Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4566
Revised: December 9, 2013
Accepted: January 14, 2014
Published online: April 28, 2014
Processing time: 182 Days and 15.3 Hours
Gastric cancer remains one of the most common causes of cancer death. However the proportion of early gastric cancer (EGC) at diagnosis is increasing. Endoscopic treatment for EGC is actively performed worldwide in cases meeting specific criteria. Endoscopic mucosal resection can treat EGC with comparable results to surgery for selected cases. Endoscopic submucosal dissection (ESD) increases the en bloc and complete resection rates and reduces the local recurrence rate. ESD has been performed with expanded indication and is expected to be more widely used in the treatment of EGC through the technological advances in the near future. This review will describe the techniques, indications and outcomes of endoscopic treatment for EGC.
Core tip: Gastric cancer remains one of the most common causes of cancer death. However the proportion of early gastric cancer (EGC) at diagnosis is increasing. Endoscopic mucosal resection is an effective treatment modality with comparable results to surgery for selected cases of EGC. Endoscopic submucosal dissection (ESD) increases en bloc and complete resection rates and reduces the local recurrence rate. Recently, favorable outcomes of ESD have been reported in patients meeting expanded criteria of endoscopic treatment for EGC. This review will describe the techniques, indications and outcomes of endoscopic treatment for EGC.
- Citation: Min YW, Min BH, Lee JH, Kim JJ. Endoscopic treatment for early gastric cancer. World J Gastroenterol 2014; 20(16): 4566-4573
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4566
Gastric cancer remains one of the most common causes of cancer death worldwide, although its incidence and mortality rate are decreasing[1,2]. Gastric cancer has become a relatively rare cancer in North America and in most Northern and Western Europe, but not in Eastern Europe, Russia and selected areas of Central and South America or East Asia[2]. Given that the high incidence of gastric cancer, the National Cancer Screening Program recommends that men and women aged 40 years and over undergo upper endoscopy or upper gastrointestinal series every other year in Korea[3] and similarly, gastric cancer screening has been conducted nationwide for all residents aged 40 years and over in Japan[4]. As a result, the proportion of early gastric cancer (EGC) at diagnosis is increasing. The prognosis of EGC is excellent with a 5-year survival rate of over 90%[5,6]. Furthermore, with the improved detection rate of EGC, the endoscopic treatment has become widespread due to advances in the instruments available and endoscopist’s experience[7-9]. This review will describe the techniques, indications and outcomes of endoscopic treatment for EGC.
The technique and instruments of endoscopic polypectomy were developed in Japan[10]. Since then, the strip biopsy was described as an extension of endoscopic snare polypectomy in 1984[11]. This technique uses a double channel endoscope. After submucosal injection under the lesion, a snare is inserted through one channel and is used to remove the lesion, while a grasper, inserted through the other channel, is used to lift the lesion. In 1988, Endoscopic mucosal resection(EMR) after circumferential pre-cutting was described[12]. In this technique, cutting around the lesion with a needle knife is done after submucosal injection using hypertonic saline mixed with diluted epinephrine, and then the lesion is removed by a snare. In 1992, EMR using transparent cap (EMR-C) was developed[13]. This technique uses a transparent plastic cap that is connected to the tip of an endoscope. After submucosal injection, the lesion is sucked into the cap while a specialized crescent shaped snare, which is deployed at the tip of the cap, is closed. With this technique intramucosal cancers 2 cm or less in diameter can be safely removed[14]. After that, EMR with ligation (EMR-L) was described[15]. In this technique, a standard variceal ligation device is used to capture the lesion. After sucking the lesion into the cap, lodged band is deployed underneath the lesion. The banded lesion is then resected by a snare. EMR-L is also safe and effective treatment modality for selected EGCs[16]. As described above, EMR-C and EMR-L are relatively simple and effective treatment modality for EGC. However, it is apt to fragment tumors that are larger than 1.5-2.0 cm in diameter, which results in incomplete histological diagnosis and possibly in an increased risk of local recurrence[17-19]. To overcome this drawback ESD, which is particularly effective for en bloc resection of tumors regardless of their size, was developed in late 1990s.
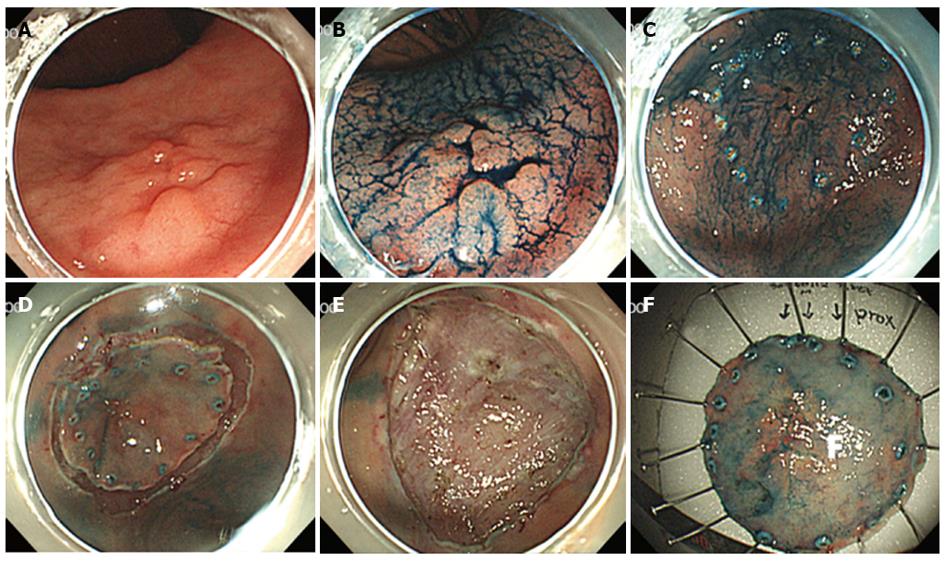
Endoscopic submucosal dissection (ESD) permits en bloc resection of larger lesions than that can be treated with EMR[20-26]. This technique consists of several steps (Figure 1)[27,28]. The marking around the lesion is done and circumferential mucosal pre-cutting is performed after submucosal injection. To distinguish clearly between the muscle layer and the submucosal layer and allow better hemostasis, normal saline mixed with diluted epinephrine and indigo-carmine is often used as submucosal injection solution. The injection is repeated a few times until the target mucosa is sufficiently raised. After lifting of the lesion, submucosal layer under the lesion is dissected with lateral movement using various knives. Several knives have been developed and used for ESD, which include needle knife, insulation-tipped diathermic knife (IT knife), hook knife, flex knife, triangle tip knife, flush knife, splash knife, IT-2 knife and dual knife[6,28-30]. After resection of the lesion, visible vessels in the artificial ulcer is treated with hemostatic devices to prevent delayed bleeding.
Determining an indication for endoscopic treatment appears to be the most important step in managing patients with EGC. To select appropriate patients with EGC and to achieve a complete resection, the exact margin and depth of tumor could be determined through endoscopic evaluations. The horizontal extent of tumor can be determined with standard endoscopy and chromoendoscopy (CE). In some cases with unclear margins even with CE, magnifying endoscopy with narrow-band imaging could be useful to identify the precise margin[31]. The depth of tumor invasion can also be assessed with standard endoscopy and CE. In addition, endoscopic ultrasonography could be used to further ascertain the depth. However, the accuracy of endoscopic ultrasonography in assessing the depth of invasion in EGC was reported to range from 71% to 78%[32,33].
The standard criteria[8] for selection of patients with EGC who are appropriate for the endoscopic treatment are below: (1) well or moderately differentiated adenocarcinoma and/or papillary carcinoma; (2) confined to the mucosa; (3) smaller than 2 cm for superficially elevated type lesions; (4) smaller than 1 cm for the flat and depressed type lesions; (5) without ulcer or ulcer scar; and (6) without venous or lymphatic involvement[34]. The rationale for this guideline is based on the knowledge that patients meeting the criteria are expected free from lymph node (LN) metastasis[35].
Expansion of the criteria for selection of patients with EGC who are appropriate for the endoscopic treatment has been proposed in Japan from clinical observations that the too strict absolute indication leads to unnecessary surgery[36]. From the surgical data involving 5265 patients who underwent gastrectomy for EGC, Gotoda et al[7] were able to further define the risk of LN metastasis in certain groups of patients with EGC and showed four groups with a low risk of LN metastasis: (1) differentiated intramucosal adenocarcinoma without lymphovascular invasion less than 3 cm in diameter, irrespective of ulcer findings; (2) differentiated intramucosal adenocarcinoma without lymphovascular invasion and ulcer findings, irrespective of tumor size; (3) undifferentiated intramucosal cancer without lymphovascular invasion and ulcer findings smaller than 2 cm in diameter; and (4) differentiated adenocarcinoma with minute submucosal penetration (SM1, cancer invasion into the upper third of the submucosa) but without lymphovascular invasion smaller than 3 cm in diameter. These results have allowed the development of expanded criteria for endoscopic treatment for EGC[8] (Table 1). In the study by An et al[37], predictive factors for LN metastasis in EGC with submucosal invasion were identified and possibility of EMR was addressed in highly selected submucosal cancers with no lymphatic involvement, SM1 invasion, and tumor size < 1 cm. In a recent study be Lee et al[38] to compare the therapeutic outcomes of conventional and expanded indications of ESD for differentiated EGC, the conventional indication group and expanded indication group did not differ with regard to the rates of local recurrence (0.7% vs 0%), metachronous recurrence (3.6% vs 3.3%) or cumulative disease-free survival. Survival outcome was similar in the subgroups classified by tumor depth and size.
| Mucosal cancer | Submucosal cancer | |||||
| No ulceration | Ulceration | SM1 | SM2 | |||
| Size (mm) | ≤ 20 | > 20 | ≤ 30 | > 30 | ≤ 30 | Any size |
| Histology | ||||||
| Intestinal type | A | B | B | D | B | D |
| Diffuse type | C | D | D | D | D | D |
The risk of LN metastasis is known to increase in undifferentiated cancer due to lymphovascular invasion[39]. In the analysis of 3843 patients who underwent gastrectomy with LN dissection for solitary undifferentiated EGC, none of the 310 intramucosal cancers 20 mm or less in size without lymphovascular invasion and ulcerative findings was associated with LN metastases[40]. Recently, favorable outcomes of endoscopic resection have been reported in selected patients with undifferentiated mucosal cancer or minimal submucosal invasion cancer (SM1)[41-43]. However, these are all single center retrospective studies. Therefore, large scale, prospective studies are warranted to confirm the feasibility of ESD for undifferentiated gastric cancer.
To expand the indication of endoscopic treatment to submucosal invasion (SM1) differentiated EGC, the histological heterogeneity of gastric cancer is the important issue to be addressed. Based on morphological features and histological background, gastric carcinoma is divided into differentiated and undifferentiated type or intestinal and diffuse type[44,45]. Gastric cancer shows remarkable heterogeneity in histological pattern, cellular phenotype, and genotype[46]. In a retrospective study to compare the clinicopathologic features of node-positive and node-negative differentiated submucosal invasion differentiated gastric cancers, histological heterogeneity was the independent risk factor for LN metastasis[47]. Thus, it is recommended to apply the endoscopic treatment to the differentiated EGC without histological heterogeneity when it is considered in submucosal invasion cancer.
Bleeding is the most common major complication of endoscopic treatment for EGC. Most bleeding occurs during the procedure or within 24 h[48]. Bleeding is divided into immediate (intraoperative) bleeding during procedure and delayed bleeding after procedure. Significant immediate bleeding occurs more often in the upper and middle thirds of the stomach than in the lower third of the stomach because of the larger diameter of the submucosal arteries in the upper and middle thirds of the stomach[49]. However, bleeding can be successfully treated in most cases through coagulation of the bleeding vessels, or placement of metallic clips for severe bleeding. In terms of delayed bleeding, the incidence rates were reported to range from 0%-15.6% in the recent review involving 28 studies with at least 300 ESD cases for EGC[50]. Delayed bleeding is associated to tumor location, larger tumor, recurrent lesion, macroscopic type (flat or depressed), old age (≥ 80 years) and longer procedure time[51-55]. At first, delayed bleeding was reported to occur more frequently after ESD for lesions in the lower and middle thirds of the stomach compared to the upper third of the stomach[55]. However, the reason remains unclear. In the recent study involving 1000 cases of ESD for early gastric neoplasms, delayed bleeding occurs more often in upper portion of the stomach than in lower portion (28.6% vs 13.8%, P = 0.003)[51]. In relation to antiplatelet drugs, there is a controversy in the risk of bleeding after ES. Two Korean retrospective studies have reported conflicting results on the risk of bleeding after ESD for gastric neoplasms[56,57].
Perforation is less common major complication of endoscopic resection for EGC than bleeding and has been reported to range from 1.2% to 5.2%[50]. Perforation is diagnosed when mesenteric fat or intra-abdominal space is directly observed during the procedure (frank perforation) or free air is found on a plain chest X-ray after the procedure without a visible stomach wall defect during the procedure (micro-perforation). Immediately recognized small perforations can be successfully treated without surgery with a combination of endoscopic clipping and broad spectrum antibiotics[58-60]. However, large perforations would require immediate surgery. In cases of micro-perforation, management is not well established. In a retrospective study by Jeong et al[61], 13 cases (3.18%) of micro-perforation after EMR for gastric neoplasms were reported. Among them, 11 cases were successfully treated only with fasting, nasogastric tube drainage and broad spectrum antibiotics. In severe pneumoperitoneum, respiratory deterioration and/or shock could occur. Decompression of the pneumoperitoneum must be performed with a puncture needle in such cases[60]. Instead of air insufflations, CO2 insufflation during procedure could minimize such pneumoperitoneum caused by a perforation[62,63].
Stenosis after gastric ESD has been reported to range from 0.7% to 1.9%[50]. In a retrospective study, stenosis occurred with 17% of cardiac resections and 7% of pyloric resections[64]. Circumferential extent of the mucosal defect of > 3/4 and longitudinal extent > 5 cm were related to stenosis with both cardiac and pyloric resections. However, all affected patients (n = 15) were successfully treated by endoscopic balloon dilation.
Aspiration pneumonia after gastric ESD has been reported to range from 0.8% to 1.6%[50]. However, the risk of aspiration pneumonia appears to increase in sedation with continuous propofol infusion with intermittent or continuous administration of an opiod[65,66]. In addition, longer procedure time (> 2 h), male gender and old age (> 75 years) are associated with occurrence of aspiration pneumonia after ESD.
Pain after endoscopic resection is usually mild and dull in nature and can be controlled by proton pump inhibitor and opioids[36].
EMR is often the procedure of choice for patients who meet the standard criteria for endoscopic resection of EGC. Studies have shown high survival and cure rates in patients with EGC who undergo EMR. In the analysis of 308 EGCs resected endoscopically, 89% of type IIa lesions less than 2 cm were resected curatively, while only 50% of those larger than 2 cm were resected completely. In type IIc, 83% of lesions less than 1 cm and 57% of those greater than 1 cm were excised completely by endoscopic resection. In type IIc, curative endoscopic resection was possible in 85% of differentiated carcinomas and 43% of undifferentiated carcinomas[67]. These successful outcomes have allowed EMR to become the standard treatment for EGC in Japan[36]. In a Japanese report of 131 patients with differentiated mucosal EGC less than 2 cm (without ulcerative change) that had been completely removed by EMR, two patients (1.5%) died of gastric cancer during the mean observation period of 58 mo. The disease-specific 5- and 10-year survival rates were 99% and 99%[68]. However, EMR is associated with risks of local recurrence, especially when resections are not performed en bloc, or when the resection margins are involved by tumor. The risk of local recurrence after EMR ranged from 2% to 35% in Japanese series[69]. In a recent cross-sectional, retrospective cohort study, maximum diameters exceeding 2 cm was the independent risk factor for piecemeal EMR and no recurrence was observed in the en bloc group[70]. In a Korean multicenter, retrospective study, complete resection rate after EMR was (77.6%) and local recurrence rate 6.0% with a median interval between EMR and recurrence of 17.9 mo (range 3.5-51.7 mo). No deaths were related to recurrence of gastric cancer during the overall median follow-up period of 39 mo[71]. ESD increases en bloc and complete resection rates and reduces the local recurrence rate. In a retrospective study, EMR and ESD were compared with each other[20]. En bloc and histologically complete resection rates were higher with ESD than with EMR, regardless of tumor size. Local recurrences were treated by incomplete EMR (en bloc, 2.9%; piecemeal, 4.4%) but no patient experienced recurrence after ESD. The outcomes of ESD show 94.9%-97.7% rates and 83.1%-97.1% 5-year survival rates[30,51,72-75]. In a retrospective study of EGC that fulfilled the expanded criteria, en bloc resection was achieved in 94.9% (559/589) and 550 of 581 lesions (94.7%) were deemed to have undergone curative resection[72]. En bloc resection was the only significant contributor to curative ESD. Patients with non-curative resection developed local recurrence more frequently. The 5-year overall and disease-specific survival rates were 97.1% and 100%, respectively[72]. In the long-term outcomes of ESD for EGC, en bloc resection rate was 97.7% for all lesions treated by ESD. The incidence of positive horizontal and vertical margins was 3.7% and 3.4%, respectively. There were no deaths related to ESD. Local recurrence was observed in five patients (1.1%), and metachronous recurrences in 7.8% of the patients. The post-treatment 5-year survival was 83.1%. There were no deaths as a result of gastric cancer associated with sites treated by ESD[75]. In a Korean multicenter, retrospective study, the rates of en bloc resection, complete en bloc resection, vertical incomplete resection and piecemeal resection were 95.3%, 87.7%, 1.8% and 4.1%, respectively[51]. The rates of delayed bleeding, significant bleeding, perforation and surgery related to complication were 15.6%, 0.6%, 1.2% and 0.2%, respectively. In other Korean single center, retrospective study, en bloc and curative resection rates were 96.7% and 88.3%, respectively[74]. The curative resection rate was significantly lower in the expanded group than in the standard group (82.1% vs 91.5%, P = 0.001). During a median follow-up of 24 mo, the local tumor recurrence rate was also higher in the expanded group than in the standard group (7.0% vs 1.8%, P = 0.025). Local recurrence was more frequent in lesions with non-curative resection than in those with curative resection (20.0% vs 1.3%, P < 0.001). The 5-year overall and disease-specific survival rates were 88% and 100%, respectively; the difference between the standard and expanded groups was not significant (P = 0.834).
EMR is an effective treatment modality with comparable results to surgery for selected cases of EGC. However, there is a risk of piecemeal resection with EMR in cases of large EGC, which is associated with higher recurrence rates. ESD increases en bloc and complete resection rates and reduces the local recurrence rate. Recently, favorable outcomes of ESD have been reported in patients meeting expanded criteria of endoscopic treatment for EGC. However, its technical difficulty requires a long learning period and technical invasiveness increases the risk of complications. Thus, further efforts are needed to make ESD easier and safer, which could be achieved through the technological advances. In addition, standardization of the pathologic diagnosis is necessary for the more reliable ESD. Finally, confirmation of more long-term outcomes under the expanded indication is warranted for establishing an appropriate indication of ESD for EGC.
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11892] [Article Influence: 792.8] [Reference Citation Analysis (6)] |
| 2. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 486] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 3. | Lee KS, Oh DK, Han MA, Lee HY, Jun JK, Choi KS, Park EC. Gastric cancer screening in Korea: report on the national cancer screening program in 2008. Cancer Res Treat. 2011;43:83-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Oliveira FJ, Ferrão H, Furtado E, Batista H, Conceição L. Early gastric cancer: Report of 58 cases. Gastric Cancer. 1998;1:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1164] [Article Influence: 46.6] [Reference Citation Analysis (5)] |
| 7. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1351] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 8. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 396] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 9. | Hotta K, Oyama T, Akamatsu T, Tomori A, Hasebe O, Nakamura N, Kojima E, Suga T, Miyabayashi H, Ohta H. A comparison of outcomes of endoscopic submucosal dissection (ESD) For early gastric neoplasms between high-volume and low-volume centers: multi-center retrospective questionnaire study conducted by the Nagano ESD Study Group. Intern Med. 2010;49:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Oguro Y, Hirashima T, Tajiri H, Yoshida S, Yamaguchi H, Yoshimori M, Itabashi M, Hirota T. Endoscopic treatment of early gastric cancer: polypectomy and laser treatment. Jpn J Clin Oncol. 1984;14:271-282. [PubMed] |
| 11. | Tada M, Shimada M, Murakami F, Mizumachi M, Arima K, Yanai H. Development of strip-off biopsy (in Japanese with English abstract). Gastroenterol Endosc. 1984;26:833-839. |
| 12. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 268] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 374] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Kume K, Yamasaki M, Kubo K, Mitsuoka H, Oto T, Matsuhashi T, Yamasaki T, Yoshikawa I, Otsuki M. EMR of upper GI lesions when using a novel soft, irrigation, prelooped hood. Gastrointest Endosc. 2004;60:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Suzuki Y, Hiraishi H, Kanke K, Watanabe H, Ueno N, Ishida M, Masuyama H, Terano A. Treatment of gastric tumors by endoscopic mucosal resection with a ligating device. Gastrointest Endosc. 1999;49:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kim HS, Lee DK, Baik SK, Kim JM, Kwon SO, Kim DS, Cho MY. Endoscopic mucosal resection with a ligation device for early gastric cancer and precancerous lesions: comparison of its therapeutic efficacy with surgical resection. Yonsei Med J. 2000;41:577-583. [PubMed] |
| 17. | Korenaga D, Haraguchi M, Tsujitani S, Okamura T, Tamada R, Sugimachi K. Clinicopathological features of mucosal carcinoma of the stomach with lymph node metastasis in eleven patients. Br J Surg. 1986;73:431-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Ell C, May A, Gossner L, Pech O, Günter E, Mayer G, Henrich R, Vieth M, Müller H, Seitz G. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 432] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, Kida M, Oida M, Saigenji K. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 537] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 21. | Takeuchi Y, Uedo N, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Higashino K, Ishihara R, Tatsuta M, Ishiguro S. Endoscopic submucosal dissection with insulated-tip knife for large mucosal early gastric cancer: a feasibility study (with videos). Gastrointest Endosc. 2007;66:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Yamamoto H, Kita H. Endoscopic therapy of early gastric cancer. Best Pract Res Clin Gastroenterol. 2005;19:909-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis. 2005;6:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Probst A, Pommer B, Golger D, Anthuber M, Arnholdt H, Messmann H. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy. 2010;42:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Cho KB, Jeon WJ, Kim JJ. Worldwide experiences of endoscopic submucosal dissection: not just Eastern acrobatics. World J Gastroenterol. 2011;17:2611-2617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Nonaka S, Oda I, Nakaya T, Kusano C, Suzuki H, Yoshinaga S, Fukagawa T, Katai H, Gotoda T. Clinical impact of a strategy involving endoscopic submucosal dissection for early gastric cancer: determining the optimal pathway. Gastric Cancer. 2011;14:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Kang KJ, Kim KM, Min BH, Lee JH, Kim JJ. Endoscopic submucosal dissection of early gastric cancer. Gut Liver. 2011;5:418-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 30. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Nagahama T, Yao K, Maki S, Yasaka M, Takaki Y, Matsui T, Tanabe H, Iwashita A, Ota A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011;74:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (2)] |
| 32. | Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 33. | Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, Okita K. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999;44:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Tsujitani S, Oka S, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery. 1999;125:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 500] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 37. | An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Lee H, Yun WK, Min BH, Lee JH, Rhee PL, Kim KM, Rhee JC, Kim JJ. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Nasu J, Nishina T, Hirasaki S, Moriwaki T, Hyodo I, Kurita A, Nishimura R. Predictive factors of lymph node metastasis in patients with undifferentiated early gastric cancers. J Clin Gastroenterol. 2006;40:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 41. | Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, Cho CM, Tak WY, Kweon YO. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, Omae M, Kubota M, Ishiyama A, Hirasawa T. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Kim YY, Jeon SW, Kim J, Park JC, Cho KB, Park KS, Kim E, Chung YJ, Kwon JG, Jung JT. Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. 2013;27:4656-4662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1968;59:251-258. [PubMed] |
| 45. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 46. | Luinetti O, Fiocca R, Villani L, Alberizzi P, Ranzani GN, Solcia E. Genetic pattern, histological structure, and cellular phenotype in early and advanced gastric cancers: evidence for structure-related genetic subsets and for loss of glandular structure during progression of some tumors. Hum Pathol. 1998;29:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Mita T, Shimoda T. Risk factors for lymph node metastasis of submucosal invasive differentiated type gastric carcinoma: clinical significance of histological heterogeneity. J Gastroenterol. 2001;36:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Dig Endosc. 2006;18:S123-S127. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 51. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 485] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 52. | Shiba M, Higuchi K, Kadouchi K, Montani A, Yamamori K, Okazaki H, Taguchi M, Wada T, Itani A, Watanabe T. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005;11:7335-7339. [PubMed] |
| 53. | Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 55. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, opera- tion time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 336] [Article Influence: 16.0] [Reference Citation Analysis (2)] |
| 56. | Lim JH, Kim SG, Kim JW, Choi YJ, Kwon J, Kim JY, Lee YB, Choi J, Im JP, Kim JS. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Cho SJ, Choi IJ, Kim CG, Lee JY, Nam BH, Kwak MH, Kim HJ, Ryu KW, Lee JH, Kim YW. Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms. Endoscopy. 2012;44:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Tsunada S, Ogata S, Ohyama T, Ootani H, Oda K, Kikkawa A, Ootani A, Sakata H, Iwakiri R, Fujimoto K. Endoscopic closure of perforations caused by EMR in the stomach by application of metallic clips. Gastrointest Endosc. 2003;57:948-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006;63:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 230] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 61. | Jeong G, Lee JH, Yu MK, Moon W, Rhee PL, Paik SW, Rhee JC, Kim JJ. Non-surgical management of microperforation induced by EMR of the stomach. Dig Liver Dis. 2006;38:605-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy. 2009;41:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Park CH, Min JH, Yoo YC, Kim H, Joh DH, Jo JH, Shin S, Lee H, Park JC, Shin SK. Sedation methods can determine performance of endoscopic submucosal dissection in patients with gastric neoplasia. Surg Endosc. 2013;27:2760-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Park CH, Kim H, Kang YA, Cho IR, Kim B, Heo SJ, Shin S, Lee H, Park JC, Shin SK. Risk factors and prognosis of pulmonary complications after endoscopic submucosal dissection for gastric neoplasia. Dig Dis Sci. 2013;58:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, Noguchi Y. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 135] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, Imanaka K, Yamada T, Yamamoto S, Yamamoto S. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 69. | Kojima T, Parra-Blanco A, Takahashi H, Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc. 1998;48:550-554; discussion 554-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 177] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Fukuda K, Fujita Y, Ninomiya K, Tano S, Katurahara M. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc. 2012;26:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY, Ryu CB, Chun HJ, Park JJ, Lee WS, Kim HS. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 530] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 73. | Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Choi MK, Kim GH, Park do Y, Song GA, Kim DU, Ryu DY, Lee BE, Cheong JH, Cho M. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013;27:4250-4258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
P- Reviewers: Chen XZ, Gu J, Kusano C S- Editor: Qi Y L- Editor: A E- Editor: Wang CH