Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4730
Revised: January 21, 2014
Accepted: March 6, 2014
Published online: April 28, 2014
Processing time: 126 Days and 12.4 Hours
AIM: To investigate roles of sphincter of Oddi (SO) motility played in pigment gallbladder stone formation in model of guinea pigs.
METHODS: Thirty-four adult male Hartley guinea pigs were divided randomly into two groups: the control group and pigment stone group. The pigment stone group was divided into 4 subgroups with 6 guinea pigs each according to time of sacrifice, and were fed a pigment lithogenic diet and sacrificed after 3, 6, 9 and 12 wk. SO manometry and recording of myoelectric activity of the guinea pigs were obtained by multifunctional physiograph at each stage. Serum vasoactive intestinal peptide (VIP), gastrin and cholecystokinin octapeptide (CCK-8) were detected at each stage in the process of pigment gallbladder stone formation by enzyme-linked immunosorbent assay.
RESULTS: The incidence of pigment gallstone formation was 0%, 0%, 16.7% and 66.7% in the 3-, 6-, 9- and 12-wk group, respectively. The frequency of myoelectric activity decreased in the 3-wk group. The amplitude of myoelectric activity had a tendency to decrease but not significantly. The frequency of the SO decreased significantly in the 9-wk group. The SO basal pressure and common bile duct pressure increased in the 12-wk group (25.19 ± 7.77 mmHg vs 40.56 ± 11.81 mmHg, 22.35 ± 7.60 mmHg vs 38.51 ± 11.57 mmHg, P < 0.05). Serum VIP was significantly elevated in the 6- and 12-wk groups and serum CCK-8 was decreased significantly in the 12-wk group.
CONCLUSION: Pigment gallstone-causing diet may induce SO dysfunction. The tension of the SO increased. The disturbance in SO motility may play a role in pigment gallstone formation, and changes in serum VIP and CCK-8 may be important causes of SO dysfunction.
Core tip: Biliary stasis is thought to be important in the development of pigment gallstones. Sphincter of Oddi (SO) motility may play an important role in the process of pigment gallstone formation. We used a guinea pig model of pigment gallstones to investigate whether SO dysfunction happens and what a role the sphincter plays in the process of pigment gallstone formation. The myoelectric activity and SO manometry were measured at different stages of stone formation. Pigment gallstone-causing diet may induce SO dysfunction. The disturbance in SO motility may play a role in pigment gallstone formation, and changes in serum vasoactive intestinal peptide and cholecystokinin octapeptide may be important causes of SO dysfunction.
- Citation: Zhang ZH, Qin CK, Wu SD, Xu J, Cui XP, Wang ZY, Xian GZ. Roles of sphincter of Oddi motility and serum vasoactive intestinal peptide, gastrin and cholecystokinin octapeptide. World J Gastroenterol 2014; 20(16): 4730-4736
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4730.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4730
In America, cholesterol gallstones or cholesterol mixed with calcium bilirubinate account for 80% of gallstones, whereas the remaining 20% are pigment or calcium bilirubinate stones[1]. In Asia, pigment gallstones are common. Their treatment, especially for intrahepatic stones, is a challenge in biliary surgery[2,3]. In Japan, cholesterol stones account for 58.3%, black gallstones for 23.7%, and pigment stones for 15.9% of gallstones[4]. In China, pigment bile duct stones are found in about 10% of gallstone patients[5].
However, the etiology and pathogenesis of pigment gallstones have not been fully clarified. Etiologically, many factors, including age, bacterial infection, malnutrition, biliary stasis, impaired intestinal barrier function, and congenital duodenal diverticulum, may be involved, but none of these has been proved unequivocally. Among these factors, biliary stasis is thought to be important. The sphincter of Oddi (SO) is the only gate through which bile is discharged into the duodenum. SO motility may play an important role in the process of pigment gallstone formation. To the best of our knowledge, no study has investigated the relationship between SO motility and pigment gallstone formation. Our study first investigated the myoelectric activity and SO manometry simultaneously in the process of pigment gallstone formation.
The aim of this study was to investigate the role of SO motility in pigment gallbladder stone formation in a guinea pig model. The myoelectric activity and SO manometry were measured at different stages of stone formation. As SO motility is controlled by neurological and hormonal factors, we detected the changes in serum vasoactive intestinal peptide (VIP), gastrin and cholecystokinin octapeptide (CCK-8) in the process of pigment gallstone formation.
Thirty-four adult male Hartley guinea pigs weighing between 230 and 270 g were provided by the Huishan Jiangnan Laboratory Animal Company (license SCXK SU:2009-0005). The guinea pigs were divided randomly into two groups (control group and pigment stone group) after 7 d with standard diet. The control group (n = 10) was fed with standard diet. The pigment stone group was divided into 4 subgroups with 6 guinea pigs each according to time of sacrifice, and was fed with a pigment lithogenic diet[6] and was sacrificed after 3, 6, 9 and 12 wk.
Preparation of pigment gallstone-causing diet: The pigment gallstone diet comprised: corn flour 136.3 g/kg, soy bean flour 90.9 g/kg, flour 90.7 g/kg, fishmeal 63.6 g/kg, whole wheat 90.9 g/kg, salt 10 g/kg, yeast powder 10 g/kg, alfalfa meal 416.5 g/kg, lard oil 20 g/kg, sucrose 20 g/kg, cellulose 20 g/kg, cholesterol 1 g/kg, cholic acid 0.4 g/kg, vitamin C 0.05 g/kg, and casein 20 g/kg (purchased from Trophic Animal Feed High-Tech Co. Ltd., Nantong, China). A greenish yellow colored, granular calculus could be seen against the contrasting clear background in the gallbladders of the guinea pigs. The calculi were tested by infrared (IR) spectrometry to verify the sample as pigment gallstones. SO manometry and myoelectric activity of the guinea pigs were determined by multifunctional physiograph at 3, 6, 9 and 12 wk.
Preparation of two polar hook metal electrode: Two plexi-glasses were cut into strips (length, 5 cm; diameter, 1 cm; thickness, 0.5 cm). Two parallel cuts along the longitudinal axes were scored at 0.2-cm intervals. Then two acupuncture needles which were treated with insulating vanish were put into the gab and the plexi-glass was conglutinated by adhesives. The anterior extremity of the acupuncture needle (1 cm long) was exposed and the insulated paint at 0.5 cm of the apical part was scraped off to expose the metal of the needle. A microelectrode was formed by bending the needle point (0.2 cm long) about 120°. The handle of the needle in the posterior extremity was exposed by about 1 cm to connect with the leads. At the midpiece of the plexi-glasses, a cut along the transverse axes was scored to fix the needle by a silk thread.
The guinea pig was anesthetized by injecting pentobarbital sodium (45 mg/kg) into the peritoneal cavity. The guinea pig was fixed in the supine position, and the skin of the superior abdomen was prepared and sterilized. A longitudinal incision was made and the papilla (entrance of the common bile duct into the duodenum) was determined. Two polar hook metal electrodes were inserted at 0.2 mm into the subserosa of the SO by megaloscope (×10 magnification). Care was taken not to insert too deep, to avoid penetrating the sphincter. The interelectrode distance was approximately 2 mm and the electrodes were hung by two silk threads to the experimental shelf to maintain the necessary tension. The experimental shelf was adjusted to maintain the necessary tension and correct angle. The outfan of the two signals were connected with the two polar of the physiological recorder (BL-420 F; Chengdu Taimeng Software, China) and a syringe needle was inserted into the legs of the guinea pigs to connect with the earth pole of the recorder. The myoelectric signal was collected by the electrode and imported into the computer after handling by the physiological recorder, and it was stored after processing with the software system that was specialized in electromyographic signals. The setup parameters were as follows: scanning speed, 500 ms/div; sensitivity, 200 μV; time parameter, 1 s; and frequency filtering, 10 Hz. The myoelectric figure was dealt with digital filtering of 10-30 Hz at last.
Preparation of manometry catheter: A pedo bi-lumen central venous catheter (4 F and 30 cm long) was chosen. The catheter was cut 0.2 cm apart from the distal end of the water out lateral aperture, and the head end was made blunt and round for inserting easily into the SO. Another lateral aperture (0.3 mm diameter) was made inferior to the water out lateral aperture. Manometry lumen (b) and water effusion lumen (a1) were marked and the SO manometry catheter was completed.
SO manometry: The a1 catheter lumen was infused with sterile water at a flow rate of 15 mL/h by a minimally compliant hydraulic capillary infusion system and connected to pressure transducers. A physiological recorder and relevant manometry program were used to record and analyze the tracings. Calibrating by air compression method and zero setting, 0 and 200 mmHg were taken for baseline calibration. The manometry catheter was inserted into the common bile duct from the duodenum and fixed by silk thread. The gallbladder duct also was ligated by silk thread. The catheter was withdrawn to the SO, and manometry was carried out. The frequency of SO phasic contraction, SO basal pressure, SO amplitude and common bile duct pressure were measured and recorded.
Four milliliters of venous blood was obtained from the guinea pigs in the early morning before they were sacrificed and placed in a test tube. The blood was centrifuged at 1500 r/min for 15 min, and serum was isolated, placed in Eppendorf tubes, and stored at -70 °C. Serum VIP, gastrin and CCK-8 were measured by enzyme-linked immunosorbent assay (ELISA). The ELISA testing kit of guinea pigs was supplied by USCN Life Science Inc., Houston, TX, United States (VIP kit: E90380Gu, lot number: 20130123; GT kit E91224Gu, lot number: 20130221; CCK-8 kit: E91044Gu, lot number: 20130123).
Statistical analysis was carried out using Student’s t test. Data were analyzed with SPSS version 11.5. The results were expressed as mean ± SD. A two-tailed P value < 0.05 was considered statistically significant.
In the guinea pigs in the control group, no pigment gallstones were found. The gallstone-formation rate in the pigment gallstone group was 0%, 0%, 16.7% and 66.7% in the 3-, 6-, 9- and 12-wk groups, respectively.
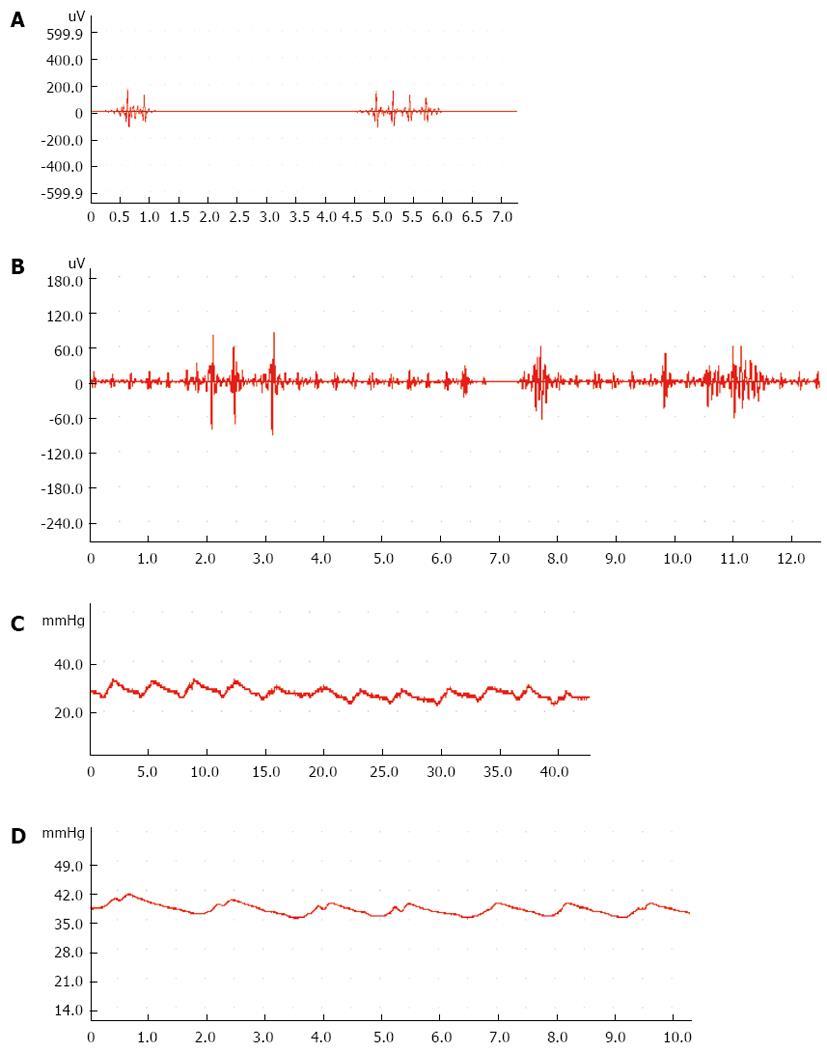
Compared with the control group, the frequency of myoelectric activity decreased significantly in the 3-wk group (P < 0.05). The amplitude of myoelectric activity had a tendency to decrease but not significantly (Table 1, Figure 1A and B).
| Groups | Amplitude | Frequency |
| Control group | 146.44 ± 81.09 | 15.86 ± 4.35 |
| 3-wk group | 125.06 ± 59.76 | 10.38 ± 2.02a |
| 6-wk group | 112.02 ± 64.69 | 12.90 ± 4.39 |
| 9-wk group | 77.81 ± 27.17 | 15.10 ± 4.13 |
| 12-wk group | 71.72 ± 35.10 | 13.70 ± 4.15 |
Compared with the control group, the frequency of the SO decreased significantly in the 9-wk group (P < 0.05). The SO basal pressure and common bile duct pressure increased significantly in the 12-wk group (P < 0.05) (Table 2, Figure 1C and D).
| Group | SO basal pressure (mmHg) | Common bile duct pressure (mmHg) | Amplitude of SO | Frequency of SO |
| Control group | 25.19 ± 7.77 | 22.35 ± 7.60 | 8.52 ± 2.27 | 11.57 ± 2.94 |
| 3-wk group | 29.72 ± 5.59 | 25.78 ± 5.30 | 11.83 ± 3.32 | 8.17 ± 3.54 |
| 6-wk group | 27.07 ± 11.11 | 24.29 ± 10.79 | 8.35 ± 2.82 | 10.50 ± 5.20 |
| 9-wk group | 33.09 ± 13.65 | 29.19 ± 13.84 | 11.71 ± 2.37 | 7.50 ± 1.73a |
| 12-wk group | 40.56 ± 11.81a | 38.51 ± 11.57a | 6.15 ± 2.97 | 8.20 ± 2.59 |
Compared with the control group, serum VIP was significantly elevated in the 6- and 12-wk groups (P < 0.05) (Table 3). Serum gastrin was significantly decreased in the 3-wk group (P < 0.001) (Table 3). Serum CCK-8 was significantly decreased in the 12-wk group (P < 0.05) (Table 3).
| Group | 3-wk | 6-wk | 9-wk | 12-wk |
| VIP | ||||
| Control (pg/mL) | 5.96 ± 2.97 | 4.37 ± 1.00 | 7.70 ± 2.08 | 8.68 ± 0.65 |
| Pigment gallstone (pg/ mL) | 10.35 ± 2.59 | 14.70 ± 3.41a | 17.02 ± 6.26 | 25.30 ± 6.56a |
| Gastrin | ||||
| Control (pg/mL) | 17.83 ± 2.35 | 18.56 ± 5.77 | 17.42 ± 6.39 | 19.41 ± 4.58 |
| Pigment gallstone(pg/mL) | 2.44 ± 0.78b | 8.51 ± 0.33 | 36.51 ± 12.83 | 23.01 ± 4.76 |
| CCK-8 | ||||
| Control (pg/mL) | 3482.63 ± 154.25 | 3650.73 ± 55.08 | 3606.74 ± 129.42 | 3599.28 ± 120.15 |
| Pigment gallstone (pg/mL) | 3524.50 ± 79.29 | 3597.64 ± 86.55 | 3498.01 ± 107.14 | 3436.09 ± 82.96a |
Pigment gallstones are classified descriptively as black stones, which are hard, and brown stones, which are soft[7]. Black stones often contain crystalline salts of calcium phosphate and/or calcium carbonate in one of its polymorphic forms, calcite, aragonite or valerite, and may also contain many metals found in bile[8]. They form in sterile gallbladder bile, and the principal risk factor is hyperbilirubinemia. Other causes of black stones are gallbladder hypomotility secondary to diabetes mellitus[9], total parenteral nutrition[10], and truncal vagotomy[11]. Parietal (gallbladder mucosa) factors may play a role in pigment stone formation[10]. We investigated many aspects relating to pigment gallstone formation and found that intestinal barrier function was correlated with pigment gallstone formation[6,11,12]. Endotoxemia and increased biliary β-glucuronidase may play important roles[13]. The structure and function of the SO are correlated significantly with bile duct pigment stones. Anatomical abnormalities and dysfunction of the SO play important roles in the formation of bile duct pigment stones[14].
Gallbladder stasis resulting from mechanical obstruction is an important antecedent in the development of pigment gallstones and sludge. Patients with cholesterol gallstones have impaired gallbladder emptying[15] and show dyspeptic symptoms with functional defects of both upper and lower gastrointestinal tracts[16,17]. Gallbladder emptying is also defective in patients with black pigment stones and such a defect is less severe than in patients with cholesterol stones[15]. Pigment stones are common in patients with cirrhosis[18], chronic hemolysis[19], ileal Crohn’s disease[20], and conditions associated with impaired gallbladder kinetics[19,21,22].
However, as the gate that regulates the flow of bile and pancreatic juice into the duodenum, and prevents the reflux of duodenal contents into the biliary and pancreatic duct, the role of SO motility in pigment gallstone formation has not been elucidated. Whether the SO dysfunction is present and what role it plays in pigment gallstone formation require further study. SO function may play an important role in cholesterol gallstone formation[23,24].
We used a guinea pig model of pigment gallstones to investigate whether SO dysfunction happens and what role the sphincter plays in the process of pigment gallstone formation. SO manometry and myoelectric activity were investigated at the same time. A MEDLINE search found that there were no studies of SO manometry and recording of myoelectric activity simultaneously in the process of gallstone formation.
We found that gallstones did not occur until 9 wk, when the incidence was 16.7%, and after 12 wk the incidence was 66.7%. The frequency of myoelectric activity decreased significantly in the 3-wk group. The amplitude of myoelectric activity tended to decrease but not significantly. As the most important indicators, SO basal pressure and common bile duct pressure increased gradually in the 12-wk group. We observed in the process of gallstone formation that SO tension increased and gallbladder stasis occurred. Disturbance of SO motor function impedes the flow of bile into the duodenum and may play an important role in gallstone formation.
The mechanism by which a cholesterol gallstone-causing diet induces SO dysfunction has not been fully elucidated. SO motility is controlled by numerous neurotransmitters and gastrointestinal hormones and their interactions[25].
VIP, an alkaline intestinal peptide composed of 28 amino acids, belongs to the secretin family. VIP relaxes gallbladder smooth muscle, decreases gallbladder pressure, and inhibits contractions induced by CCK[26,27]. VIP is thought to work as a neurotransmitter of the vagus nerve terminals[28,29]. Previously, we found that VIP2-R mRNA expression level in controls was lower than in patients with gallbladder polyps or gallstones[30]. The study of the relationship between VIP and the SO showed that the myenteric plexuses of the sphincter and duodenum are in direct continuity with many interconnecting nerve trunks, some of which show nitric oxide synthase activity and VIP immunoreactivity[31]. VIP increases the phasic activity of the sphincter[32]. In the present study, serum VIP level in the pigment gallstone group was higher than that of the control group. We suggest that the role of VIP in the formation of pigment gallstones may be as follows. First, VIP decreases the resting pressure in the gallbladder, causing its dilation and bile stasis. Second, it inhibits the contraction induced by CCK. Third, it regulates secretion of mucoproteins of the gallbladder mucosa and alters the bile components. Fourth, increased plasma VIP results in SO contraction, thus preventing bile flow from entering the duodenum. We noted that the frequency of SO decreased significantly in the 9-wk group and the SO basal pressure and common bile duct pressure increased significantly in the 12-wk group. Elevation of VIP may play an important role in mechanism of SO dysfunction.
There have been few studies about the effect of gastrin on the SO[33,34]. A few reports show that gastrin can increase the sphincter of Oddi basal pressure amplitude. Chen suggested that patients with post-cholecystectomy pain had SO dysfunction with characteristics of high tension, which was related to elevation of serum gastrin[35]. In our study, we found that serum gastrin decreased significantly in the 3-wk group. This may have resulted from changes in diet and did not necessarily have any relationship with the formation of pigment gallstones.
As the most important neural or hormonal factors which control the motility of biliary tract, CCK may play an important role in gallstone formation. Many studies have evaluated CCK-8 and its effect on the SO. Zhang et al[24] found that gallbladder cholestasis was observed during early stages of gallstone formation in Ch rabbits. Cholesterol gallstone model rabbits CCK-8 could not improve gallbladder cholestasis in the Ch group. Another study found that cyclic myoelectric activity of the SO at phases 2 and 3 of the migrating motor complex, and the excitatory response to CCK-8 were dramatically decreased in animals with chronic cholangitis[36]. We found that the levels of serum CCK-8 were significantly decreased. As a hormone that stimulates SO motility, decreasing its level may play a role in sphincter dysfunction and gallstone formation.
In conclusion, our study found that a pigment gallstone-causing diet may induce SO dysfunction. The tension of the SO increased. Disturbance of SO motility may play a role in gallstone formation. The mechanism by which a cholesterol gallstone-causing diet induces SO dysfunction has not been fully elucidated. The disturbance of serum VIP and CCK-8 may be important causes of SO dysfunction. Control of diet and regulation of SO motility are important in the prevention of pigment gallstone formation.
Biliary stasis is thought to be important in the development of pigment gallstones. Sphincter of Oddi (SO) motility may play an important role in the process of pigment gallstone formation. This study investigated the role of SO motility in pigment gallbladder stone formation in a guinea pig model. The myoelectric activity and SO manometry were measured at different stages of stone formation. The changes in serum vasoactive intestinal peptide (VIP), gastrin and cholecystokinin octapeptide (CCK-8) were detected in the process of pigment gallstone formation. There were no prior studies of SO manometry and recording of myoelectric activity simultaneously in the process of gallstone formation.
SO manometry and recording of myoelectric activity are the two most important methods in study of SO motility. SO manometry (SOM) is recognized as the standard diagnostic modality for sphincter of Oddi dysfunction (SOD). In this study, the authors clarified the impact of SO motility on the formation of pigment gallstone formation.
This study showed that SO motility played an important role in gallstone formation. This result is similar to those of previous reports. This is the first study to investigate SO manometry and recording of myoelectric activity simultaneously. SO motility is controlled by numerous neurotransmitters and gastrointestinal hormones and their interactions. Serum VIP, gastrin and CCK8 were detected at each stage in the process of pigment gallbladder stone formation by enzyme-linked immunosorbent assay. The disturbance of serum VIP and CCK8 may be important causes of SO dysfunction.
The study results suggest that disturbance of SO motility may play a role in gallstone formation. The disturbance of serum VIP and CCK8 may be important causes of SO dysfunction. Control of diet and regulation of SO motility are important in the prevention of pigment gallstone formation.
SO dysfunction is a painful syndrome that presents as recurrent episodes of right upper quadrant biliary pain, or recurrent idiopathic pancreatitis. SOM is the only available method to measure SO motor activity directly. Additionally, it is the only modality for diagnosis of suspected SOD which has been demonstrated to be reproducible and predictive of positive therapeutic outcome results.
The study investigated the roles of sphincter of Oddi motility played in pigment gallbladder stone formation in model of guinea pigs. The myoelectric activity and pressure of SO were measured at different stages of stone formation. The result shows that disturbance of SO motility may play a role in gallstone formation. The disturbance of serum VIP and CCK8 may be important causes of SO dysfunction. Control of diet and regulation of SO motility are important in the prevention of pigment gallstone formation.
| 1. | Goldman L, Bennett JC. Textbook of Medicine. Philadelphia: WB Saunders Co 2008; 829. |
| 2. | Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386-393. [PubMed] |
| 3. | Yang T, Lau WY, Lai EC, Yang LQ, Zhang J, Yang GS, Lu JH, Wu MC. Hepatectomy for bilateral primary hepatolithiasis: a cohort study. Ann Surg. 2010;251:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol. 2006;20:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (2)] |
| 5. | Zhu X, Zhang S, Huang Z. [The trend of the gallstone disease in China over the past decade]. Zhonghua Wai Ke Zazhi. 1995;33:652-658. [PubMed] |
| 6. | Su Y, Wu S, Fan Y, Jin J, Zhang Z. The preliminary experimental and clinical study of the relationship between the pigment gallstone and intestinal mucosal barrier. J Gastroenterol Hepatol. 2009;24:1451-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 7. | Cahalane MJ, Neubrand MW, Carey MC. Physical-chemical pathogenesis of pigment gallstones. Semin Liver Dis. 1988;8:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Suzuki N, Nakamura Y, Kobayashi N, Sato T. On metal elements in pure pigment gallstones. Tohoku J Exp Med. 1975;116:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Tsunoda K, Shirai Y, Wakai T, Yokoyama N, Akazawa K, Hatakeyama K. Increased risk of cholelithiasis after esophagectomy. J Hepatobiliary Pancreat Surg. 2004;11:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Cariati A, Piromalli E. Role of parietal (gallbladder mucosa) factors in the formation of black pigment gallstones. Clin Res Hepatol Gastroenterol. 2012;36:e50-e1; author reply e50-e1;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Wu SD, Su Y, Fan Y, Jin JZ, Zhang ZH. [Relationship between pigment gallstone and intestinal barrier function: experiment with guinea pigs and clinical observations]. Zhonghua Yi Xue Zazhi. 2008;88:1498-1502. [PubMed] |
| 12. | Su Y, Wu SD, Jin JZ, Zhang ZH, Fan Y. Role of intestinal barrier in pathogenesis of pigment gallstone in a guinea pig model. Hepatobiliary Pancreat Dis Int. 2006;5:443-448. [PubMed] |
| 13. | Fan Y, Wu SD, Sun L, Fu BB, Su Y. Possible relationship between intestinal barrier function and formation of pigment gallstones in hamsters. Hepatobiliary Pancreat Dis Int. 2008;7:529-532. [PubMed] |
| 14. | Wu SD, Yu H, Wang HL, Su Y, Zhang ZH, Sun SL, Kong J, Tian Y, Tian Z, Wei Y. [The relationship between Oddi’s sphincter and bile duct pigment gallstone]. Zhonghua Wai Ke Zazhi. 2007;45:58-61. [PubMed] |
| 15. | Portincasa P, Di Ciaula A, Baldassarre G, Palmieri V, Gentile A, Cimmino A, Palasciano G. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol. 1994;21:430-440. [PubMed] |
| 16. | Portincasa P, Di Ciaula A, Vendemiale G, Palmieri V, Moschetta A, Vanberge-Henegouwen GP, Palasciano G. Gallbladder motility and cholesterol crystallization in bile from patients with pigment and cholesterol gallstones. Eur J Clin Invest. 2000;30:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Alvaro D, Angelico M, Gandin C, Ginanni Corradini S, Capocaccia L. Physico-chemical factors predisposing to pigment gallstone formation in liver cirrhosis. J Hepatol. 1990;10:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Sakata R, Ueno T, Sata M, Sujaku K, Tamaki S, Torimura T, Tanikawa K. Formation of black pigment gallstone in a hamster model of experimental cirrhosis. Eur J Clin Invest. 1997;27:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Trotman BW. Pigment gallstone disease. Gastroenterol Clin North Am. 1991;20:111-126. [PubMed] |
| 20. | Brink MA, Slors JF, Keulemans YC, Mok KS, De Waart DR, Carey MC, Groen AK, Tytgat GN. Enterohepatic cycling of bilirubin: a putative mechanism for pigment gallstone formation in ileal Crohn’s disease. Gastroenterology. 1999;116:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Kurihara N, Ide H, Omata T, Yonamine S, Mashima Y, Tanno M, Chiba K, Yamada H. [Evaluation of gallbladder emptying in patients with chronic liver disease by 99mTc-EHIDA hepatobiliary scintigraphy]. Radioisotopes. 1989;38:269-274. [PubMed] |
| 22. | Pompili M, Rapaccini GL, Caturelli E, Curró D, Montuschi P, D’Amato M, Aliotta A, Grattagliano A, Cedrone A, Anti M. Gallbladder emptying, plasma levels of estradiol and progesterone, and cholecystokinin secretion in liver cirrhosis. Dig Dis Sci. 1995;40:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Chapman WC, Peterkin GA, LaMorte WW, Williams LF. Alterations in biliary motility correlate with increased gallbladder prostaglandin synthesis in early cholelithiasis in prairie dog. Dig Dis Sci. 1989;34:1420-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Zhang XY, Cui GB, Ma KJ, Wang S, Wei YN, Du P, Chen BY, Guo W, Wang XJ, Huang HD. Sphincter of Oddi dysfunction in hypercholesterolemic rabbits. Eur J Gastroenterol Hepatol. 2008;20:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Woods CM, Saccone GT. Neurohormonal regulation of the sphincter of Oddi. Curr Gastroenterol Rep. 2007;9:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Greaves RR, O’Donnell LJ, Battistini B, Forget MA, Farthing MJ. The differential effect of VIP and PACAP on guinea pig gallbladder in vitro. Eur J Gastroenterol Hepatol. 2000;12:1181-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Kalfin R, Milenov K. The effect of vasoactive intestinal polypeptide (VIP) on the canine gallbladder motility. Comp Biochem Physiol C. 1991;100:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Alcón S, Morales S, Camello PJ, Salido GM, Miller SM, Pozo MJ. Relaxation of canine gallbladder to nerve stimulation involves adrenergic and non-adrenergic non-cholinergic mechanisms. Neurogastroenterol Motil. 2001;13:555-566. [PubMed] |
| 29. | Björck S, Fahrenkrug J, Jivegård L, Svanvik J. Release of immunoreactive vasoactive intestinal peptide (VIP) from the gallbladder in response to vagal stimulation. Acta Physiol Scand. 1986;128:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Zhang ZH, Wu SD, Gao H, Shi G, Jin JZ, Kong J, Tian Z, Su Y. Expression of pituitary adenylate cyclase-activating polypeptide 1 and 2 receptor mRNA in gallbladder tissue of patients with gallstone or gallbladder polyps. World J Gastroenterol. 2006;12:1468-1471. [PubMed] |
| 31. | Simula ME, Brookes SJ, Meedeniya AC, Toouli J, Saccone GT. Distribution of nitric oxide synthase and vasoactive intestinal polypeptide immunoreactivity in the sphincter of Oddi and duodenum of the possum. Cell Tissue Res. 2001;304:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Pálvölgyi A, Sári R, Németh J, Szabolcs A, Nagy I, Hegyi P, Lonovics J, Szilvássy Z. Interplay between nitric oxide and VIP in CCK-8-induced phasic contractile activity in the rabbit sphincter of Oddi. World J Gastroenterol. 2005;11:3264-3266. [PubMed] |
| 33. | Becker JM, Moody FG, Zinsmeister AR. Effect of gastrointestinal hormones on the biliary sphincter of the opossum. Gastroenterology. 1982;82:1300-1307. [PubMed] |
| 34. | Cox MR, Padbury RT, Snelling TL, Schloithe AC, Harvey JR, Toouli J, Saccone GT. Gastrin-releasing peptide stimulates gallbladder motility but not sphincter of Oddi motility in Australian brush-tailed possum. Dig Dis Sci. 1998;43:1275-1284. [PubMed] |
| 35. | Chen XX, Mo JZ, Liu WZ. [A study on motility of sphincter of Oddi in postcholecystectomy syndrome]. Zhonghua Nei Ke Zazhi. 1991;30:337-39, 381. [PubMed] |
| 36. | Liu YK, Li ZH, Liu NZ, He Q, Lin H, Wang XJ, Li XW, Dong JH. Reduced myoelectric activity in the sphincter of Oddi in a new model of chronic cholangitis in rabbits: an in vivo and in vitro study. Neurogastroenterol Motil. 2010;22:927-934, e238-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
P- Reviewers: Li ZF, Morioka D S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Zhang DN