Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4393
Revised: December 30, 2013
Accepted: February 17, 2014
Published online: April 21, 2014
Processing time: 200 Days and 5 Hours
AIM: To compare the surgical outcomes between living-donor and deceased-donor liver transplantation in patients with hepatic carcinoma.
METHODS: From January 2007 to December 2010, 257 patients with pathologically confirmed hepatic carcinoma met the eligibility criteria of the study. Forty patients who underwent living-donor liver transplantation (LDLT) constituted the LDLT group, and deceased-donor liver transplantation (DDLT) was performed in 217 patients. Patients in the LDLT group were randomly matched (1:2) to patients who underwent DDLT using a multivariate case-matched method, so 40 patients in the LDLT group and 80 patients in the DDLT group were enrolled into the study. We compared the two groups in terms of clinicopathological characteristics, postoperative complications, long-term cumulative survival and relapse-free survival outcomes. The modified Clavien-Dindo classification system of surgical complications was used to evaluate the severity of perioperative complications. Furthermore, we determined the difference in the overall biliary complication rates in the perioperative and follow-up periods between the LDLT and DDLT groups.
RESULTS: The clinicopathological characteristics of the enrolled patients were comparable between the two groups. The duration of operation was significantly longer (553 min vs 445 min, P < 0.001) in the LDLT group than in the DDLT group. Estimated blood loss (1188 mL vs 1035 mL, P = 0.055) and the proportion of patients with intraoperative transfusion (60.0% vs 43.8%, P = 0.093) were slightly but not significantly greater in the LDLT group. In contrast to DDLT, LDLT was associated with a lower rate of perioperative grade II complications (45.0% vs 65.0%, P = 0.036) but a higher risk of overall biliary complications (27.5% vs 7.5%, P = 0.003). Nonetheless, 21 patients (52.5%) in the LDLT group and 46 patients (57.5%) in the DDLT group experienced perioperative complications, and overall perioperative complication rates were similar between the two groups (P = 0.603). No significant difference was observed in 5-year overall survival (74.1% vs 66.6%, P = 0.372) or relapse-free survival (72.9% vs 70.9%, P = 0.749) between the LDLT and DDLT groups.
CONCLUSION: Although biliary complications were more common in the LDLT group, this group did not show any inferiority in long-term overall survival or relapse-free survival compared with DDLT.
Core tip: Several retrospective studies from single centers have shown that living-donor liver transplantation (LDLT) might be associated with higher rates of tumor recurrence than deceased-donor liver transplantation (DDLT). In this study, we compared the surgical outcomes between LDLT and DDLT in patients with hepatic carcinoma using a multivariate case-matched method, which minimized the disparity from case selection and thus made our results statistically more persuasive than others. Our results suggest that LDLT did not show any inferiority in long-term overall survival or relapse-free survival in comparison with DDLT.
- Citation: Wan P, Zhang JJ, Li QG, Xu N, Zhang M, Chen XS, Han LZ, Xia Q. Living-donor or deceased-donor liver transplantation for hepatic carcinoma: A case-matched comparison. World J Gastroenterol 2014; 20(15): 4393-4400
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4393.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4393
Liver cancer is the sixth most common cancer and the third leading cause of cancer-related death, accounting for 7% of all cancers worldwide[1]. In the United States and Japan, the incidence of liver cancer is highest in the elderly population (approximately 70 years), with a strong male preponderance[2,3], while the mean age of patients with liver cancer in the Chinese population is appreciably younger. Patients who develop liver cirrhosis after hepatitis B virus (HBV) infection have a high risk of developing liver cancer. Owing to the high incidence of HBV infection, China has a large population of patients with HBV-related cirrhosis, which makes Chinese cases of and deaths from liver cancer constitute 55% of the global total[4].
A significant proportion of patients with liver cancer are accompanied by serious liver cirrhosis or liver dysfunction, so radical liver resection is limited in such patients. Liver transplantation (LT) is the best option for patients with a hepatic tumor ≤ 5 cm in diameter and concurrent decompensated cirrhosis (Child-Pugh class B or C)[5]. However, due to the severe shortage of donor organs, a considerable number of patients with liver cancer die from a lack of donors every year. As a solution, living-donor liver transplantation (LDLT) is currently an effective alternative. Currently, approximately 70% of patients undergoing LDLT are from Japan, South Korea, Hong Kong and Taiwan, but worries exist that LDLT may be associated with higher rates of tumor recurrence[6-10] and biliary complications[11,12] than deceased-donor liver transplantation (DDLT). Which group has better postoperative results is not certain, as results from various centers differ from one another[13-16]. Whether LDLT can have more benefits on perioperative and long-term outcomes than DDLT is still disputable. Based on our long-term follow-up experience, we carried out this study to compare the two approaches of LT for primary liver cancer patients in terms of perioperative and long-term survival outcomes.
From January 2007 to December 2010, 281 patients underwent either LDLT or DDLT for clinically diagnosed primary liver cancer in the Department of Liver Surgery, Ren Ji Hospital, Shanghai, China. Twenty-four patients were excluded for the following reasons: (1) 11 patients who received preoperative downstaging treatment had complete tumor necrosis; (2) 7 patients had possible metastatic disease before LT; (3) coexistence of gallbladder carcinoma was pathologically found with hepatocellular carcinoma (HCC) in 2 patients after LT; (4) one patient underwent additional left nephrectomy for concurrent renal carcinoma; (5) one patient underwent combined liver-kidney transplantation; (6) one patient was pathologically diagnosed with hepatic diffuse large B cell lymphoma after LT; and (7) one patient was pathologically diagnosed with hepatic sarcomatoid carcinoma after surgery.
A total of 257 patients with pathologically confirmed hepatic carcinoma met the eligibility criteria of the study, of whom 40 consecutive patients who underwent LDLT constituted the LDLT group, leaving 217 patients in the DDLT pool from which the case-matching was performed. To minimize any disparity in case selection between the two groups, we used a 1:2 multivariate matching analysis of similar patient cohorts. Each case in the LDLT group was exactly matched to 2 cases from the DDLT pool. We set 4 exact matching variables and 3 relative matching variables. Gender, age (± 5 years), within or outside the Milan criteria (the Pittsburgh staging system was used to classify the cases outside the Milan criteria[17]) and history of hepatic surgery were selected as exact matching variables by which each case in the LDLT group should have been absolutely matched to the corresponding 2 cases in the DDLT pool. After more than two matches were identified, we used relative matching variables to select the best candidates for the DDLT group. Child-Pugh classification, MELD score (± 5 points), and serum alpha-fetoprotein (< 400 or ≥ 400 ng/mL) were defined as relative matching variables. After two rounds of matching, a random selection through the use of computer software was performed to determine the final members of the DDLT group. Finally, 40 patients in the LDLT group and 80 patients in the DDLT group were enrolled into the study.
The clinicopathological characteristics and surgical data of the two groups were retrospectively reviewed from our prospectively collected database of LT. All the surgeons involved in the study were from the same surgical team. Preoperative down-staging treatment for size reduction of the tumors included transcatheter arterial chemoembolization, radiofrequency ablation, percutaneous ethanol injection and stereotactic body radiation therapy (gamma knife), thereby facilitating LT. Data of oncological characteristics (tumor within or outside the Milan criteria, tumor size, tumor number, vascular invasion and tumor pathological type) were based on the intraoperative findings and confirmed pathologically after LT. Tumor size was measured with the maximal tumor diameter in the resected specimens. The duration of the operation was collected from operation or anesthesia records. Follow-up data were obtained through outpatient visits or telephone inquiries. All of the living organs were donated with informed consent. Cadaveric donors involved in the study were obtained from brain-dead or no-heart beating donors.
All the surgical procedures were performed by specialists with experience with the LT technique in the Department of Liver Surgery, Ren Ji Hospital, Shanghai, China. Surgery was performed using standard techniques. Classic orthotopic LT was the only surgery type in the DDLT group. All patients undergoing LDLT were operated on using right liver grafts without the middle hepatic vein. Biliary tract reconstruction was performed using a duct-to-duct anastomosis. The posterior anastomosis line was sutured continuously with 7-0 Prolene, while an interrupted suture was applied to the anterior anastomosis line.
The definition of postoperative complication introduced by Copeland et al[18] does not cover many common complications after LT, so complications that were confirmed clinically were also included. Perioperative complications occurring during the initial hospital stay for LT were compared between the two groups. The modified Clavien-Dindo classification system of surgical complications was used to evaluate the severity of perioperative complications[19]. As for biliary complications, a bile leak was diagnosed through abdominal drains or reexploration findings, while an anastomotic stenosis was detected on the basis of an overt dilatation of the intrahepatic duct according to computed tomography or ultrasonography, or on the basis of direct visualization using endoscopic retrograde cholangiopancreatography (ERCP). Wound infection not requiring pharmacological treatment was defined as a grade I complication, so it was not included in postoperative bacterial infection of grade II. Suspected cases of acute rejection were confirmed by liver biopsy. Postoperative hypertension was defined as hypertension lasting longer than 3 d after surgery in the absence of a history of hypertension. If a patient died of a grade IV complication, the reason of death was not listed in the complications, but other coexistent complications were still recorded. Furthermore, we compared the overall biliary complication rates in the perioperative and long-term follow-up periods between the LDLT and DDLT groups.
After LT, a triple drug regimen of tacrolimus or cyclosporine (CsA) combined with methylprednisolone and/or mycophenolate mofetil (MMF) was used. Immunosuppression was started during surgery with 500 mg methylprednisolone, followed by tapering from 240 mg on postoperative day 1 to 40 mg on postoperative day 6. Maintenance prednisone at an initial dose of 20 mg daily was gradually reduced every week and was withdrawn 3 mo post-transplantation. The initial dose of tacrolimus was 0.06-0.15 mg/kg every 24 h with a target trough level of 8-10 ng/mL during the first 30 d. MMF was administered orally after LT at 0.5-0.75 g twice a day. If tacrolimus did not reach the target level, it was replaced with CsA at 6-10 mg/kg per day. The target C0 and C2 levels for CsA were 150-200 ng/mL and 800-1200 ng/mL, respectively.
Statistical analyses were performed with SPSS for Windows version 13.0 to compare the differences between the LDLT group and DDLT group. Categorical data were analyzed with the χ2 test. The test of normality for all related variables was checked by the Shapiro-Wilk method, and a nonparametric statistical method (Mann-Whitney test) was applied to the variables without a normal distribution. The Kaplan-Meier method was used to estimate the cumulative overall survival (OS) and relapse-free survival (RFS). A log-rank method was used to test the equality of survival distributions between the two groups. P values < 0.05 were considered statistically significant.
The clinical characteristics of the LDLT and DDLT groups are summarized in Table 1. Matching in terms of age, sex distribution, MELD score, Child-Pugh score and proportion of patients with previous hepatic resection was well achieved in all cases. Twelve patients (30.0%) in the LDLT group and 29 patients (36.3%) in the DDLT group received down-staging treatment before LT, with no significant difference between the two groups. One hundred and sixteen patients (96.7%) suffered cirrhosis due to HBV infection. One patient in the LDLT group had no cirrhosis, and the DDLT group included one case with alcoholic cirrhosis, one case with autoimmune cirrhosis and a third case without cirrhosis. No significant difference was seen in the overall distribution of different causes of liver cirrhosis. The proportions of patients who had particular comorbidities, including hypertension, diabetes mellitus, cardiac diseases, cerebrovascular diseases and previous history of abdominal surgery were also similar between the two groups. Table 2 shows the outcomes of preoperative serological detection in the two groups; there was no significant difference in serum alpha-fetoprotein, blood group or the proportion of patients with positive hepatitis B virus surface antigen (HBVsAg) or positive hepatitis B virus DNA (HBV DNA). No patient with positive hepatitis C virus antibody was found in either group.
| Variable | LDLT (n = 40) | DDLT ( n= 80) | P value |
| Age (yr) | 48.6 ± 9.7 | 49.5 ± 8.9 | 0.614 |
| Gender | > 0.999 | ||
| Male | 34 (85.0) | 68 (85.0) | |
| Female | 6 (15.0) | 12 (15.0) | |
| MELD score | 0.354 | ||
| 6-9 | 7 (17.5) | 22 (27.5) | |
| 10-19 | 28 (70.0) | 49 (61.2) | |
| 20-29 | 3 (7.5) | 8 (10.0) | |
| 30-40 | 2 (5.0) | 1 (1.3) | |
| Child-Pugh score | 0.898 | ||
| A | 12 (30.0) | 25 (31.2) | |
| B | 18 (45.0) | 38 (47.5) | |
| C | 10 (25.0) | 17 (21.3) | |
| Previous hepatic resection | 3 (7.5) | 6 (7.5) | > 0.999 |
| Preoperative down-staging treatment | 12 (30.0) | 29 (36.3) | 0.496 |
| Liver cirrhosis | > 0.999 | ||
| Hepatitis B cirrhosis | 39 (97.5) | 77 (96.3) | |
| Alcoholic cirrhosis | 0 | 1 (1.3) | |
| Autoimmune cirrhosis | 0 | 1 (1.3) | |
| No cirrhosis | 1 (2.5) | 1 (1.3) | |
| Comorbidities | |||
| Hypertension | 4 (10.0) | 8 (10.0) | > 0.999 |
| Diabetes mellitus | 4 (10.0) | 8 (10.0) | > 0.999 |
| Cardiac disease | 0 | 1 (1.3) | > 0.999 |
| Cerebrovascular disease | 1 (2.5) | 4 (5.0) | 0.872 |
| Previous abdominal surgery | 11 (27.5) | 13 (16.3) | 0.146 |
| Variable | LDLT (n = 40) | DDLT (n = 80) | P value |
| Serum alpha-fetoprotein | 0.331 | ||
| < 400 ng/mL | 25 (62.5) | 57 (71.2) | |
| ≥ 400 ng/mL | 15 (37.5) | 23 (28.8) | |
| Blood group | 0.132 | ||
| A | 10 (25.0) | 25 (31.3) | |
| B | 11 (27.5) | 28 (35.0) | |
| AB | 2 (5.0) | 9 (11.3) | |
| O | 17 (42.5) | 18 (22.5) | |
| HBVsAg positive | 40 (100.0) | 76 (95.0) | 0.369 |
| HBV DNA | 0.297 | ||
| < 1000 copies/mL | 20 (50.0) | 32 (40.0) | |
| ≥ 1000 copies/mL | 20 (50.0) | 48 (60.0) | |
| HCV antibody positive | 0 | 0 | > 0.999 |
As shown in Table 3, the duration of operation was significantly longer (P < 0.001) in the LDLT group than in the DDLT group. Estimated blood loss (P = 0.055) and the proportion of patients with intraoperative transfusion (P = 0.093) were slightly but not significantly greater in the LDLT group. Tumor pathological type, tumor size, tumor number and the proportion of patients with vascular invasion were similar between the two groups. The mean tumor sizes in the LDLT and DDLT groups were 3.8 ± 1.9 cm and 4.1 ± 2.3 cm, respectively. The proportions of patients who met the Milan criteria and of patients staged by the Pittsburgh system who did not meet the Milan criteria were exactly matched; 60% of the entire cohort of patients with hepatic carcinoma met the Milan criteria, and 10% of the enrolled patients fell within stage IV according to the Pittsburgh staging system[17].
| Variable | LDLT ( n= 40) | DDLT (n = 80) | P value |
| Duration of operation (min) | 553 ± 105 | 445 ± 75 | < 0.001 |
| Estimated blood loss (mL) | 1188 ± 824 | 1035 ± 1072 | 0.055 |
| Intraoperative transfusion | 24 (60.0) | 35 (43.8) | 0.093 |
| Tumor pathological type | 0.557 | ||
| HCC | 39 (97.5) | 79 (98.8) | |
| ICC | 0 | 1 (1.3) | |
| cHCC-CC | 1 (2.5) | 0 | |
| Tumor size (cm) | 3.8 ± 1.9 | 4.1 ± 2.3 | 0.708 |
| Tumor number | 0.238 | ||
| Single | 27 (67.5) | 62 (77.5) | |
| Multiple | 13 (32.5) | 18 (22.5) | |
| Vascular invasion | 3 (7.5) | 4 (5.0) | 0.890 |
| Within Milan criteria | 24 (60.0) | 48 (60.0) | > 0.999 |
| Outside Milan criteria (Pittsburgh staging) | > 0.999 | ||
| I | 12 (30.0) | 24 (30.0) | |
| IVa | 2 (5.0) | 4 (5.0) | |
| IVb | 2 (5.0) | 4 (5.0) |
Details of all perioperative complications are listed in Table 4. Multiple complications were possible in a single patient, so the sum of the individual complications was not equal to the total number of patients with complications. Twenty-one patients (52.5%) in the LDLT group and 46 patients (57.5%) in the DDLT group experienced perioperative complications. No significant difference was observed between the 2 groups in terms of overall number of patients with complications, whereas a significantly higher incidence of grade II complications was noted in the DDLT group than in the LDLT group, and postoperative bacterial infection accounted for the majority of grade II complications. The 3 most common complications during the initial hospital stay for LT were postoperative bacterial infection, intra-abdominal bleeding and biliary complications. Most intra-abdominal bleeding (5 out of 8) and biliary complications (5 out of 7) were solved through reoperation or biliary stent placement by ERCP.
| Grade | LDLT ( n= 40) | DDLT (n = 80) | P value |
| Grade I | 3 (7.5) | 5 (6.3) | > 0.999 |
| Wound infection | 3 | 4 | |
| Mental symptom | 0 | 1 | |
| Grade II | 18 (45.0) | 52 (65.0) | 0.036 |
| Postoperative bacterial infection | 11 | 35 | |
| Virus infection | 2 | 2 | |
| Bile leak | 2 | 0 | |
| Hypertension | 1 | 2 | |
| Mental symptom | 1 | 4 | |
| Acute rejection | 0 | 5 | |
| Peripheral nerve injury | 0 | 1 | |
| Intra-abdominal bleeding | 1 | 2 | |
| Subdural hematoma | 0 | 1 | |
| Grade IIIa | 3 (7.5) | 0 | 0.063 |
| Biliary stricture | 1 | 0 | |
| Hepatic artery thrombosis | 1 | 0 | |
| Wound dehiscence | 1 | 0 | |
| Grade IIIb | 2 (5.0) | 10 (12.5) | 0.333 |
| Intra-abdominal bleeding | 0 | 5 | |
| Bile leak | 2 | 1 | |
| Wound dehiscence | 0 | 2 | |
| Biliary stricture | 0 | 1 | |
| Hepatic artery thrombosis | 0 | 1 | |
| Grade IVa | 0 | 2 (2.5) | 0.552 |
| Renal insufficiency | 0 | 2 | |
| Grade IVb | 1 (2.5) | 1 (1.3) | > 0.999 |
| MODS | 1 | 1 | |
| Grade V | 1 (2.5) | 4 (5.0) | 0.872 |
| Death | 1 | 4 | |
| Overall | 21 (52.5) | 46 (57.5) | 0.603 |
It is worth highlighting here that during the perioperative period, the LDLT group had more biliary complications (12.5% vs 2.5%, P = 0.073), while postoperative bacterial infection (27.5% vs 43.8%, P = 0.084) and intra-abdominal bleeding (2.5% vs 8.8%, P = 0.365) seemed to be more common in the DDLT group. However, only a slight difference existed in the 3 kinds of complications between the two groups. In the long-term follow-up period after the first hospital stay for LT, 6 patients in the LDLT group (15.0%) and 4 patients in the DDLT group (5.0%) experienced biliary stricture, so the LDLT group had a higher proportion of patients suffering biliary stricture (P = 0.129). Consequently, total biliary complications in the LDLT group reached a rate of 27.5%, which was significantly higher than that in the DDLT group (27.5% vs 7.5%, P = 0.003) (Table 5).
| Biliary complications | LDLT (n = 40) | DDLT (n = 80) | P value |
| Perioperative period | 5 (12.5) | 2 (2.5) | 0.073 |
| Bile leak | 4 | 1 | |
| Biliary stricture | 1 | 1 | |
| Long-term follow-up period | 6 (15.0) | 4 (5.0) | 0.129 |
| Biliary stricture | 6 | 4 | |
| Overall | 11 (27.5) | 6 (7.5) | 0.003 |
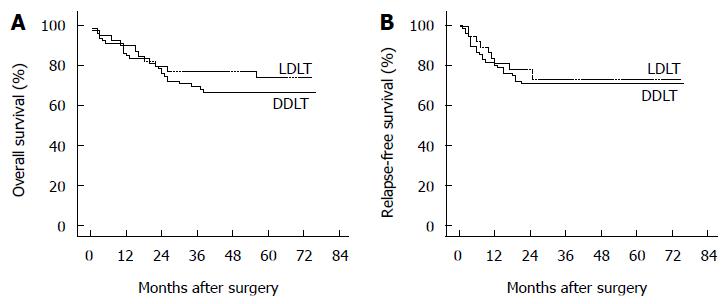
The median follow-up period of this cohort of patients was 50 mo (range, 1-76 mo) from the surgery day. Table 6 shows the details of survival outcomes between the two groups. With a median follow-up period of 57 mo (range, 1-75 mo) in the LDLT group and 48 mo (range, 1-76 mo) in the DDLT group, the two groups were comparable in the follow-up period (P = 0.236). The 1- and 5-year OS rates in the two groups were, respectively, 89.8% vs 84.9% and 74.1% vs 66.6% (Figure 1A, P = 0.372). Moreover, the RFS rate also did not differ between the two groups. The 1- and 5-year RFS rates were 81.2% vs 80.3% and 72.9% vs 70.9% in the LDLT vs DDLT group, respectively (Figure 1B, P = 0.749). In the LDLT group, recurrence occurred in 10 patients (25.0%) and caused 5 deaths (12.5%). In contrast, 22 recurrences (27.5%) occurred and 16 patients (20%) died of recurrence in the DDLT group. Three patients with relapse in the LDLT group (7.5%) and 3 patients with that in the DDLT group (3.8%) were still alive by the end of follow-up. In patients treated with LDLT, 3 deaths were caused by biliary complications and 2 deaths were caused by severe bacterial infection. On the other hand, 10 deaths from non-recurrent causes in the DDLT group included the following causes: severe bacterial infection (n = 4), cerebral hemorrhage (n = 2), biliary complication (n = 1), gastrointestinal tract hemorrhage (n = 1), severe coagulation disorder (n = 1) and hemothorax after thoracentesis (n = 1).
| Variable | LDLT (n = 40) | DDLT (n = 80) | P value |
| Survival status | |||
| Alive without relapse | 27 (67.5) | 51 (63.8) | |
| Alive with disease | 3 (7.5) | 3 (3.8) | |
| Died of relapse | 5 (12.5) | 16 (20.0) | |
| Died of other causes | 5 (12.5) | 10 (12.5) | |
| Overall survival | 0.372 | ||
| 1-yr | 89.8% | 84.9% | |
| 5-yr | 74.1% | 66.6% | |
| Relapse-free survival | 0.749 | ||
| 1-yr | 81.2% | 80.3% | |
| 5-yr | 72.9% | 70.9% |
In recent decades, more and more patients with end-stage liver diseases have been saved thanks to the rapid progress in LT technology. Mazzaferro et al[5] put forward the Milan criteria for LT in HCC patients in 1996. Nevertheless, patients’ chance of cure has been restricted by such a strict indication, and whether the criteria could be expanded to enable more patients to qualify as transplant candidates has been a moot point. The EASL-EORTC clinical practice guidelines have mentioned that the criteria of “up-to-seven” in patients without microvascular invasion could be considered for LT for HCC[1,20]. Additionally, extended indications for LT in patients with hepatic carcinoma having similar survival outcomes with the Milan criteria have been reported by some transplant centers[21-24].
Theoretically speaking, LDLT calls for preservation of the inferior vena cava and a long internal structure of the hepatoduodenal ligament, which seems to render it less radical and thus less effective than DDLT. Moreover, it may expose the living donors to the risk of surgery. Therefore, most reported expanded indications are currently based on DDLT. However, several studies from Japan have shown encouraging outcomes of extended criteria for LDLT[25-27]. In the current study, tumors were staged according to the intraoperative findings and pathological outcomes of the resected specimens. Patients with tumor invasion were confirmed after LT. The Pittsburgh staging system seemed to have a direct correlation with the tumor-free survival rate based on the clinical data in our medical center, so we used it to match the patients of the two groups who did not meet the Milan criteria. Although 40% of the enrolled patients did not meet the Milan criteria, the OS and RFS turned out to be favorable on the whole.
Organs from donation after cardiac death (DCD) cannot meet the needs of LT. Consequently, a large number of patients die of a shortage of liver donors every year, making living donors another important source of organs. However, animal experiments have suggested that the regenerating liver might have a potential effect on the growth of HCC[28,29], and LDLT has been criticized for its higher HCC recurrence rate than DDLT. Based on our experience, the long-term follow-up results do not support such a conclusion. Therefore, we carried out this multivariate case-matched comparison to maximize the comparability of the LDLT and DDLT groups. Our results show similar overall and relapse-free survival rates between the two groups, and LDLT conferred a 7.5% survival benefit compared with DDLT, so LDLT did not compromise the survival of patients or increase tumor recurrence. In addition, for emergency patients with fulminant hepatic failure, LDLT is often an optimal choice and can provide a timely graft to save their lives.
Patients with HCC, now widely accepted as legitimate transplant candidates, require special consideration to achieve timely transplantation. A study by Mizuno et al[30] showed that the median period between the registration for LDLT and the occurrence of extrahepatic metastasis, macroscopic vascular invasion or rupture of HCC was 12.2 mo (range, 3.8-32.9 mo), indicating that the waiting period suitable for a liver transplant is quite limited for HCC patients. Many patients with hepatic carcinoma lose their chance of LT or die of tumor progression during the waiting period, whereas the waiting period has greatly shortened and the survival has greatly improved with the advent of LDLT[31]. In the present study, the median preoperative waiting time for LT in the LDLT group was 14 d (range, 5-63 d), which was significantly less than the 45 d (range, 20-235 d) in the DDLT group. However, LDLT can only be feasible when a suitable volunteer is available, even though it is in theory a good alternative to DDLT. In Hong Kong, the policy of a 6-mo wait for cadaveric liver allocation benefits the HCC patients who have practically no chance of undergoing LDLT. These modifications of the cadaveric liver allocation policy could result in transplants for as many HCC patients as possible but would not deprive non-HCC patients of a fair chance of undergoing LT[32].
Another important criticism of LDLT is that biliary complications in patients who undergo LDLT are more common than in DDLT patients[11,12]. Our data showed a similar pattern that LDLT was associated with a significantly increased rate of biliary complications compared with DDLT. In the 5 patients of the LDLT group who died of non-recurrent causes, 3 deaths (60%) were caused by biliary complications, which may be a weak point of LDLT. There were 5 bile leak cases in this study, all of them diagnosed within the first month, of whom 2 underwent reexploration. Continuous abdominal drainage was the most important treatment modality for bile leaks. Unlike bile leaks, the stenoses (10 out of 12 cases) were mostly detected in the outpatient clinic after worsening of liver function, symptoms of cholangitis, or intrahepatic duct dilatation on computed tomography scans or magnetic resonance cholangiopancreatography. However, they could be successfully controlled by placement of a biliary stent through ERCP in most cases.
By and large, the LDLT and DDLT groups were comparable in the total number of patients with perioperative complications, but it should be noted that complications occurring in the DDLT group seemed to be more serious than those in the LDLT group. For example, in the DDLT group 10 patients (12.5%) underwent surgical or endoscopic interventions under general anesthesia due to grade IIIb complications, and 7 patients (8.8%) suffered complications higher than grade IIIb. Our data of perioperative complications and follow-up results revealed that DDLT tended to result in more bleedings after surgery, including intra-abdominal bleeding, cerebral hemorrhage and gastrointestinal tract hemorrhage. In our comparison, 5 intra-abdominal bleedings needed a second operation, and another 4 patients died of hemorrhage in the DDLT group. A slight but not significant difference was observed in these findings, which prompts the question whether a difference exists in the coagulation function among liver grafts from different sources. Further large-scale, prospective, randomized trials are needed to address the question. Postoperative bacterial infection is always the most common complication in the field of LT. In our comparative study the DDLT group showed a significantly higher proportion of patients with grade II complications, which were mainly composed of postoperative bacterial infections. At times, bacterial infections after LT are not accompanied by fever, so identifying and preventing the development of infections after LT is particularly important.
In conclusion, grade II complications occurred more frequently in the DDLT group than in the LDLT group, but the overall proportions of patients with perioperative complications were comparable between the two groups. Although LDLT led to a higher rate of biliary complications, it did not show any inferiority in long-term OS or RFS. Large-scale, prospective, randomized controlled trials are needed to reveal the inherent characteristics of complications between the two groups.
Liver cancer is the sixth most common cancer and the third cause of cancer-related death, accounting for 7% of all cancers worldwide. Liver transplantation (LT) is the best option for patients with a hepatic tumor ≤ 5 cm in diameter and concurrent decompensated cirrhosis. Living-donor liver transplantation (LDLT) is currently an effective alternative to alleviate the organ shortage, but worries exist that LDLT may be associated with higher rates of tumor recurrence and biliary complications than deceased-donor liver transplantation (DDLT). Whether LDLT can have more benefits on perioperative and long-term outcomes than DDLT is still disputable.
Due to a severe shortage of cadaveric donor organs, LDLT is currently an effective alternative to alleviate organ shortage, but it has been criticized for its underlying higher cancer recurrence rate than DDLT. As for the surgical selection for patients with hepatic carcinoma, the research hotspot is whether LDLT will result in more postoperative complications or tumor recurrences than DDLT.
It remains controversial whether LDLT can achieve similar or better long-term survival than DDLT. In the present study, the authors used a multivariate case-matched method to compare the results of LDLT and DDLT in patients with hepatic carcinoma. In spite of a higher rate of biliary complications in the LDLT group, DDLT was associated with more grade II complications, while the overall perioperative complication rates were similar between the two groups. Furthermore, compared with the DDLT group, the LDLT group had comparable long-term follow-up survival. The present data do not suggest that LDLT results in more tumor recurrences than DDLT.
The study results suggest that LDLT not only could greatly shorten the waiting period of patients with hepatic carcinoma but also would not compromise long-term survival or increase tumor recurrence, so LDLT is a favorable alternative to solve the problem of organ shortage.
Liver transplantation is a surgical technique that removes a diseased liver and replaces it with a healthy donor liver in patients with end-stage liver disease. Case-matched comparison is a comparative study between groups in which each case is matched by one or more comparable cases (1:1 or 1:N) in terms of several measurable parameters.
The study used a special statistical method to compare the surgical outcomes of LDLT and DDLT in patients with hepatic carcinoma, which is a good way to establish greater parity between the two surgery groups. The results suggested that LDLT could have similar survival outcomes as DDLT. This study has significant merits.
| 1. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (4)] |
| 2. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2135] [Article Influence: 79.1] [Reference Citation Analysis (1)] |
| 3. | Tanaka H, Imai Y, Hiramatsu N, Ito Y, Imanaka K, Oshita M, Hijioka T, Katayama K, Yabuuchi I, Yoshihara H. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13561] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 5. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Full Text] |
| 6. | Ng KK, Lo CM, Chan SC, Chok KS, Cheung TT, Fan ST. Liver transplantation for hepatocellular carcinoma: the Hong Kong experience. J Hepatobiliary Pancreat Sci. 2010;17:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 7. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 8. | Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Ghobrial RM, Fair JH, Olthoff KM, Kam I. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Reichman TW, Katchman H, Tanaka T, Greig PD, McGilvray ID, Cattral MS, Renner EL, Selzner M, Ghanekar A, Levy G. Living donor versus deceased donor liver transplantation: a surgeon-matched comparison of recipient morbidity and outcomes. Transpl Int. 2013;26:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Zimmerman MA, Baker T, Goodrich NP, Freise C, Hong JC, Kumer S, Abt P, Cotterell AH, Samstein B, Everhart JE. Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: a report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl. 2013;19:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 13. | Lei J, Yan L, Wang W. Comparison of the outcomes of patients who underwent deceased-donor or living-donor liver transplantation after successful downstaging therapy. Eur J Gastroenterol Hepatol. 2013;25:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, Shang Y, Kong Y, Guo Z, He X. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 15. | Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, McGilvray ID, Levy G, Greig PD, Renner EL. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl. 2012;18:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 16. | Bhangui P, Vibert E, Majno P, Salloum C, Andreani P, Zocrato J, Ichai P, Saliba F, Adam R, Castaing D. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology. 2011;53:1570-1579. [PubMed] |
| 17. | Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538-543. [PubMed] |
| 18. | Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1162] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 19. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1616] [Article Influence: 89.8] [Reference Citation Analysis (1)] |
| 21. | Li J, Yan LN, Yang J, Chen ZY, Li B, Zeng Y, Wen TF, Zhao JC, Wang WT, Yang JY. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol. 2009;15:4170-4176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 22. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (1)] |
| 23. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 24. | Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, Qian JM, Zhou J, Xu Y, Qiu SJ. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol. 2009;135:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 25. | Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, Ogawa K, Sakamoto S, Ogura Y, Egawa H. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 26. | Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Aishima S, Terashi T, Shimada M, Maehara Y. Extended indication for living donor liver transplantation in patients with hepatocellular carcinoma. Transplantation. 2007;83:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 27. | Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 28. | Picardo A, Karpoff HM, Ng B, Lee J, Brennan MF, Fong Y. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998;124:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Shi JH, Huitfeldt HS, Suo ZH, Line PD. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl. 2011;17:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Mizuno S, Yokoi H, Shiraki K, Usui M, Sakurai H, Tabata M, Sugimoto K, Takei Y, Yamakado K, Takeda K. Prospective study on the outcome of patients with hepatocellular carcinoma registered for living donor liver transplantation: how long can they wait? Transplantation. 2010;89:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Berg CL, Gillespie BW, Merion RM, Brown RS, Abecassis MM, Trotter JF, Fisher RA, Freise CE, Ghobrial RM, Shaked A. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 32. | Chan SC, Sharr WW, Chok KS, Chan AC, Lo CM. Wait and transplant for stage 2 hepatocellular carcinoma with deceased-donor liver grafts. Transplantation. 2013;96:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
P- Reviewer: Mizuno S S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Liu XM