Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4037
Revised: October 10, 2013
Accepted: November 1, 2013
Published online: April 14, 2014
Processing time: 239 Days and 11.3 Hours
AIM: To investigate whether seasonal changes had an effect on the incidence of acute appendicitis (AA) or nonspecific abdominal pain (NSAP).
METHODS: We carried out a national register study of all patients with a hospital discharge diagnosis of AA and acute NSAP in Finland. Data were analyzed for the whole country and correlated to seasonal and weather parameters (temperature, humidity). Moreover, additional sub-analyses were performed for five geographically different area of Finland.
RESULTS: The observation period spanned 21 years, with 186558 appendectomies, of which 137528 (74%) cases were reported as AA. The incidence of AA declined for 32% over the study period. The average incidence of the NSAP was 34/10000 per year. The mean annual temperature, but not relative humidity, showed clear geographical variations. The incidence of AA decreased significantly during the cold months of the year. No correlation was detected between temperature and incidence of NSAP. Humidity had a statistically significant impact on NSAP.
CONCLUSION: The incidence of acute appendicitis is declining in Finland. We detected a clear seasonality in the incidence of AA and NSAP.
Core tip: The incidence of acute appendicitis (AA) varies between countries and the etiology of the disease is still unclear. The aim of this study was to investigate whether seasonal changes had an effect on the incidence of AA or nonspecific abdominal pain (NSAP) in Finland between 1987 and 2007. We found that the incidence of AA, but not NSAP, was higher during the warm period of the year. In comparison to AA, NSAP was influenced by humidity and the incidence was lower during a period with lower levels of humidity.
- Citation: Ilves I, Fagerström A, Herzig KH, Juvonen P, Miettinen P, Paajanen H. Seasonal variations of acute appendicitis and nonspecific abdominal pain in Finland. World J Gastroenterol 2014; 20(14): 4037-4042
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4037.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4037
Acute appendicitis (AA) and nonspecific abdominal pain (NSAP) remain the two most common surgical emergencies in acute care units[1-3]. NSAP has been evaluated in the literature as a self-limited diagnosis of acute abdominal pain for which no serious or definite cause is established and thus is no useful surgical treatment available[3]. Although AA has been recognized as a clinical entity for over 100 years[4], the differential diagnosis between AA and NSAP can still be challenging. Moreover, the etiology of the appendicitis still remains unclear and multifactorial. The disease has been attributed to a variety of possible causes, including mechanical obstruction[5], inadequate dietary fiber[6], smoking[7], air pollution[8] and familial susceptibility[9,10]. Population-based epidemiological studies about the impact of seasonal variables on appendicitis have been published, but so far there is no clear consensus about the connection between the seasonal variations and AA or NSAP[11,12].
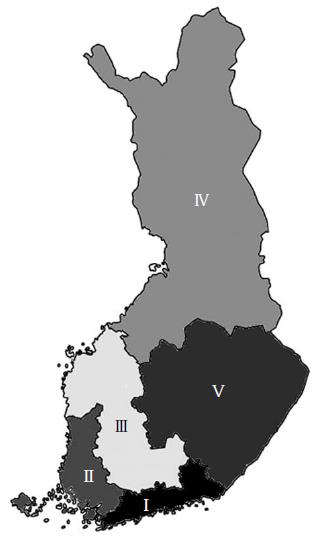
The main factor influencing Finland’s climate is the country’s geographical position between the 60th and 70th northern parallels on the Eurasian continent, which shows characteristics of both maritime and continental climate. The mean temperature in Finland is several degrees (as much as 10 °C in winter) higher than that of other areas at these latitudes, e.g., Siberia and south Greenland. The mean daily temperature can vary in Finland from below -30 °C during winter months (November-March) to more than +25 °C in the summer months (June-August). Moreover, because of the geographical shape and location of Finland there are large disparities in local temperatures inside the country. Finland has an excellent health register and is divided into five, geographically different University Hospital Districts (UHD) serving as a good base for comparing incidences and investigating various seasonal impacts on diseases. We have previously reported a declining incidence trend of AA, but not of NSAP[13]. The aim this study was to determine whether those trends could be explained by possible seasonal changes in temperature and humidity.
A population-based register study was performed to assess the incidence of AA and NSAP in Finland between the years 1987 and 2007. Diagnoses were classified according to the World Health Organization International Classification of Diseases, version 9 and 10 (ICD-9 and ICD-10). The study population was recruited from the National Institute for Health and Welfare (NIHW) registry to retrieve the data on discharge diagnoses as well as surgical procedures of the whole country. Population data from 1987 to 2007 were retrieved from the Official Statistics of Finland. More detailed data about the material and methods can be found in our previously published study[13].
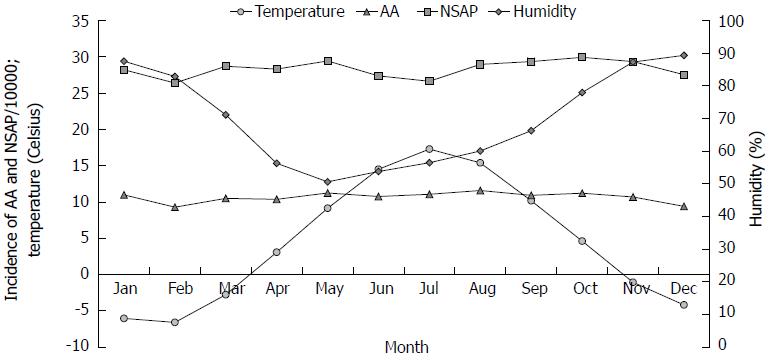
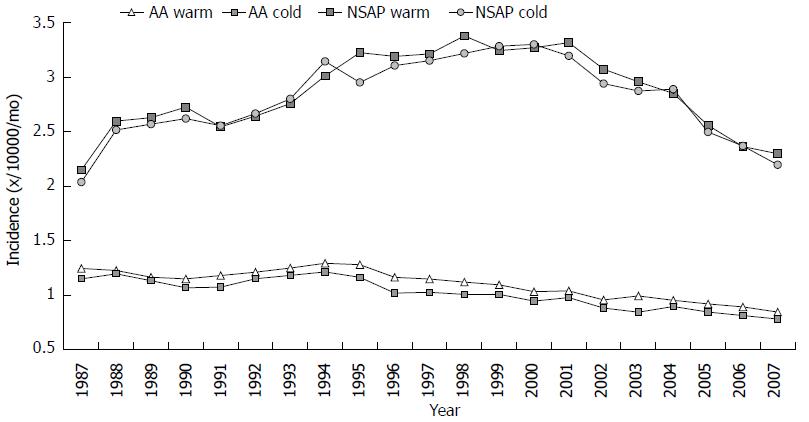
Weather data for the years 1987 to 2007 were retrieved from the Finnish Meteorological Institute[14] for each of the five UHD. Temperature and humidity levels were obtained from the main measurement points in each of the UHD, and were expressed as mean values for each month. Moreover, the temperature of the whole country was calculated as mean temperature value of each of the UHD. According to the measurement results, the period from March to September was defined as “warm period”, and from October to February as “cold period”.
Poisson regression analysis was used to assess association between temperature, humidity and incidences (AA and NSAP) for monthly data. In the Poisson regression model, incidences were as dependent variable per 10000 inhabitants. Temperature and humidity were explanatory variables. For the statistical analysis we used SPSS for Windows, release 18.0 (SPSS, Inc., Chicago, IL, United States).
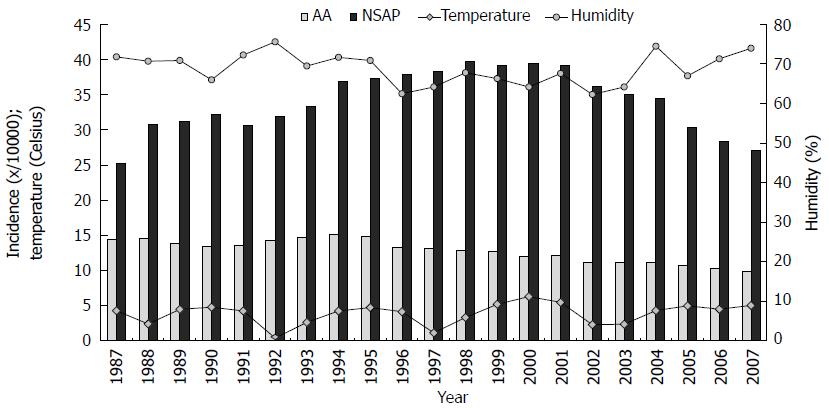
During the study period, the mean annual temperature and relative humidity varied from 0.4 °C-6.3 °C (mean 3.9 °C) and 62%-76% (mean 69%), respectively (Figure 1). We observed cyclical temperature changes over a period of 4-5 years, but not in humidity. Lower levels of humidity were registered between 1996 and 2003. The mean temperature over the 21-year study period was below zero degrees from November to March (a cold period) (-4.1; SD 1.44 °C), and above zero degrees in the warm period from April to October (10.6; SD 0.72 °C) (Figure 2). Furthermore, a relative humidity showed higher values during the cold months. There was a large geographical disparity between temperatures of the different UHDs. The biggest difference between the mean temperatures was observed between the UHD of Oulu (Figure 3, area IV) 2.4 °C (min, -0.8 °C; max, 4.5 °C) and the UHD of Tampere (Figure 3, area III) 5.0 °C (min, 1.6 °C; max, 7.55 °C), P = 0.006. During the study period, the annual temperature varied from +1.6 to +7.55 (median 1.7 °C) and -0.75 to +4.5 (median 3.5 °C) in the UHD of Tampere and Oulu, respectively, with no statistically significant difference in the incidence of AA between the hospital districts.
The incidence of AA declined for 32%, from 14.5 to 9.8 per 10000 inhabitants. The average incidence of NSAP was 34.0 per 10000 inhabitants. The incidence rose between the years 1987 and 1998 (from 25.2 to 39.8/10000 per year), after which it fell to 27.1/10000 per year. There was a clear difference in the incidence of AA between cold and warm months (Figures 2 and 4). The AA incidence was higher during the warm months throughout the study period. An increase of 10-Celsius degrees in temperature increased the incidence of AA by 4%, 1.04 (95%CI: 1.019-1.061). The incidence of NSAP did not change during the year, and the association between temperature and NSAP incidence was not statistically significant (Figure 4). Furthermore, humidity levels had no effect on incidence of AA, but had an effect on the incidence of NSAP. An increase of 10% in humidity decreased NSAP incidence by 0.8%, 0.992 (95%CI: 0.984-0.999).
In our study we found a significant association between temperature and the incidence of AA, but not for NSAP. Previously we showed that both the incidence of appendicitis and the rate of appendectomies decreased in Finland between 1987 and 2007[13]. In addition to an annual decline, there was also seasonal fluctuation in the incidence of AA. During the 21-year study period, the diagnostic accuracy for appendicitis had steadily improved. The incidence of NSAP also decreased during the last study decade.
The seasonality of several infectious and respiratory diseases is well known[15,16]. Cardiac and psychological diseases also have a seasonal behavior[17]. Furthermore, the association between AA and viral diseases has been investigated[18]. Saps et al[19] described the effect of seasonality on NSAP in children. The predisposing factors of appendicitis are thought to be multifactorial. The influence of seasonal variables on the incidence of AA has been discussed in many studies[11,12,20-22]. The highest incidence of AA was found in summer and the lowest levels in winter. Moreover, the same trends in AA incidence have been shown in Nigeria[23], where the annual temperature is higher than in Finland or the United States. Unfortunately, those papers did not publish exact temperature data[11,12,20-23]. The reasons for increased incidence of AA during the warm period are not clear, although various speculations have been proposed. Examples include the effect of dehydration, lower bowel movements, infections or allergens on the reactivity of the lymphoid tissue in the appendix, and the effects of diet, humidity or changes in atmospheric pressure. Furthermore, changes in the temperature or other factors mentioned have been shown in the geographical variation of AA in the United States[20,22].
Our current study looked at a 21-year time period to investigate the fluctuation of temperature and humidity in warm and cold months, and their effects on the incidence of AA and NSAP (Figures 1, 2 and 4). Temperature had a statistically significant impaction on the incidence of AA. Although, several studies have had the same results[11,12,24,25], some additional considerations may be important. In the summertime the effect of a higher temperature on the human body is more straightforward than in winter. In Finland, winter temperatures are below zero degrees, however, people usually dress warmly. Furthermore, they spend more time inside. Therefore, the true effect of a low temperature on the human body core temperature is minor. Moreover, in a study from Nigeria, the incidence of the AA was reported to be higher during the cooler and rainy season than during the warmer season. However, the temperature in Nigeria has high levels all year round. It is possible that humidity plays a larger role in the incidence of AA in Nigeria, yet the magnitude of humidity during the rainy season in Nigeria is far greater than that in Finland[23]. Additional infections cause might further contribute to the differences between the two countries.
Another consideration is that patients with milder forms of appendicitis might not seek medical attention during the winter period[26]. On the contray, in the summer period young doctors may tend to operate on patients with lower abdominal pain with less strong indications.
Humidity had a statistically significant impact on the incidence of NSAP. The mechanism, however, continues to be unclear. There are no studies to date about seasonal variations of NSAP. It is possible that different extrinsic factors such as allergens, viral and bacterial infections might play a role during humidity changes and the etiology of NSAP. Clearly this issue needs further study.
The major strength of our study was the use of a population-based register. Moreover, the Finnish Meteorological Institute provided high-quality observational data. As a population-based retrospective study, however, there are also weaknesses. We used a discharge diagnoses in our analyses without histological confirmation. A 6% overestimation of appendicitis by surgeons has been reported[27]. In contrast, according to Kraemer et al[28], there was no evidence to support the assumption that the macroscopic diagnosis of appendicitis is unreliable. Furthermore, the small difference between the mean temperatures in the warm and cold period and the low mean annual temperature could be considered as a possible bias when generalizing our conclusions. However, in the reports from warmer countries (United States[22], Canada[11], Iran[12], and South Africa[16]), a similar incidence peak of AA during the warmer period was observed. Although those studies did not report exact temperatures and respective differences between the warm and cold periods, it seems reasonable to assume that higher temperatures are associated with a higher risk of developing AA. Whether temperature plays a minor or major role in the development of AA when compared to other risk factors, or whether it is an independent risk factor at all, remains to be solved in further studies.
In sum, our findings showed that AA occurs more commonly in the warmer months of the year, indicating that the temperature may have an effect in the pathogenesis of appendicitis. No connection was seen between the temperature and NSAP.
In summary, the incidence of appendicitis but not NSAP was significantly higher during the warm time of the year. This difference remained unchanged during the whole 21-year study period. The humidity had effect on the incidence of NSAP but not on AA.
Acute appendicitis and nonspecific abdominal pain are two most common surgical emergencies. The etiology of both diseases still remains unclear. Many studies have reported that seasonal and weather parameters might have an effect on those diseases. Clear consensus, however, is still missing.
There are several publications about the seasonality of acute appendicitis, but no agreement exists about the impact of seasonal variables on appendicitis. To the best of our knowledge there are no studies about the seasonality of non-specific abdominal pain.
Appendicitis is an inflammation of the blind-ended part of the bowel connected to the cecum. Abdominal pain is a common symptom associated with transient disorders or serious disease. Diagnosing the cause of abdominal pain can be difficult because of the multifactorial nature.
That is a good study in which the authors tried to find an answer whether temperature or humidity have an effect on acute appendicitis and nonspecific abdominal pain. Strength of the study was long examination period with large number of patients.
| 1. | Sheridan WG, White AT, Havard T, Crosby DL. Non-specific abdominal pain: the resource implications. Ann R Coll Surg Engl. 1992;74:181-185. [PubMed] |
| 2. | Miettinen P, Pasanen P, Lahtinen J, Alhava E. Acute abdominal pain in adults. Ann Chir Gynaecol. 1996;85:5-9. [PubMed] |
| 3. | Laurell H, Hansson LE, Gunnarsson U. Diagnostic pitfalls and accuracy of diagnosis in acute abdominal pain. Scand J Gastroenterol. 2006;41:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Fitz RH. Perforating inflammation of the vermiform appendix with special reference to its early diagnosis and treatment. Am J Med Sci. 1886;92:321-346. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Jones BA, Demetriades D, Segal I, Burkitt DP. The prevalence of appendiceal fecaliths in patients with and without appendicitis. A comparative study from Canada and South Africa. Ann Surg. 1985;202:80-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Adamidis D, Roma-Giannikou E, Karamolegou K, Tselalidou E, Constantopoulos A. Fiber intake and childhood appendicitis. Int J Food Sci Nutr. 2000;51:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Oldmeadow C, Wood I, Mengersen K, Visscher PM, Martin NG, Duffy DL. Investigation of the relationship between smoking and appendicitis in Australian twins. Ann Epidemiol. 2008;18:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Kaplan GG, Dixon E, Panaccione R, Fong A, Chen L, Szyszkowicz M, Wheeler A, MacLean A, Buie WD, Leung T. Effect of ambient air pollution on the incidence of appendicitis. CMAJ. 2009;181:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Ergul E. Heredity and familial tendency of acute appendicitis. Scand J Surg. 2007;96:290-292. [PubMed] |
| 10. | Sadr Azodi O, Andrén-Sandberg A, Larsson H. Genetic and environmental influences on the risk of acute appendicitis in twins. Br J Surg. 2009;96:1336-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Al-Omran M, Mamdani M, McLeod RS. Epidemiologic features of acute appendicitis in Ontario, Canada. Can J Surg. 2003;46:263-268. [PubMed] |
| 12. | Noudeh YJ, Sadigh N, Ahmadnia AY. Epidemiologic features, seasonal variations and false positive rate of acute appendicitis in Shahr-e-Rey, Tehran. Int J Surg. 2007;5:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ilves I, Paajanen HE, Herzig KH, Fagerström A, Miettinen PJ. Changing incidence of acute appendicitis and nonspecific abdominal pain between 1987 and 2007 in Finland. World J Surg. 2011;35:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Available from: http://en.ilmatieteenlaitos.fi. |
| 15. | Koopmans M, Brown D. Seasonality and diversity of Group A rotaviruses in Europe. Acta Paediatr Suppl. 1999;88:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011;11:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Alexander P. Association of monthly frequencies of diverse diseases in the calls to the public emergency service of the city of Buenos Aires during 1999-2004 with meteorological variables and seasons. Int J Biometeorol. 2013;57:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Alder AC, Fomby TB, Woodward WA, Haley RW, Sarosi G, Livingston EH. Association of viral infection and appendicitis. Arch Surg. 2010;145:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Saps M, Blank C, Khan S, Seshadri R, Marshall B, Bass L, Di Lorenzo C. Seasonal variation in the presentation of abdominal pain. J Pediatr Gastroenterol Nutr. 2008;46:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910-925. [PubMed] |
| 21. | Luckmann R, Davis P. The epidemiology of acute appendicitis in California: racial, gender, and seasonal variation. Epidemiology. 1991;2:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Anderson JE, Bickler SW, Chang DC, Talamini MA. Examining a common disease with unknown etiology: trends in epidemiology and surgical management of appendicitis in California, 1995-2009. World J Surg. 2012;36:2787-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Oguntola AS, Adeoti ML, Oyemolade TA. Appendicitis: Trends in incidence, age, sex, and seasonal variations in South-Western Nigeria. Ann Afr Med. 2010;9:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Wei PL, Chen CS, Keller JJ, Lin HC. Monthly variation in acute appendicitis incidence: a 10-year nationwide population-based study. J Surg Res. 2012;178:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Stein GY, Rath-Wolfson L, Zeidman A, Atar E, Marcus O, Joubran S, Ram E. Sex differences in the epidemiology, seasonal variation, and trends in the management of patients with acute appendicitis. Langenbecks Arch Surg. 2012;397:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Vons C, Barry C, Maitre S, Pautrat K, Leconte M, Costaglioli B, Karoui M, Alves A, Dousset B, Valleur P. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 460] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 27. | Andersson R, Hugander A, Thulin A, Nyström PO, Olaison G. Indications for operation in suspected appendicitis and incidence of perforation. BMJ. 1994;308:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 165] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Kraemer M, Ohmann C, Leppert R, Yang Q. Macroscopic assessment of the appendix at diagnostic laparoscopy is reliable. Surg Endosc. 2000;14:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
P- Reviewers: Augustin G, Martellucci J, Mentes O S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN