Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4076
Revised: February 25, 2014
Accepted: March 19, 2014
Published online: April 14, 2014
Processing time: 118 Days and 3 Hours
AIM: To investigate the correlation between nerve growth factor-tropomyosin-receptor-kinase (NGF-TrkA) signaling pathway and prognosis in intrahepatic cholangiocarcinoma (IHCC).
METHODS: NGF and TrkA expression in 83 samples of IHCC was assessed by immunohistochemistry. Correlations between NGF-TrkA expression and clinicopathological features were analyzed by χ2 test. Moreover, we evaluated the association between NGF-TrkA and overall survival by univariate and multivariate analysis. With experiments in vitro, we investigated the crucial role of NGF-TrkA on proliferation and invasion of IHCC cells with recombinant NGF-β stimulation.
RESULTS: We found that NGF and TrkA expression was significantly related with differentiation (P = 0.024) and intraneural invasion (P = 0.003), respectively. Additionally, double higher expression of NGF and TrkA was identified as an independent prognostic factor in IHCC (P = 0.003). Moreover, we demonstrated that NGF-TrkA signaling pathway can promote IHCC proliferation and invasion.
CONCLUSION: NGF-TrkA double higher expression is an independent prognostic factor in IHCC. NGF-TrkA pathway can promote IHCC progression, indicating that NGF-TrkA may become a potential drug target.
Core tip: For the first time, we systemically investigated nerve growth factor (NGF) and tropomyosin-receptor-kinase (TrkA) expression in 83 intrahepatic cholangiocarcinoma(IHCC) samples by immunohistochemistry, and then analyzed the expression relationship with clinicopathological features, which resulted in finding that NGF and TrkA were significantly associated with differentiation (P = 0.024) and intraneural invasion (P = 0.003) respectively. Moreover, we found that NGF and TrkA double higher expression had poorer prognosis than others. NGF-TrkA double higher expression was further confirmed as an independent prognostic factor by multivariate analysis. With function assays we demonstrated that NGF-TrkA signaling pathway played a crucial role in cholangiocarcinoma proliferation and invasion, indicating NGF-TrkA pathway could be a promising potential drug target of intrahepatic cholangiocarcinoma.
- Citation: Yang XQ, Xu YF, Guo S, Liu Y, Ning SL, Lu XF, Yang H, Chen YX. Clinical significance of nerve growth factor and tropomyosin-receptor-kinase signaling pathway in intrahepatic cholangiocarcinoma. World J Gastroenterol 2014; 20(14): 4076-4084
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4076
Cholangiocarcinoma (CCA) is highly malignant tumor that arises from biliary tract. The 7th union for international cancer control/american joint committee on cancer (UICC/AJCC) divided CCA into the intrahepatic, perihilar and distant cholangiocarcinoma according to their location and different clinical features. Incidence of CCA just accounts for 3% of gastrointestinal cancer[1,2], but the morbidity and mortality of cholangiocarcinoma, especially intrahepatic cholangiocarcinoma (IHCC), are increasing worldwide[3]. The IHCC is featured of silent clinical signatures, early regional invasiveness, distant metastasis and poor prognosis[4-6]. Currently, radical resection of tumor and involved liver is the only curative treatment for IHCC. However, when patients are hospitalized because of jaundice or pain, it is usually too late for surgery owing to the early invasiveness and metastasis, which make the resectability rate quite low and variable (18%-70%)[7]. Hence, both effective pre- and post-operational biomarkers are urgently needed for early and individualized treatment. Unfortunately, the process of researching mechanisms and biomarkers of IHCC was very slow, though some breakthroughs were realized in molecular analysis and classification[8,9].
Nerve growth factor (NGF) was the first discovered critical member in the neurotrophin polypeptide family[10], which is comprised of brain-derived neurotrophic factor, neurotrophins NT-3, NT-4/5, NT-6, and NT-7[11]. NGF functions as a signaling molecule by binding with two known receptor: the common p75 neurotrophin receptor (p75NTR) which binds all of the neurotrophins with almost equal affinity, and specific tyrosine kinase receptors called tropomyosin related kinases (Trks) which binds neurotrophin specially[12,13]. Dysregulation of NGF was found in many kinds of tumors including neuronal tumors and non-neuronal tumors like prostate, lung, and breast cancers[14]. Moreover, rearrangements or aberrant expression of Trk genes were found in a variety of other cancers such as papillary thyroid carcinomas, secretory breast cancers, pediatric sarcomas and leukemias[15]. For example, tropomyosin-receptor-kinase receptor, the only Trk receptor which can bind with NGF, is demonstrated to be correlated with breast cancers, thyroid carcinomas and neuroblastomas[16-19]. NGF has been proved to activate Raf-MAPK signaling pathway, which is well acknowledged to be related with carcinogenesis[20]. For a long period of time, NGF and its receptors are considered as a potential molecular target of tumorgenesis. In regard to CCA, NGF itself was indicated to promote CCA cell line progression in study in vitro[21]. In perihilar CCA, NGF-β was proved to be associated with lymph node metastasis and nerve infiltration[22]. However, the relation between NGF and IHCC has not been reported in clinical study, and no article has proved the role of NGF-tropomyosin-receptor-kinase (NGF-TrkA) signaling in IHCC.
In our study, we investigated expression of NGF and TrkA in 83 samples of IHCC by IHC, and further explored the relationship of NGF/TrkA expression with clinicopathologic parameters and overall survival rates. To explain why NGF-TrkA signaling pathway was associated with poor prognosis of IHCC, we used two IHCC cell lines and performed tumor function assays in vitro, including proliferation and invasion assay.
All the 83 IHCC samples were obtained from the tumor resection between 2002 and 2010. All the samples were obtained from the Department of Pathology of Qilu Hospital and Yishui Central Hospital, Shandong Province. The diagnosis was confirmed by the routine pathology and histopathological samples were reviewed by a senior pathologist to select suitable areas for immunohistochemical detection. The overall survival time was calculated from the operation to the date of death or censored at the date of the last follow-up examination. Clinical data, including age, gender and other clinicopahtologic features were abstracted from the patients’ medical records. Pathologic tumor-node-metastasis (pTNM) staging was based on the 7th staging classification of International Union Against Cancer (2009).
This study was approved of the Institutional Clinical Ethics Review Board with prior patient consents. The clinical follow-up was at least 3 mo after surgery, with the median follow-up time 25.1 mo (from 3 to 96 mo). Criteria of the validation cohort included: (1) available formalin-fixed tumor tissues; (2) available clinical follow-up data and complete medical records; and (3) no history of previous anticancer therapy and other malignancies.
Human intrahepatic cholangiocarcinoma cell lines RBE and QBC939 were bought from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cell line HUCCT-1 was purchased from RIKEN Bioresourse Center (Saitama, Japan). All the cells were cultured in the RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco) and 1% ampicillin/streptomycin in 5% CO2 resuscitation.
Recombinant human β-NGF was obtained from Sino Biological Inc. (Beijing, China). Matrigel pre-coated transwell was from BD Biosciences. All other reagents were bought from Sigma Company. TrkA antibody was purchased from Santa Cruz (Cat No. sc-118), NGF antibody was from Abcam Company (Cat No. ab52918), phosphor-TrkA-Y490, phosphor-AKT-S473 and phosphor-ERK-T202/204 antibodies were obtained from Cellsignaling Technology.
Streptavidin peroxidase complex method was used for immunohistochemical (IHC) staining referring to previous study[14,23,24]. Adhesive-coated slide was used to transfer tissue microarray sections, and then samples were de-paraffinized and rehydrated with xylene and graded alcohol. Slides were then incubated in 3% hydrogen peroxide for 60 min to quench endogenous activity, and then immersed in citrate buffer (pH = 6.0) for antigen retrieval. Microwave oven was used to heat the buffer for 15 min for satisfied antigen retrieval. The sections were then blocked with 1% BSA in PBS containing 10% normal serum for 30 min at 37 °C. Slides were incubated in the corresponding primary antibodies (at the dilution of 1:50) overnight at 4 °C. Secondary antibodies labeled with streptoavidin-biotin-peroxidase reagent were used after removal of primary antibody and PBS washing. For visualization, slides were incubated in the 3,3’-diaminobenzidine solution until desired staining was approached. Lastly, slides were counterstained with hematoxylin and mounted.
The score of IHC staining was based on the multiply of staining intensity and area. The staining intensity of all tested proteins was scored as negative (0), weak (1), moderate (2) and strong (3), and scores of stained area was defined as follows: 1, < 10% of cells were positive; 2, 10%-50% of cells were positive; 3, > 50% of cells were positive. The mean score of NGF and TrkA was 3.4 and 2.5 respectively. The samples were divided into higher and lower groups according to the average score, namely the cut-off.
Cells were lysed first after treatment on ice with the lysis buffer(1% NP-40, 10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L sodium vanadate, 10 μg of leupeptin, 1 μg of aprotinin, 1 μg of pepstatin, 1 μg of antipain, and 30 μg of phenylmethylsulfonyl fluoride per mL). Cells were centrifuged at 10000 g at 4 °C for 15 min after scraping and the superior was added with loading buffer with equal volume. Protein concentration was tested by a BCA kit(Merck Company) and equal quality of protein was added to run SDS-PAGE gel. Protein in the gel was subsequently transferred into a PVDF membrane(PALL, United States) and incubated in 5% skimmed milk to block unspecific binding and then incubated in primary antibody with dilution at 1:1000 in 4 °C overnight. Corresponding secondary antibody was added after washing the membrane 3 times and protein was visualized by adding ECL (Millipore Company, United States).
MTT assay was used to measure the proliferative activity of CCA cells. In brief, RBE cells were split into a 96-well plate with density at 5000 cells per well and then starved in serum-free medium overnight before stimulation. 100 ng/mL NGF-β was used to stimulate cells for 48 h. After stimulation, 10 uL MTT at 10 mg/mL concentration was added into medium and incubated for 4-6 h. Subsequently, medium was decanted and crystals were dissolved by 100 μL DMSO and incubated for 15 min for complete solution. Absorbance at 490 nm was read by a microplate reader and all data were standardized by compare with base line, and three independent experiments were performed to confirm results.
Cell invasive activity was evaluated by transwell assay with matrigel-precoated transwell chambers (BD Company, United States). RBE cells were split into the chambers and incubated in serum-free medium for 6 h before 100 ng/mL NGF stimulation. After starvation, medium was changed to RPMI1640 with 1% fetal bovine serum, and NGF was added into lower filter. After 24 h, cells on upper filter surface were removed using a cotton swab and invasive cells were stained by crystal violet. Cell numbers were counted at × 200 magnification of at least five random visual fields and three independent experiments were performed to confirm results.
Both of the oligo siRNA and scramble RNA of NGF (sc-43970) and TrkA (sc-36726) were purchased from Santa Cruz Biotechnology. Growing cells were transiently transfected with either 50 μmol/L of siRNA or the scrambled siRNA control with Lipofectamine 2000™ (Invitrogen) according to the manual. Forty-eight hours following transfection, transfected cells were scraped and immunoblotting was used to confirm the successful knock down.
All the statistical analyses were carried out with SPSS 17.0 software (IBM company, United States). The association between protein expression and clinicopathologic parameters was evaluated by χ2 test. Kaplan-Meier method was used to analyze correlation between survival rate and NGF-TrkA expression, and Cox Regression Model was used for multivariate analysis to determine the independent prognostic factors. P values < 0.05 was considered to be significant. The statistical comparisons between control and tested group were made with the Student t tests (The software Graph Pad Prism 5 was also used for statistical analysis).
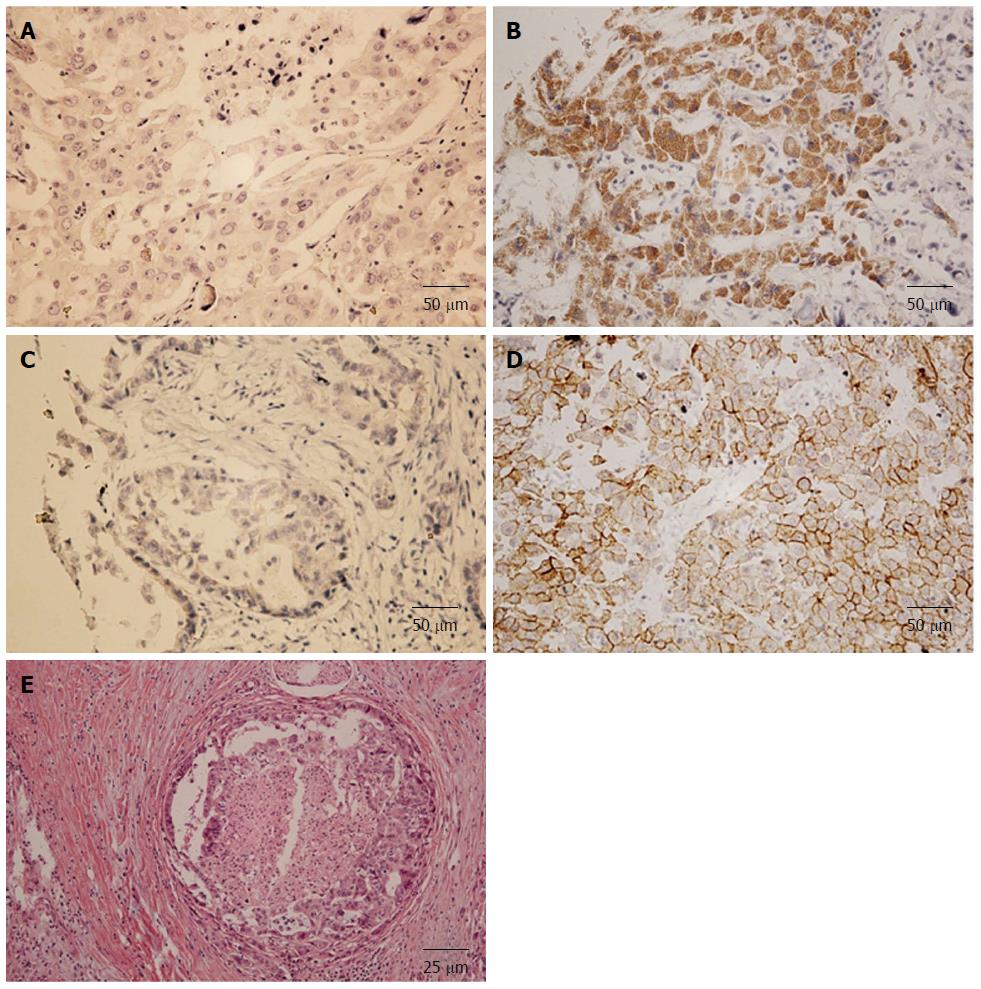
As a secreted growth factor, NGF was mostly found in cytoplasm (Figure 1A and B), and TrkA was found on membrane or in cytoplasm (Figure 1C and D). According to the criteria described before, expressions of NGF and TrkA were divided into higher and lower expressive groups by the average cut-off. NGF was observed higher expressed in 27.7% (23/83) IHCC samples while TrkA was overexpressed in 20.5% (17/83) samples. Double NGF and TrkA higher expression was defined as both NGF and TrkA had higher score than corresponding cut-off. The percent of double NGF and TrkA higher expression was 15.6% (13/83).
To further investigate the clinical and pathological importance of NGF and TrkA receptor, correlations between NGF, TrkA and clinicopathologic features were analyzed by Chi-Square method (Table 1). Well differentiation has more cases with higher NGF expression and poor differentiation has less cases with higher expression (P = 0.024) (Table 1). Moreover, TrkA expression was closely related to intraneural invasion (P = 0.003) (Figure 1E), which indicated that intraneural invasion may be resulted from the TrkA signaling pathway ectopic activation. This kind of TrkA activation may be caused by TrkA overexpression and consistent NGF stimulation, which probably come from the neuron or cancer cell autocrine.
| Clinicopathologic parameters | n | NGF | P value1 | TrkA | P value1 | |||
| low | high | low | high | |||||
| Age | < 65 yr | 62 | 44 | 18 | 0.640 | 49 | 13 | 0.850 |
| ≥ 65 yr | 21 | 16 | 5 | 13 | 4 | |||
| Gender | Male | 44 | 30 | 14 | 0.373 | 32 | 12 | 0.099 |
| Female | 39 | 30 | 9 | 34 | 5 | |||
| Tumor size | < 5 cm | 37 | 24 | 13 | 0.220 | 28 | 9 | 0.438 |
| ≥ 5 cm | 46 | 36 | 10 | 38 | 8 | |||
| Differentiation | Well | 19 | 9 | 10 | 0.024 | 20 | 5 | 0.228 |
| Moderately | 39 | 32 | 7 | 34 | 5 | |||
| Poorly | 25 | 19 | 6 | 12 | 7 | |||
| T stage | T1 | 40 | 29 | 11 | 0.200 | 33 | 7 | 0.428 |
| T2 | 20 | 17 | 3 | 16 | 4 | |||
| T3 | 23 | 14 | 9 | 17 | 6 | |||
| T4 | 0 | 0 | 0 | 0 | 0 | |||
| N stage | N0 | 57 | 40 | 17 | 0.520 | 46 | 11 | 0.695 |
| N1 | 26 | 20 | 6 | 20 | 6 | |||
| M stage | M0 | 79 | 57 | 22 | 0.900 | 63 | 16 | 0.823 |
| M1 | 4 | 3 | 1 | 3 | 1 | |||
| TNM stage | I | 31 | 22 | 9 | 0.948 | 26 | 5 | 0.470 |
| II | 10 | 9 | 1 | 8 | 2 | |||
| III | 16 | 9 | 7 | 12 | 4 | |||
| IVa | 22 | 17 | 5 | 17 | 5 | |||
| IVb | 4 | 3 | 1 | 3 | 1 | |||
| Satellites | N | 63 | 45 | 18 | 0.754 | 51 | 12 | 0.572 |
| P | 20 | 15 | 5 | 15 | 5 | |||
| Macrovascular invasion | N | 80 | 57 | 23 | 0.158 | 63 | 17 | 0.236 |
| P | 3 | 3 | 0 | 3 | 0 | |||
| Microvascular invasion | N | 67 | 48 | 19 | 0.786 | 52 | 15 | 0.357 |
| P | 16 | 12 | 4 | 14 | 2 | |||
| Intraneural invasion | N | 73 | 53 | 20 | 0.864 | 62 | 11 | 0.003 |
| P | 10 | 7 | 3 | 4 | 6 | |||
| HBV | N | 73 | 51 | 22 | 0.146 | 57 | 16 | 0.347 |
| P | 10 | 9 | 1 | 9 | 1 | |||
| Cirrhosis | N | 67 | 48 | 19 | 0.786 | 53 | 14 | 0.847 |
| P | 16 | 12 | 4 | 13 | 3 | |||
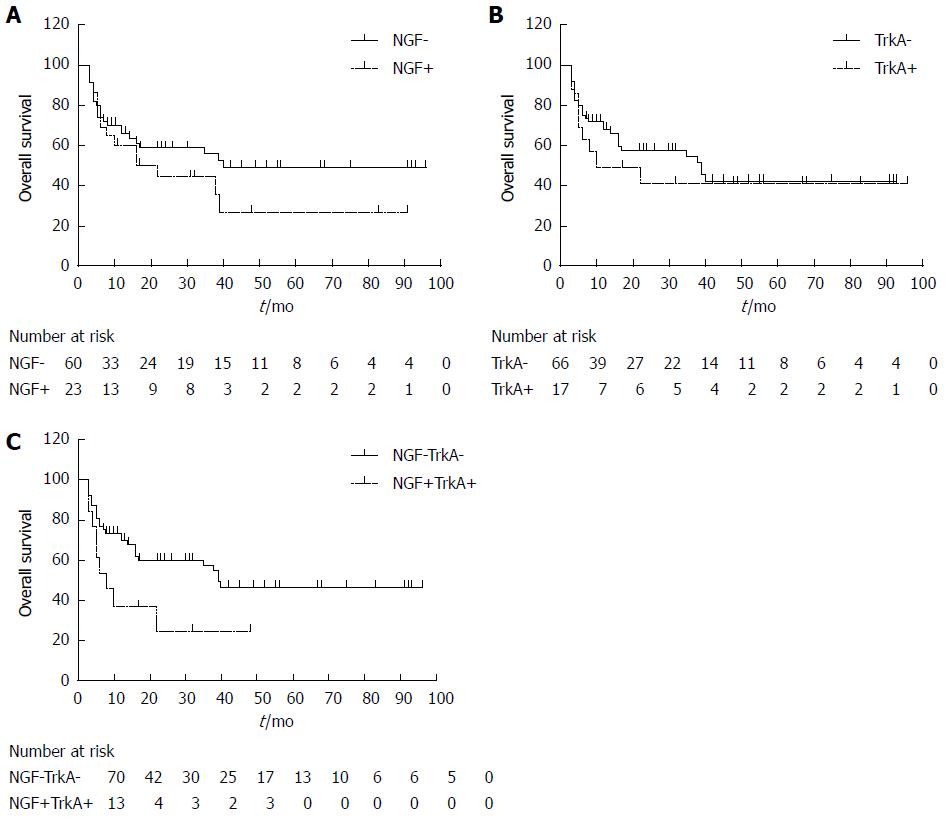
In univariate analysis, T stage (P = 0.002), N stage (P = 0.004) and TNM stage (P = 0.004) were significantly associated with the 5-year overall survival rate (Table 2). Unexpectedly, expression of NGF and TrkA alone had no significant influence on survival rate (P = 0.201 and 0.483 respectively) (Figure 2A and B). However, considering that NGF and TrkA may affect cell survival as a network, we further divided IHCC into group of NGF/TrkA higher-expression (both NGF and TrkA have higher score than the cut-off) and group of others (including both lower expression, only NGF higher and only TrkA higher), subsequently investigated the correlation between these two groups. When expressions of NGF and TrkA were both higher, they had a significant relation with survival rate (P = 0.030), indicating that the NGF-TrkA pathway may be involved in the CCA progression in an autocrine loop way, as previously reported in breast cancer[14].
| Clinicopathologic parameters | Median | Survival | P value1 | |
| Age | < 65 yr | 46.2 | 39.5% | 0.776 |
| ≥ 65 yr | 59.9 | 60.3% | ||
| gender | male | 46.7 | 42.6% | 0.364 |
| female | 51.5 | 43.5% | ||
| Tumor size | < 5 cm | 57.4 | 50.2% | 0.029 |
| ≥ 5 cm | 40.6 | 36.1% | ||
| Differentiation | Well | 54.5 | 49.9% | 0.587 |
| Moderately | 47.8 | 40.4% | ||
| Poorly | 43.6 | 40.7% | ||
| T stage | T1 | 52.7 | 49.0% | 0.411 |
| T2 | 58.9 | 60.0% | ||
| T3 | 33.0 | 23.4% | ||
| T4 | 0.0 | 0.0% | ||
| N stage | N0 | 58.7 | 53.3% | 0.002 |
| N1 | 17.6 | 14.0% | ||
| M stage | M0 | 51.8 | 45.5% | 0.004 |
| M1 | 7.8 | 0.0% | ||
| TNM stage | I | 62.4 | 60.4% | 0.004 |
| II | 73.0 | 77.8% | ||
| III | 46.6 | 36.8% | ||
| IV | 17.2 | 13.6% | ||
| Satellites | Negative | 50.0 | 47.1% | 0.132 |
| Positive | 31.3 | 31.4% | ||
| Macrovascular invasion | Negative | 49.7 | 42.9% | 0.290 |
| Positive | 24.3 | 33.3% | ||
| Microvascular invasion | Negative | 49.5 | 43.9% | 0.445 |
| Positive | 44.7 | 41.8% | ||
| Intraneural invasion | Negative | 47.2 | 41.5% | 0.678 |
| Positive | 55.7 | 52.5% | ||
| NGF | Negative | 54.2 | 49.4% | 0.201 |
| Positive | 36.4 | 29.6% | ||
| TrkA | Negative | 48.9 | 42.7% | 0.483 |
| Positive | 44.6 | 41.6% | ||
| NGF + TrkA | Negative | 53.1 | 46.5% | 0.030 |
| Positive | 18.0 | 24.6% | ||
All the suspicious prognostic factors were enrolled in the Cox regression model to identify the independent prognostic factors, including tumor size, differentiation, T, N, M stage, satellites, macrovascular invasion, microvascualr invasion, intraneural invasion and NGF-TrkA double higher expression (Table 3). In IHCC, NGF and TrkA double higher expression was a significant prognostic factor (P = 0.010), meanwhile N stage was also defined as a prognostic parameter (P = 0.015). Other clinicopathologic parameters were not proved to be significantly related with prognosis in our experiments.
| Factor | Category | HR | 95%CI | P value1 |
| Tumor size | < 5 cm | 1.00 | ||
| ≥ 5 cm | 2.00 | 0.8-3.9 | 0.157 | |
| Differentiation | Well | 1.00 | ||
| Moderately | 0.74 | 0.3-2.1 | 0.578 | |
| Poorly | 1.65 | 0.6-4.3 | 0.335 | |
| T stage | T1 + T2 | 1.00 | 0.550 | |
| T3 + T4 | 1.25 | 0.6-2.6 | ||
| N stage | N0 | 1.00 | 0.015 | |
| N1 | 3.15 | 1.3-7.9 | ||
| M stage | M0 | 1.00 | 0.200 | |
| M1 | 2.18 | 0.7-7.2 | ||
| Satellites | Negative | 1.00 | 0.533 | |
| Positive | 0.76 | 0.3-1.8 | ||
| Macrovascular invasion | Negative | 1.00 | 0.245 | |
| Positive | 2.61 | 0.5-13.2 | ||
| Microvascualr invasion | Negative | 1.00 | 0.092 | |
| Positive | 2.15 | 0.9-5.2 | ||
| Intraneural invasion | Negative | 1.00 | 0.927 | |
| Positive | 0.95 | 0.3-2.9 | ||
| NGF + TrkA | Negative | 1.00 | 0.010 | |
| Positive | 2.87 | 1.3-6.4 |
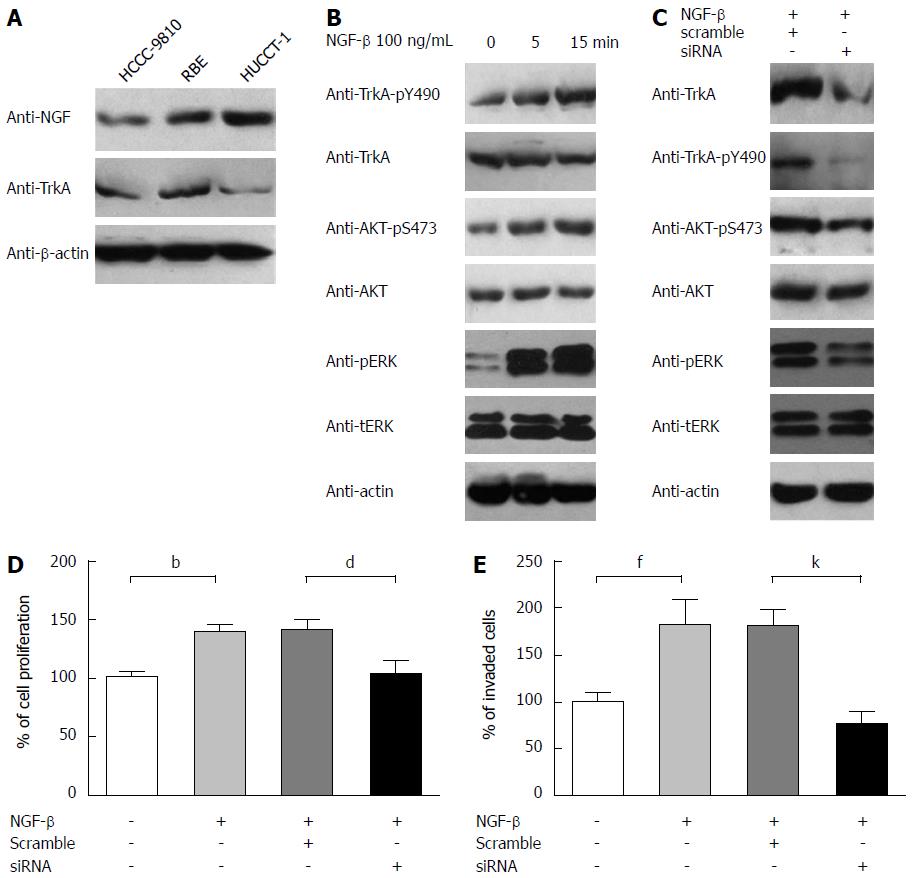
It was previously indicated that overexpression of NGF-β may play an important role in progression of the perihilar CCA cell line QBC939[21], but TrkA role in the progression was not involved. To further explore the insight into the molecular mechanism and confirm the phenomenon found in our clinical study, we investigated NGF-TrkA signaling pathway in CCA cell lines with experiments in vitro. First of all, the levels of NGF and TrkA expressions were detected in three cell lines: HUCCT-1, RBE and HCCC9810 by immunoblotting (Figure 3A). Both NGF and TrkA can be detected in these three cell lines although the abundance was different. Interestingly, HUCCT-1 had the most NGF expression but the least TrkA expression. To activate TrkA receptor and detect downstream targeted molecules; we used 100 ng/mL recombinant NGF-β for stimulation. After starved in serum-free medium for 12 h and then stimulated for 5 or 15 min, phosphorylation levels of TrkA, AKT and ERK were detected by immunoblotting. Consistent with previous study, NGF can significantly activate the PI3K-AKT pathway and Ras-MAPK pathway in a time-related pattern (Figure 3B). The phosphorylated level of TrkA-pY490, AKT-pS473 and ERK-pT202/pY204 markedly increased with 5 min NGF stimulation, and 15 min stimulation had a more significant change, indicating that NGF can trigger the PI3K-AKT and Ras-MAPK pathway by inducing TrkA phosphorylation. Furthermore, we knocked down TrkA by siRNA and evaluated the influence on cellular signaling and tumor progression. As expected, TrkA expression was knocked down successfully (Figure 3C), and the down-regulation of TrkA resulted in reduction of AKT-pS473 and ERK-pT202/pY204 phosphorylation, which suggested the crucial role of TrkA in NGF-induced MAPK and PI3K/AKT activation. Function assays including proliferation and invasion were carried out to identify NGF and TrkA role in CCA progression. RBE cells were starved in serum-free medium and then incubated with or without 100 ng/mL NGF-β for 48 h. The proliferation rate of control group without NGF stimulation was set as baseline and proliferation rate of other groups was evaluated by comparing with the ratio to baseline (Figure 3D). It was obvious that NGF could accelerate IHCC cells proliferation while TrkA knockdown significantly decreased this tendency, which demonstrating NGF/TrkA signaling pathway was required in IHCC proliferation.
Based on the founding that TrkA expression was associated with intraneural filtration, we speculated that TrkA was essential on invasion, so we performed transwell assay to evaluate the invasive ability of CCA cells. After split into matrigel-precoated well, RBE cells were then starved for 6 h and then activated by 100 ng/mL NGF-β, cell numbers were counted randomly in 8 visual fields and the number of control group without NGF-β was set as baseline. Similarly with proliferation, we can see that NGF-β markedly increased the invasive ability of RBE and TrkA knockdown reversed this increase significantly, which indicated that TrkA played crucial role in NGF-induced CCA invasion (Figure 3E).
The study on oncogenic role of NGF and its receptor was mostly focused in neural tumor such as neuroblastoma and sporadically reported in oral cancer, pancreatic cancer, colon cancer and breast cancer[25-28]. NGF was identified as a prognostic biomarker and mostly reported to be related with intraneural invasion, which is consistent with our study. Moreover, it is a breakthrough of CCA that NGF-β was identified to be associated with lymph and nerve invasion in perihilar cholangiocarcinoma, though the cases number was small and no prognostic data available[22]. However, more underlying molecular mechanism of why NGF related to poor prognosis of cancer need further investigation. NGF functioned mostly by interacting with its two receptors: p75NTR and Trk receptor. TrkA is distinguishing in the Trk receptors because it functions by autophosphorylating and activating of various signaling cascades. Proteins interacting directly with the TrkA include SHC, PLC 1, SH2B and IAPs, which can activate downstream signaling pathway, including RAS-MEKK-MAPK, and PI3K-PDK-AKT pathway. Activation of both MAPK and PI3K-AKT pathway can promote survival by affecting apoptosis-related protein BAD and BCL-2[29]. Moreover, NGF can promote intraneural invasion through activating STAT3 signaling[30]. Besides proliferation and invasion, NGF signaling pathway was proved to promote other oncogenic process like angiogenesis[31], which also need further investigation in CCA. In our study, we first found that NGF/TrkA signaling pathway can relate to poor prognosis in CCA and demonstrated that NGF/TrkA signaling pathway can promote CCA proliferation and invasion, which can provide new insight to CCA biomarker and progression, and help find a new molecular drug target for CCA.
Based on our clinical and experimental finding, we highly speculated that NGF and TrkA affected the progression of IHCC in an autocrine or paracrine way. The NGF which stimulated TrkA receptor of CCA cells could be secreted by neurocytes or by CCA cells themselves. Up-regulation of NGF in matrix could enhance invasive activity of CCA cells and promote the intraneural filtration, which can explain that TrkA expression was associated with intraneural invasion. NGF and TrkA network as paracrine loop could promote IHCC cells progression and finally lead to poor prognosis. In addition, more molecular insights into CCA tumorigenesis could be performed surrounding NGF and its receptors, and more experiments including animal model should be performed in the future.
The molecular significance of this signaling pathway was gradually revealed, which provides us a new inspiration for finding new chemical therapy. There are several potent small-molecular inhibitors of NGF and TrkA now, such as GW441756 or GNF-5837. More interestingly, the anti-NGF antibody tanezumab, which acts by sequestering NGF and preventing its binding to either of TrkA and p75, is in clinical trial for osteoarthritis and inflammation pain now. We hope our finding can increase the interest of NGF/TrkA signaling inhibitor as a potential chemical drug for CCA treatment.
In summary, for the first time, we demonstrated that NGF-TrkA double higher expression was associated with poor prognosis in IHCC and NGF-TrkA signaling pathway could promote IHCC cell line progression. This might provide a new insight into the molecular mechanism of IHCC progression. We hope our study could trigger the interest of NGF or TrkA as a potential drug target and help find new therapy of IHCC.
Nerve growth factor (NGF) and its receptor tropomyosin-receptor-kinase (TrkA) have been identified to be correlated to tumorgenesis and prognosis in several kinds of cancers. However,the importance of NGF-TrkA signaling pathway in intrahepatic cholangiocarcinoma (IHCC) is poorly elucidated.
Recently, there is several high-level article focused on molecular classification of IHCC.
The authors found that NGF and TrkA double higher expression was an independent prognostic factor in IHCC; NGF/TrkA signaling pathway can activate MAPK and PI3K pathway in IHCC cell lines; NGF/TrkA signaling pathway played crucial role in IHCC proliferation and invasion.
Considering NGF/TrkA could promote IHCC progression, it may help develop new potential drugs for chemical therapy of IHCC.
This article is original and while the nerve growth factor has been studied in relation to other types of cancers, cholangiocarcinoma data is sparse.
| 1. | Gatto M, Bragazzi MC, Semeraro R, Napoli C, Gentile R, Torrice A, Gaudio E, Alvaro D. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 2. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 855] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 3. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 908] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 4. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 687] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 5. | Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Gansler T, Ganz PA, Grant M, Greene FL, Johnstone P, Mahoney M, Newman LA, Oh WK, Thomas CR, Thun MJ. Sixty years of CA: a cancer journal for clinicians. CA Cancer J Clin. 2010;60:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Morise Z, Sugioka A, Tokoro T, Tanahashi Y, Okabe Y, Kagawa T, Takeura C. Surgery and chemotherapy for intrahepatic cholangiocarcinoma. World J Hepatol. 2010;2:58-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 449] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 9. | Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, Zhao X, Andersen JB, Ye QH, Jia HL. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56:1792-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Geldof AA, De Kleijn MA, Rao BR, Newling DW. Nerve growth factor stimulates in vitro invasive capacity of DU145 human prostatic cancer cells. J Cancer Res Clin Oncol. 1997;123:107-112. [PubMed] |
| 11. | Zhu ZW, Friess H, Wang L, Bogardus T, Korc M, Kleeff J, Büchler MW. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer Res. 2001;7:105-112. [PubMed] |
| 12. | Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203-233. [PubMed] |
| 13. | Schecterson LC, Bothwell M. Neurotrophin receptors: Old friends with new partners. Dev Neurobiol. 2010;70:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Dollé L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene. 2003;22:5592-5601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Wang T, Yu D, Lamb ML. Trk kinase inhibitors as new treatments for cancer and pain. Expert Opin Ther Pat. 2009;19:305-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Brodeur GM, Minturn JE, Ho R, Simpson AM, Iyer R, Varela CR, Light JE, Kolla V, Evans AE. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244-3250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Marano N, Dietzschold B, Earley JJ, Schatteman G, Thompson S, Grob P, Ross AH, Bothwell M, Atkinson BF, Koprowski H. Purification and amino terminal sequencing of human melanoma nerve growth factor receptor. J Neurochem. 1987;48:225-232. [PubMed] |
| 18. | Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864-17870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Lagadec C, Meignan S, Adriaenssens E, Foveau B, Vanhecke E, Romon R, Toillon RA, Oxombre B, Hondermarck H, Le Bourhis X. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 2009;28:1960-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Niault TS, Baccarini M. Targets of Raf in tumorigenesis. Carcinogenesis. 2010;31:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Yue XJ, Xu LB, Zhu MS, Zhang R, Liu C. Over-expression of nerve growth factor-β in human cholangiocarcinoma QBC939 cells promote tumor progression. PLoS One. 2013;8:e62024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Xu LB, Liu C, Gao GQ, Yu XH, Zhang R, Wang J. Nerve growth factor-beta expression is associated with lymph node metastasis and nerve infiltration in human hilar cholangiocarcinoma. World J Surg. 2010;34:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser SS, Francis H, Mancino MG, Ueno Y, Barbaro B. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198-1209. [PubMed] |
| 24. | Davidson B, Reich R, Lazarovici P, Nesland JM, Skrede M, Risberg B, Tropé CG, Flørenes VA. Expression and activation of the nerve growth factor receptor TrkA in serous ovarian carcinoma. Clin Cancer Res. 2003;9:2248-2259. [PubMed] |
| 25. | Zhu Y, Li Y, Haraguchi S, Yu M, Ohira M, Ozaki T, Nakagawa A, Ushijima T, Isogai E, Koseki H. Dependence receptor UNC5D mediates nerve growth factor depletion-induced neuroblastoma regression. J Clin Invest. 2013;123:2935-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Noh SJ, Bae JS, Jamiyandorj U, Park HS, Kwon KS, Jung SH, Youn HJ, Lee H, Park BH, Chung MJ. Expression of nerve growth factor and heme oxygenase-1 predict poor survival of breast carcinoma patients. BMC Cancer. 2013;13:516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Liebl F, Demir IE, Rosenberg R, Boldis A, Yildiz E, Kujundzic K, Kehl T, Dischl D, Schuster T, Maak M. The severity of neural invasion is associated with shortened survival in colon cancer. Clin Cancer Res. 2013;19:50-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Yu EH, Lui MT, Tu HF, Wu CH, Lo WL, Yang CC, Chang KW, Kao SY. Oral carcinoma with perineural invasion has higher nerve growth factor expression and worse prognosis. Oral Dis. 2014;20:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1600] [Cited by in RCA: 1649] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 30. | Guo K, Ma Q, Li J, Wang Z, Shan T, Li W, Xu Q, Xie K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol Cancer Ther. 2013;12:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Romon R, Adriaenssens E, Lagadec C, Germain E, Hondermarck H, Le Bourhis X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol Cancer. 2010;9:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
P- Reviewers: Kaiser GM, Wu ZL, Vasilieva LE S- Editor: Ma YJ L- Editor: A E- Editor: Ma S