer underwent laparoscopic-assisted total gastrectomy with D2 LN dissection plus spleen-preserving splenic hilar LN dissection in the Department of Gastric Surgery, Fujian Medical University Union Hospital. All subjects were preoperatively confirmed as having upper- or middle-third AGC by analyses of endoscopic biopsy specimens. Preoperative imaging was routinely performed following endoscopic examination, including CT scanning, ultrasonography (US) of the abdomen, and endoscopic US. All patients also underwent intraoperative diagnostic laparoscopy, including a complete examination of the peritoneal cavity and liver. Patients preoperatively diagnosed with T1 and T4b gastric cancer were excluded. Patients with enlarged and integrated splenic hilar LNs were not considered candidates for surgery. The surgical procedure, including its advantages and risks, was explained to all candidates for surgery. The ethics committee of Fujian Union Hospital approved this retrospective study. Written consent was given by the patients for their information to be stored in the hospital database and used for research.
All patients underwent abdominal helical CT (Discovery CT750 HD), with scans ranging from the top of the diaphragm to the lower edge of the liver. An average of 100 mL of nonionic contrast agent was infused at a rate of 2 mL/s. CT data acquisition was triggered using the bolus tracking technique, with the region of interest (ROI) placed in the abdominal aorta just below the diaphragmatic dome. The trigger threshold level was set at a CT value of 100 Hounsfield units. Abdominal CT scans were performed at a slice thickness of 5 mm. During each phase, scanning was performed in a single breath-hold. 3DCT images of the splenic vessels were individually reconstructed using the original scanning images. The CT data were downloaded to an offline workstation for image post-processing and analysis, and 3DCT reconstructions were performed by a team of professional radiologists. The patients were assigned to two groups in accordance with their wishes: 231 patients underwent CT with 3D angiography (group 3DCT), and 81 underwent CT without 3D angiography (group NO-3DCT). The following were compared between the two groups: patient demographic and clinical characteristics, including age, sex, BMI, tumour size, depth of invasion, LN metastasis, and TNM stage[10]; operative data, including operation time, blood loss, number of positive and retrieved splenic hilar LNs, and number of positive and retrieved LNs; and postoperative data, including postoperative hospital stay, day of first flatus, day of first fluid diet, day of first semifluid diet, and postoperative complications. The operation time at the splenic hilum was defined as the time of splenic hilar LN dissection, and the blood loss at the splenic hilum was defined as the volume of blood lost during dissection of the splenic hilar LNs. The patients in each group were further subdivided by BMI ( ≥ 23 vs < 23 kg/m2) and the number of operations performed by their surgeon ( ≤ 40 vs > 40 cases). According to patients’ wishes, patients in each subgroup were also distributed into 3DCT and No-3DCT groups, and their surgical outcomes were compared. High (≥ 23 kg/m2) and normal (< 23 kg/m2) BMIs were defined according to the World Health Organisation definitions for individuals in the western Pacific region[11].
Splenic hilar LN dissection was performed according to the guidelines of the Japanese Classification of Gastric Carcinoma[10]. At our institution, laparoscopic spleen-preserving splenic hilar LN dissection has become highly standardised[12,13], with all operations performed by a senior surgeon (Chang-Ming Huang) who had performed > 500 laparoscopic-assisted gastrectomies before starting to perform this operation.
Patient positioning: The patient was placed in the reverse Trendelenburg position with the head elevated approximately 15-20 degrees and tilted left-side up approximately 20-30 degrees. The surgeon stood between the patient’s legs, and the assistant and camera operator were both on the patient’s right side.
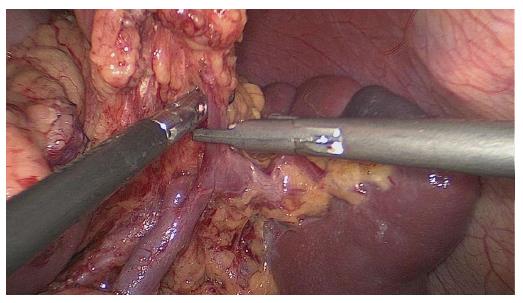
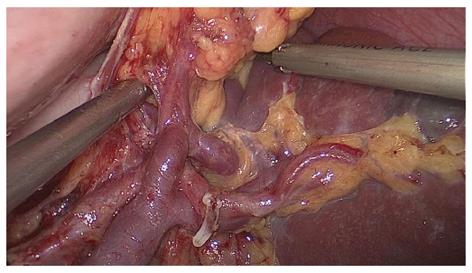
Splenic hilar LN dissection: Before the operation, the assistant placed the greater omentum behind the stomach to keep the visual field clear, pulled the body of the stomach towards the upper right, and tensed the splenogastric ligament while the surgeon gently pressed the tail of the pancreas towards the lower left, thus exposing the splenic hilum. The surgeon opened the pancreatic envelope, ultrasonically separated the membrane of the body and tail of the pancreas to reach the posterior pancreas space at the superior border of the pancreas, and opened the vascular envelope at the end of the splenic arteries. The surgeon dissected the lymphatic fatty tissue on the surface of the inferior splenic lobar artery from the lower pole of the spleen. The left gastroepiploic artery issuing from the inferior splenic lobar artery was vascularised and clamped after cutting its origin (Figure 1). The assistant gently pulled the lymphatic fatty tissue on the surface of the inferior splenic lobar artery. Starting from the root of the left gastroepiploic artery, the surgeon, using the non-functioning face of the ultrasonic scalpel, closed the surface of the inferior splenic lobar artery. The surgeon used the ultrasonic scalpel to carefully dissect the lymphatic fatty tissue and vascularise the inferior splenic lobar artery. As the latter was gradually revealed, the two branches of the short gastric arteries issuing from it were skeletonised and divided at their roots, resulting in the complete vascularisation of the inferior splenic lobar artery (Figure 2). The assistant then pulled up the fatty tissues and gastric tissues, and the surgeon dissected the lymphatic fatty tissue on the surface of the superior splenic lobar artery, starting from its root towards the upper pole of the spleen, similar to the procedure used to vascularise the inferior splenic lobar artery. One branch of the short gastric artery issuing from the superior splenic lobar artery was skeletonised and divided at its root, thus concluding the dissection of the LNs at the front of the splenic vessels.
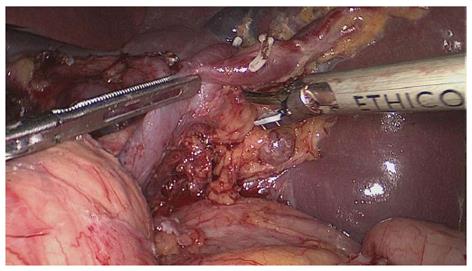
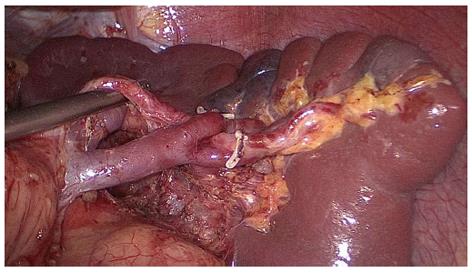
The assistant pulled the root of the inferior splenic lobar artery towards the upper right, revealing the lymphatic fatty tissue behind the splenic hilum, which was pulled by the surgeon towards the lower left to maintain the tension (Figure 3). This lymphatic fatty tissue behind the splenic hilum was dissected. Finally, the splenic hilar LNs were dissected completely (Figure 4).
Statistical analyses were performed using the SPSS 18.0 statistical software package. Means were compared using t-tests and categorical data using Chi-square tests. Proportions were compared by the χ2 test or Fisher’s exact test. P values < 0.05 were considered statistically significant.
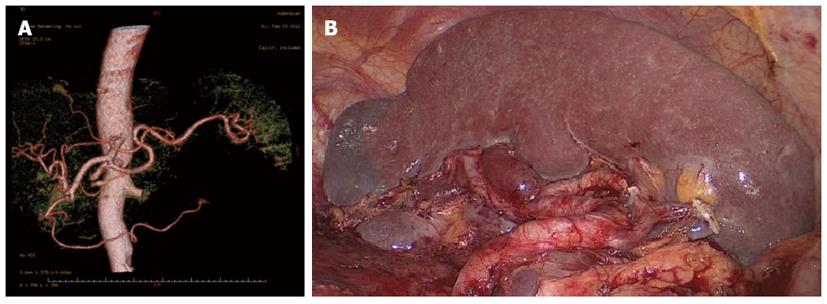
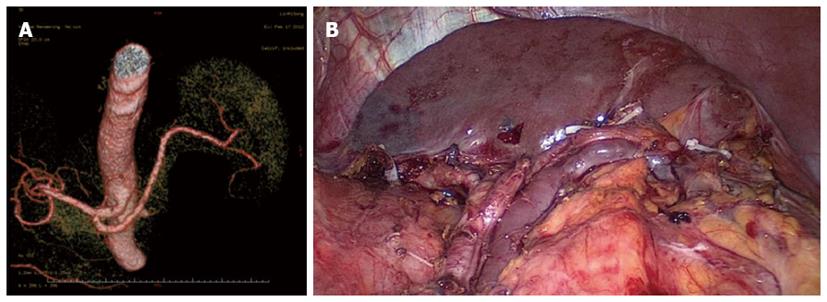
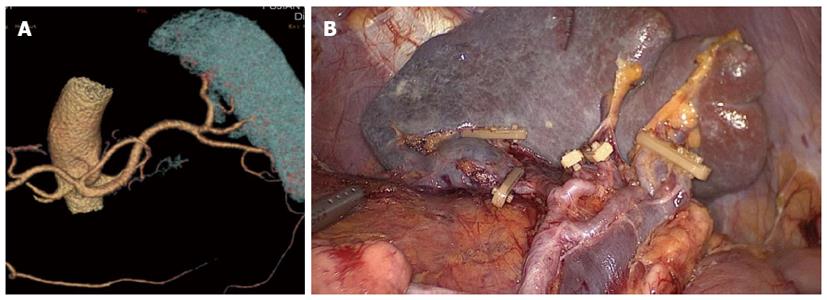
Between January 2010 and December 2012, 312 patients with upper- or middle-third gastric cancer underwent laparoscopic-assisted total gastrectomy with D2 LN dissection and spleen-preserving splenic hilar LN dissection. Of these, 231 patients underwent CT scans with 3D angiography, and 81 underwent CT scans without 3D angiography. The mean age of the 312 patients was 60.86 ± 10.51 years. The clinicopathologic characteristics were similar among patients in both the 3DCT and NO-3DCT groups (Table 1). The types of splenic lobe vessels observed preoperatively in group 3DCT were in accordance with their intraoperative conditions (Figures 5-7). The mean operation time (173.65 ± 27.12 min vs 189.56 ± 48.36 min; P = 0.007), mean operation time at the splenic hilum (19.70 ± 5.59 min vs 24.47 ± 9.98 min; P = 0.001), and mean blood loss at the splenic hilum (13.62 ± 4.50 mL vs 17.92 ± 9.08 mL; P = 0.001) were lower in patients who were not evaluated by 3DCT. In contrast, the number of retrieved and positive splenic hilar LNs, number of retrieved and positive LNs, and mean blood loss were similar in the two groups (P > 0.05 each). There were also no significant differences between these two groups with regards to days to first flatus, first fluid diet, and first semifluid diet, postoperative hospital stay, and complications (P > 0.05) (Tables 2 and 3).
| Patient characteristics | Group NO-3DCT | Group 3DCT | P value |
| No. of patients | 81 | 231 | |
| Age (yr) | 59.48 ± 10.82 | 61.34 ± 10.38 | 0.542 |
| Gender | 0.484 | ||
| Male | 58 | 178 | |
| Female | 23 | 52 | |
| BMI, kg/m2 | 21.67 ± 3.24 | 21.95 ± 2.74 | 0.273 |
| Tumour size, mm | 57.53 ± 23.66 | 55.76 ± 27.88 | 0.251 |
| Histology | 0.262 | ||
| Differentiated | 29 | 98 | |
| Undifferentiated | 52 | 133 | |
| Depth of invasion | 0.098 | ||
| pT1a | 5 | 10 | |
| pT1b | 6 | 9 | |
| pT2 | 1 | 22 | |
| pT3 | 29 | 87 | |
| pT4a | 40 | 103 | |
| Lymph node metastasis | 0.090 | ||
| pN0 | 25 | 45 | |
| pN1 | 13 | 37 | |
| pN2 | 12 | 43 | |
| pN3 | 31 | 106 | |
| TNM stage | 0.487 | ||
| Ia | 6 | 15 | |
| Ib | 4 | 12 | |
| IIa | 15 | 25 | |
| IIb | 9 | 27 | |
| IIIa | 12 | 31 | |
| IIIB | 12 | 52 | |
| IIIC | 23 | 69 | |
| Group NO-3DCT | Group 3DCT | P value | |
| Operation time, min | 189.56 ± 48.36 | 173.65 ± 27.12 | 0.007 |
| Operation time at splenic hilum, min | 24.47 ± 9.98 | 19.70 ± 5.59 | 0.001 |
| Blood loss, mL | 57.34 ± 30.78 | 50.18 ± 28.89 | 0.064 |
| Blood loss at the splenic hilum, mL | 17.92 ± 9.08 | 13.62 ± 4.50 | 0.001 |
| No. of positive No. 10 lymph nodes | 0.25 ± 0.79 | 0.24 ± 0.85 | 0.889 |
| No. of retrieved No. 10 lymph nodes | 2.48 ± 2.18 | 2.85 ± 2.33 | 0.225 |
| No. of positive lymph nodes | 7.97 ± 10.65 | 9.10 ± 10.08 | 0.414 |
| No. of retrieved lymph nodes | 39.47 ± 13.51 | 43.19 ± 15.64 | 0.067 |
| Group NO-3DCT | Group 3DCT | P value | |
| Postoperative hospital stay, d | 11.03 ± 2.84 | 12.29 ± 6.49 | 0.094 |
| Day of first flatus, d | 3.95 ± 1.07 | 4.19 ± 1.04 | 0.079 |
| Day of first fluid diet, d | 4.29 ± 1.12 | 4.55 ± 1.70 | 0.205 |
| Day of first semifluid diet, d | 7.85 ± 1.92 | 8.15 ± 3.52 | 0.463 |
| Blood transfusion, n | 0 | 0 | 1.000 |
| Postoperative complications, n | 11 | 35 | 0.731 |
| Pulmonary infection, n | 7 | 11 | 0.141 |
| Abdominal infection, n | 1 | 12 | 0.085 |
| Chylous fistula, n | 1 | 2 | 0.770 |
| Wound problem, n | 0 | 3 | 0.303 |
| Anastomotic leakage, n | 0 | 3 | 0.303 |
| Anastomotic bleeding, n | 1 | 3 | 0.965 |
| Septicaemia, n | 0 | 1 | 0.554 |
| Postoperative mortality, n | 0 | 0 | 1.000 |
Subgroup analyses of the patients with a BMI ≥ 23 kg/m2 showed that those who were evaluated by preoperative 3DCT had a significantly shorter mean operation time at the splenic hilum (20.27 ± 5.84 min vs 26.17 ± 11.01 min; P = 0.003). Of the patients with a BMI < 23 kg/m2, those who underwent 3DCT had a significantly lower overall operation time (171.8 ± 26.32 vs 188.09 ± 52.63 min, P = 0.028), operation time at the splenic hilum (19.39 ± 5.46 vs 23.74 ± 9.56 min, P = 0.001), and blood loss at the splenic hilum (13.27 ± 4.96 vs 17.98 ± 8.12 ml, P = 0.000) than did the patients in group NO-3DCT (Table 4). To determine the impact of 3DCT on these parameters, we assessed the effect of the ‘‘learning curve’’ for laparoscopic spleen-preserving splenic hilar LN dissection. A previous report from our department indicated that the learning curve for laparoscopic spleen-preserving splenic hilar LN dissection reached a plateau after 40 operations[13]. When patients were divided into those treated by surgeons who had performed ≤ 40 or > 40 of these operations, we found that of the patients operated on by surgeons who had performed > 40 operations, those who had undergone 3DCT had a significantly lower operation time (18.63 ± 4.40 min vs 23.85 ± 7.92 min, P = 0.000) and blood loss (13.10 ± 4.17 mL vs 15.10 ± 4.42 mL, P = 0.005) at the splenic hilum than patients who had not undergone 3DCT. In contrast, there were no significant differences between these two groups when operated on by surgeons who had performed < 40 such operations (Table 5).
| BMI < 23 kg/m2 (n = 201) | BMI≥23 kg/m2 (n = 111) | |||||
| Group NO-3DCT | Group 3DCT | P value | Group NO-3DCT | Group 3DCT | P value | |
| No. of patients | 56 | 145 | 25 | 86 | ||
| Age, yr | 57.73 ± 11.59 | 60.95 ± 11.43 | 0.745 | 63.40 ± 7.72 | 61.99 ± 8.37 | 0.807 |
| Operation time, min | 188.09 ± 52.63 | 171.38 ± 26.32 | 0.028 | 187.92 ± 32.00 | 177.44 ± 28.17 | 0.121 |
| Operation time at splenic hilum, min | 23.74 ± 9.56 | 19.39 ± 5.46 | 0.001 | 26.17 ± 11.01 | 20.27 ± 5.84 | 0.003 |
| Blood loss, mL | 61.27 ± 35.94 | 51.83 ± 22.66 | 0.073 | 48.33 ± 7.61 | 49.41 ± 34.73 | 0.670 |
| Blood loss at splenic hilum, mL | 17.98 ± 8.12 | 13.27 ± 4.96 | 0.000 | 17.78 ± 11.28 | 14.29 ± 3.39 | 0.054 |
| No. of retrieved lymph nodes | 38.38 ± 12.25 | 46.18 ± 16.86 | 0.002 | 42.09 ± 16.15 | 37.86 ± 11.48 | 0.172 |
| No. of retrieved No. 10 lymph nodes | 2.67 ± 2.27 | 3.20 ± 2.42 | 0.162 | 2.04 ± 1.94 | 2.23 ± 2.06 | 0.692 |
| Postoperative hospital stay, d | 10.69 ± 2.29 | 11.62 ± 5.84 | 0.256 | 11.79 ± 3.77 | 13.42 ± 7.36 | 0.299 |
| Postoperative complications, n | 9 | 18 | 0.495 | 2 | 17 | 0.169 |
| Less than 40 cases (n = 40) | More than 40 cases (n = 272) | |||||
| Group NO-3DCT | Group 3DCT | P value | Group NO-3DCT | Group 3DCT | P value | |
| No. of patients | 18 | 22 | 59 | 213 | ||
| Age, yr | 61.05 ± 10.14 | 59.22 ± 13.46 | 0.827 | 63.81 ± 8.03 | 60.96 ± 10.52 | 0.053 |
| Operation time, min | 205.00 ± 67.36 | 199.44 ± 46.99 | 0.769 | 176.40 ± 27.83 | 173.39 ± 27.59 | 0.466 |
| Operation time at the splenic hilum, min | 30.27 ± 11.58 | 28.92 ± 11.61 | 0.779 | 23.85 ± 7.92 | 18.63 ± 4.40 | 0.000 |
| Blood loss, mL | 69.32 ± 37.49 | 66.35 ± 33.10 | 0.368 | 49.18 ± 23.25 | 50.29 ± 29.97 | 0.799 |
| Blood loss at the splenic hilum, mL | 30.27 ± 8.17 | 32.38 ± 13.61 | 0.514 | 15.10 ± 4.42 | 13.10 ± 4.17 | 0.005 |
| No. of retrieved lymph nodes | 34.97 ± 10.67 | 25.33 ± 10.26 | 0.140 | 43.54 ± 14.61 | 43.47 ± 15.56 | 0.980 |
| No. of retrieved No. 10 lymph nodes | 2.81 ± 2.00 | 2.33 ± 1.87 | 0.313 | 2.74 ± 2.33 | 2.88 ± 2.33 | 0.719 |
| Postoperative hospital stay, d | 12.87 ± 1.78 | 11.91 ± 2.59 | 0.112 | 11.93 ± 5.27 | 12.15 ± 6.37 | 0.809 |
| Postoperative complications, n | 3 | 3 | 0.789 | 8 | 32 | 0.779 |
Advances in surgical concepts, improvements in anatomical techniques, and progress in organ retention have indicated the increased feasibility of spleen-preserving splenic LN dissection[14-16]. Laparoscopic splenic LN dissection has also been shown to be safe and feasible compared with open surgery. Its advantages over open surgery include its minimal invasiveness and the ability to leave the spleen intact without mobilising it while similarly dissecting the splenic LNs[17-19].
The identification of the splenic vessels is often critical in performing laparoscopic spleen-preserving splenic hilar LN dissection. Clinically, the vessels in the splenic hilum are particularly intricate and variable and are covered with much fatty lymphoid tissue; in addition, the spleen often adheres to the omentum or peritoneum. The areas adjacent to the splenic hilum are complex and located in a narrow, but very deep, operating space, making it difficult and time consuming to identify the proper vessels in each patient and to complete splenic regional LN dissection during both open and laparoscopic procedures. Moreover, the laparoscopic technique has significant limitations, being essentially two dimensional with a loss of depth perception and spatial orientation. It also lacks the surgeon’s intuitive touch and exposure, with laparoscopic grasping forceps used only for traction and separation in the local area. The inability to manipulate tissue and the limited view of the operative field hinder the identification of vessels and procedure-specific anatomical landmarks. This difficulty results in longer operating times and an increased risk of visceral and vascular injuries. The latter can cause major complications, such as massive bleeding and bowel ischaemia, particularly for obese patients. In general, laparoscopic spleen-preserving splenic hilar LN dissection is much more difficult in obese patients because of their narrower abdominal cavity, greater accumulation of fatty lymphoid tissue covering the vessels, and limited visualisation of surgical fields[20]. Preoperative 3DCT evaluation of vascular anatomy may therefore aid the safe and rapid ligation of vessels and dissection of the splenic hilar LNs, particularly in obese patients.
The importance of the preoperative assessment of vessel anatomy has been recognised since the era of interventional percutaneous angiography. Improvements in CT scanner technology have enabled the accurate reconstruction of the images of various vessels[21]. 3DCT has been shown to be clinically useful in various diagnostic fields, including abdominal surgery, in which 3DCT has been utilised for the preoperative evaluation of aortic aneurysms and pancreatic cancers and for the preoperative assessment and planning of liver and kidney transplants[22]. Recent advances in 3DCT have also enabled its use in gastric resection. In addition, 3D angiography prior to laparoscopic gastrectomy has been shown to be useful in the detection of perigastric vessels, including the left gastric artery and left gastric vein[23-26]. 3DCT imaging of the vascular anatomy was found to be of critical importance in reducing the risks associated with laparoscopic gastric cancer surgery[9] and for reducing blood loss during surgery[26]. The overlapping of different phase images with 3D reconstruction also helps to identify the anatomical correlations between arteries and veins[27]. Prior knowledge of the particular gastric vascular anatomy in a patient can aid accurate surgical planning, reducing the risk of complications. To our knowledge, no previous study has assessed the detectability of the splenic vessels or analysed their anatomic variety to properly plan laparoscopic spleen-preserving splenic hilar LN dissection. 3DCT not only produces high-quality images, which provide excellent visualisation of the vessel anatomy, but can also detect subtle vascular abnormalities. 3D reconstructions have enabled the planning of dissections and the localisation of topographical landmarks for the identification of the splenic vessels, helping to avoid lengthy and harmful dissections while searching for splenic blood vessels or those with non-typical courses and even simplifying their identification in obese patients. These 3D images can be rotated, magnified, and examined in different planes to evaluate the anatomical relationships and anomalies of the splenic vessels and their collateral vessels, as well as their presence or absence[28].
The types of splenic lobe vessels preoperatively detected by 3DCT were in accordance with intraoperative conditions. Intraoperative complications, such as vascular and visceral injuries and bleeding, as well as the mean operation time and blood loss at the splenic hilum, were significantly reduced in patients who underwent 3DCT compared to those who did not. 3DCT enabled surgeons to determine the distribution of the splenic vessels preoperatively, avoiding intraoperative searching for missing vessels and helping to identify those that were present. The subgroup analysis showed that the operation time at the splenic hilum was significantly lower in patients with a BMI ≥ 23 kg/m2 who had undergone 3DCT than in those who had not. Moreover, both the operation time at the splenic hilum and blood loss were significantly lower in patients with a BMI < 23 kg/m2 who had undergone 3DCT. In patients with a higher BMI, visualisation of the surgical fields is limited, the anatomical level of the splenic vessels is unclear due to excessive fat accumulation, and the blood supply is rich in soft tissues. All of these factors can result in uncontrollable bleeding and make surgical fields unclear, increasing the risks and operation time during laparoscopic splenic hilar LN dissection. Thus, precise preoperative knowledge of the splenic vascular anatomy can reduce stress and operation time and avoid unnecessary injury to blood vessels. In patients with a normal BMI without obesity, the variation of the splenic hilar blood vessels is the most important influencing factor when undergoing splenic hilar LN dissection. With the ability to preoperatively confirm the splenic vascular anatomic variation by 3DCT, surgical difficulties and operation time would be dramatically decreased, as would splenic vascular injuries. 3DCT presents more advantages in patients with a normal BMI.
We also performed a subgroup analysis based on the experience of the surgeon in performing laparoscopic spleen-preserving splenic hilar LN dissection for upper- and middle-third gastric cancer. Twenty-two patients underwent 3DCT and had surgeons who had completed less than 40 operations; 59 patients did not undergo 3DCT and had surgeons who had completed more than 40 operations. Both sets of patients were allocated into the appropriate subgroup according to their wishes. Both the operation time and blood loss at the splenic hilum in patients operated on by surgeons who had performed more than 40 such operations were significantly lower with 3DCT than those in patients without 3DCT. Although the operation time and blood loss were also lower with versus without 3DCT during operations performed by surgeons with less surgical experience, those differences were not statistically significant. Operative technique has been shown to affect the operation time and blood loss at the splenic hilum but not vessel reconstruction. However, surgeons gain additional experience with the improvement of surgical technology, and splenic vascular anatomic variation has gradually become the essential factor affecting operation time and blood loss. 3DCT can have significant effects. Without sufficient knowledge of splenic anatomy, surgeons may injure splenic vessels during LN dissection, increasing operation time and blood loss. By contrast, with the wide application of 3DCT, all these injuries could be avoided during an operation performed by an informed surgeon. Therefore, 3DCT could be beneficial for splenic hilar LN dissection.
In conclusion, preoperative 3DCT analysis of splenic vessels is precise and informative for surgeons, enabling laparoscopic total gastrectomy with spleen-preserving splenic LN dissection to be performed more easily and safely.
The JGCA guidelines recommend splenic hilar lymph node (LN) dissection in patients with upper- and middle-third advanced gastric cancer. However, the surgery is made more difficult by anatomic complications of the vessels around the stomach, particularly the splenic vessels, which are located in a narrow, deep space. The inability to intuitively judge the shape of the splenic vessels increases the likelihood of vascular injury and bleeding. Preoperative assessment of the splenic vascular anatomy at the splenic hilum is important for the safe and rapid performance of laparoscopic spleen-preserving splenic hilar LN dissection.
Vascular anatomy can be mapped preoperatively using computed tomography (CT) angiography, followed by processing of the images with rendering software to reconstruct 3D images of the splenic vessels. These models can be rotated and viewed from different angles to identify the course of each splenic vessel and its relationship to other anatomical structures.
The results of the current study demonstrate that with the help of computed tomography with 3D imaging (3DCT), reduced operation time and blood loss at the splenic hilum can be achieved. Furthermore, the operation time at the splenic hilum in patients with a body mass index (BMI) ≥ 23 kg/m2 was also shorter in group 3DCT than in group NO-3DCT, as was the overall operation time, operation time at the splenic hilum, and blood loss at the splenic hilum in 3DCT group patients with a BMI < 23 kg/m2. After 40 operations, the operation time and blood loss at the splenic hilum were significantly lower in patients who underwent 3DCT.
Preoperative 3DCT analysis of splenic vessels is precise and informative for surgeons, enabling laparoscopic total gastrectomy with spleen-preserving splenic LN dissection to be performed more easily and safely. Preoperative 3DCT can be used for surgical guidance to reduce the risks of splenic LN dissection.
BMI: Body mass index was used as an objective index to indicate massive obesity. The cutoff value was chosen according to the World Health Organisation Western Pacific Region: The Asia-Pacific Perspective.
This is an interesting study in which the authors evaluate the efficacy of 3DCT for assessing the distribution of splenic vessels in patients with upper- and middle-third gastric cancer. This finding has important clinical implications for gastrointestinal surgeons in spleen-preserving splenic LN dissection, particularly for beginners.
| 1. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Lee J, Kim W. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol. 2009;100:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Shuang J, Qi S, Zheng J, Zhao Q, Li J, Kang Z, Hua J, Du J. A case-control study of laparoscopy-assisted and open distal gastrectomy for advanced gastric cancer. J Gastrointest Surg. 2011;15:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Kim MC, Jung GJ, Kim HH. Morbidity and mortality of laparoscopy-assisted gastrectomy with extraperigastric lymph node dissection for gastric cancer. Dig Dis Sci. 2007;52:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (2)] |
| 6. | Lee JH, Ahn SH, Park do J, Kim HH, Lee HJ, Yang HK. Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg. 2012;36:2394-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Lee MS, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 9. | Lee SW, Shinohara H, Matsuki M, Okuda J, Nomura E, Mabuchi H, Nishiguchi K, Takaori K, Narabayashi I, Tanigawa N. Preoperative simulation of vascular anatomy by three-dimensional computed tomography imaging in laparoscopic gastric cancer surgery. J Am Coll Surg. 2003;197:927-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 11. | World Health Organization Western Pacific Region, International Association for the Study of Obesity, International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney, Australia: Health Communications Australia Pty Limited 2000; 17-18. |
| 12. | Jia-Bin W, Chang-Ming H, Chao-Hui Z, Ping L, Jian-Wei X, Jian-Xian L. Laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer in left approach: a new operation procedure. World J Surg Oncol. 2012;10:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Lu J, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX. Learning curve of laparoscopy spleen-preserving splenic hilar lymph node dissection for advanced upper gastric cancer. Hepatogastroenterology. 2013;60:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kwon SJ. Prognostic impact of splenectomy on gastric cancer: results of the Korean Gastric Cancer Study Group. World J Surg. 1997;21:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Sano T, Yamamoto S, Sasako M. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol. 2002;32:363-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Hur H, Jeon HM, Kim W. Laparoscopic pancreas- and spleen-preserving D2 lymph node dissection in advanced (cT2) upper-third gastric cancer. J Surg Oncol. 2008;97:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (3)] |
| 18. | Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Okabe H, Obama K, Kan T, Tanaka E, Itami A, Sakai Y. Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg. 2010;211:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Lascano CA, Kaidar-Person O, Szomstein S, Rosenthal R, Wexner SD. Challenges of laparoscopic colectomy in the obese patient: a review. Am J Surg. 2006;192:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Sakaguchi T, Suzuki S, Morita Y, Oishi K, Suzuki A, Fukumoto K, Inaba K, Kamiya K, Ota M, Setoguchi T. Analysis of anatomic variants of mesenteric veins by 3-dimensional portography using multidetector-row computed tomography. Am J Surg. 2010;200:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Chen Z, Molloi S. Automatic 3D vascular tree construction in CT angiography. Comput Med Imaging Graph. 2003;27:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kumano S, Tsuda T, Tanaka H, Hirata M, Kim T, Murakami T, Sugihara E, Abe H, Yamashita H, Kobayashi N. Preoperative evaluation of perigastric vascular anatomy by 3-dimensional computed tomographic angiography using 16-channel multidetector-row computed tomography for laparoscopic gastrectomy in patients with early gastric cancer. J Comput Assist Tomogr. 2007;31:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Matsuki M, Tanikake M, Kani H, Tatsugami F, Kanazawa S, Kanamoto T, Inada Y, Yoshikawa S, Narabayashi I, Lee SW. Dual-phase 3D CT angiography during a single breath-hold using 16-MDCT: assessment of vascular anatomy before laparoscopic gastrectomy. AJR Am J Roentgenol. 2006;186:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Usui S, Hiranuma S, Ichikawa T, Maeda M, Kudo SE, Iwai T. Preoperative imaging of surrounding arteries by three-dimensional CT: is it useful for laparoscopic gastrectomy? Surg Laparosc Endosc Percutan Tech. 2005;15:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Natsume T, Shuto K, Yanagawa N, Akai T, Kawahira H, Hayashi H, Matsubara H. The classification of anatomic variations in the perigastric vessels by dual-phase CT to reduce intraoperative bleeding during laparoscopic gastrectomy. Surg Endosc. 2011;25:1420-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Chen JK, Johnson PT, Horton KM, Fishman EK. Unsuspected mesenteric arterial abnormality: comparison of MDCT axial sections to interactive 3D rendering. AJR Am J Roentgenol. 2007;189:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 28. | Matsuki M, Kani H, Tatsugami F, Yoshikawa S, Narabayashi I, Lee SW, Shinohara H, Nomura E, Tanigawa N. Preoperative assessment of vascular anatomy around the stomach by 3D imaging using MDCT before laparoscopy-assisted gastrectomy. AJR Am J Roentgenol. 2004;183:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
P- Reviewers: Lee HJ, Leuratti L S- Editor: Wen LL L- Editor: O’Neill M E- Editor: Zhang DN