Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4017
Revised: December 20, 2013
Accepted: January 8, 2014
Published online: April 14, 2014
Processing time: 188 Days and 16.7 Hours
AIM: To identify objective and subjective predictors for the reliable diagnosis of gastroesophageal reflux disease (GERD) and the response to proton pump inhibitor (PPI) therapy.
METHODS: Retrospectively, 683 consecutive patients suspected for GERD who underwent pH-metry/impedance measurement (pH/MII) were analyzed. All patients had previously undergone standard PPI treatment (e.g., pantoprazole 40 mg/d or comparable). Four hundred sixty patients were at least 10 d off PPIs (group A), whereas 223 patients were analyzed during their ongoing PPI therapy (group B). In addition, all patients completed a standardized symptom- and lifestyle-based questionnaire, including the therapeutic response to previous PPI trials on a 10-point scale. Uni- and multivariance analyses were performed to identify criteria associated with positive therapeutic response to PPIs.
RESULTS: In group A, positive predictors (PPs) for response in empirical PPI trials were typical GERD symptoms (heartburn and regurgitation), a positive symptom index (SI) and pathological results in pH/MII, along with atypical symptoms, including hoarseness and fullness. In group B, regular alcohol consumption was associated with the therapeutic response. The PPs for pathological results in pH/MII in group A included positive SI, male gender, obesity, heartburn and regurgitation. In group B, the PPs were positive SI and vomiting. Analyzing for positive SI, the PPs were pathological pH and/or MII, heartburn regurgitation, fullness, nausea and vomiting in group A and pathological pH and/or MII in group B.
CONCLUSION: Anamnestic parameters (gender, obesity, alcohol) can predict PPI responses. In non-obese, female patients with non-typical reflux symptoms, pH/MII should be considered instead of empirical PPIs.
Core tip: The response rates to proton pump inhibitors in reflux disease vary. Empirical proton pump inhibitor therapy poses a substantial economic burden. Positive predictors of the therapeutic response are necessary. This study provides the highest number of reflux patients. Anamnestic, objective and subjective parameters predicting the therapeutic response were evaluated.
- Citation: Becker V, Grotz S, Schlag C, Nennstiel S, Beitz A, Haller B, Schmid RM, Meining A, Bajbouj M. Positive predictors for gastroesophageal reflux disease and the therapeutic response to proton-pump inhibitors. World J Gastroenterol 2014; 20(14): 4017-4024
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4017.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4017
Gastroesophageal reflux disease (GERD) is one of the most prevalent gastrointestinal disorders worldwide[1-4]. In western countries, approximately 40% of the adult population suffers occasionally from reflux symptoms; approximately 20% report symptoms at least once per week[5,6]. Symptoms of GERD are subdivided into typical/esophageal (heartburn, regurgitation) and atypical/extraesophageal symptoms (chronic cough, hoarseness, recurrent sinusitis, globus sensation in the throat, burning feeling on the tongue, dental erosion, fullness)[7]. Symptom overlap is common[8].
The most effective therapeutic approaches for GERD symptoms are proton pump inhibitor (PPI) trials[9,10]. Therapy response rates for PPIs vary but are more satisfactory in patients with erosive reflux disease (ERD) and typical reflux symptoms. However, the data are conflicting in patients with non-erosive reflux disease (NERD) and/or atypical/extraesophageal symptoms and functional disorders (FD)[11]. Nonetheless, the discrimination between NERD and FD is challenging. Distinguishing between patients who adequately respond to PPIs and those who remain symptomatic is a matter of debate. PH/MII is considered a useful tool for answering this question[12,13]. This technique enables the reliable detection and quantification of non-acidic, weakly acidic and acid reflux episodes in the esophagus with high sensitivity for all types of reflux episodes[14]. Combined esophageal pH/MII monitoring patterns can also discriminate between NERD and FD[15,16].
Separate from the classifications, the ultimate clinical goal in all patients is most likely symptom relief after PPI therapy. Knowing the reliable and specific anamnestic findings and/or parameters of pH/MII for predicting symptom relief might lead to more selective PPI therapies than empiric PPI tests. The benefit of PPI tests is controversially discussed[17,18]. These trials pose an extensive economic burden and contribute substantially to overall health-care expenditures[19]. However, with higher response rates to PPIs, unnecessary treatment may be avoided, which could result in tremendous savings in resources.
Therefore, the aim of our study was to identify objective and subjective parameters that might predict the therapeutic response to PPIs in patients with suspected GERD for guiding therapy, particularly in the primary-care setting.
This retrospective study included 683 consecutive patients who underwent pH/MII for suspected GERD between January 2007 and December 2011 at the Technische Universität München, Munich, Germany. The indication to perform pH/MII was suspected GERD with typical and/or atypical reflux symptoms. The inclusion criteria were a previous standard PPI trial [e.g., pantoprazole 40 mg/d (or comparable) within the last 6 mo], with positive or negative symptom relief, and endoscopy of the upper gastrointestinal tract within the last 12 mo to exclude malignancy. The exclusion criteria were a history of previous gastric or esophageal surgery or severe esophageal motility disorders. Informed consent for data evaluation was obtained from all patients. The study was approved by the Ethics Commission of the Technischre Universität München.
Before pH/MII, all patients were asked to complete a lifestyle- and symptom-based questionnaire to query their personal characteristics (weight, height, age, relevant disorders, smoking and drinking habits) and symptoms (heartburn, regurgitation, globus sensations, burning feeling on the tongue, chronic cough, hoarseness, fullness, nausea, vomiting and halitosis) on a 10-point scale. A subjective response to PPI therapy was defined as a symptom reduction of at least 3 points on the 10-point scale.
Combined pH/MII was performed using an ambulatory, multi-channel, intra-luminal impedance system consisting of a portable data logger and a combined pH-impedance catheter (Tecnomatix ZAN S 61 C 01 E, Sandhill Scientific, Highlands Ranch, CO, United States). Six impedance electrodes and a distal antimony-pH probe were placed at pre-defined spots on this catheter (3.0, 5.0, 7.0, 9.0, 15.0 and 17.0 cm; pH probe, 5.0 cm). The catheter was inserted with the antimony pH probe located 5 cm above the manometrically defined lower esophageal sphincter. Data recording was performed for 22-24 h. The stored data were then uploaded to a personal computer and individually analyzed using a commercially available software system (BioView, Sandhill Scientific). Gastroesophageal reflux detected from impedance changes was defined based on previous reports[20,21].
Reflux episodes were defined as either acidic or non-acidic, when a retrograde bolus movement was detected via impedance and the pH value was below or above 4, respectively. Furthermore, the content of the reflux episode was characterized according to its composition (gas, fluid or mixed). Following the suggested reference values published by Shay et al[22] and Zerbibb et al[14], the MII was considered pathological when more than 73 fluid and/or mixed reflux episodes occurred in the esophagus over the 22- to 24-h recording period. The esophageal pH measurement was considered pathological when the period during which the pH was below 4 was more than 4% overall. Meals were excluded from the analysis.
The patients were asked to indicate their predominant symptoms during the course of the measurements to assess the symptom index (SI). The SI was assessed as positive when at least half of each specific symptom’s duration was associated with reflux episodes over a 5-minute interval.
Alcohol consumption was defined as equal to or more than 15 g per day (on more than 3 d per week); cigarette consumption was defined as equal to or more than 10 cigarettes per day.
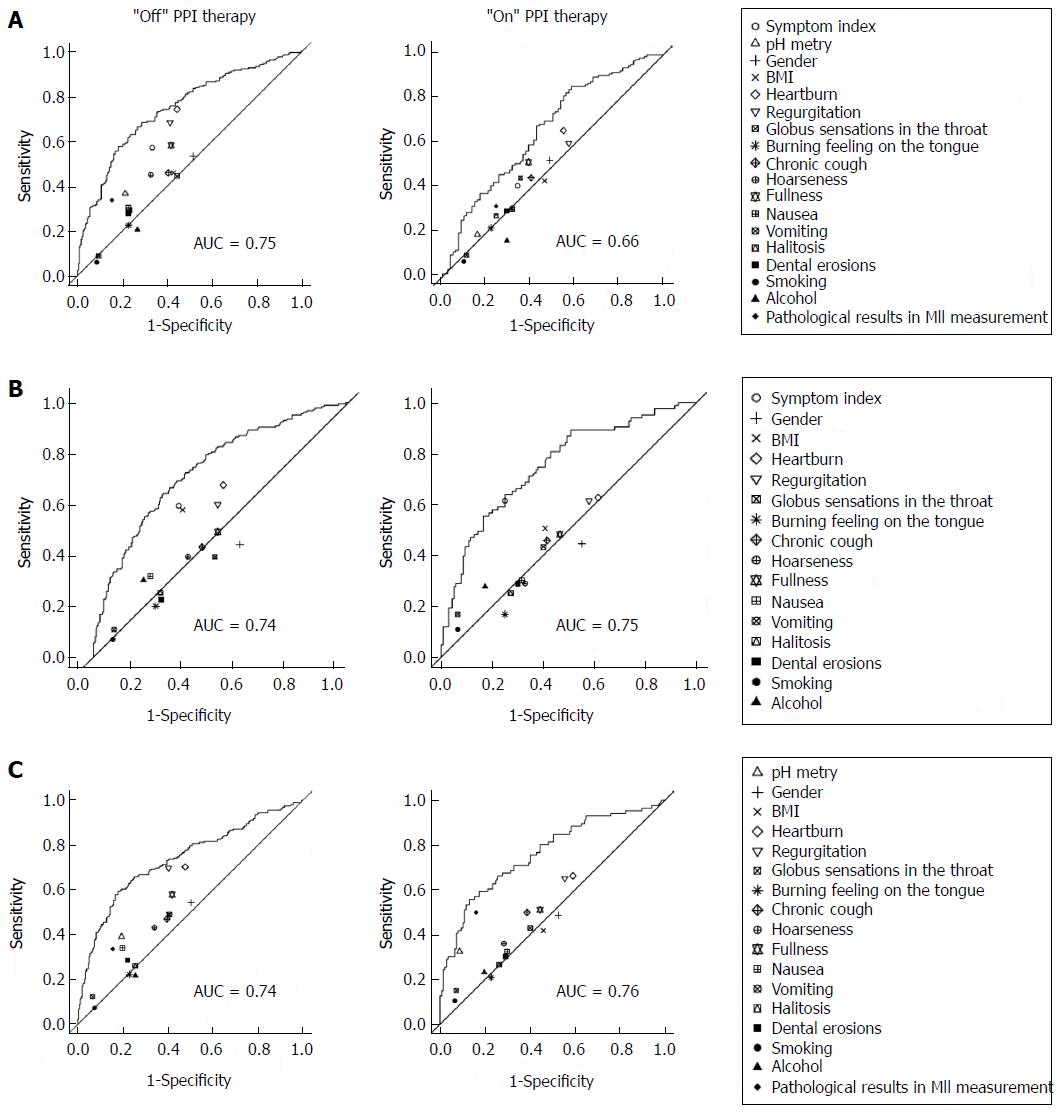
For the qualitative data, absolute and relative frequencies are presented; for the quantitative data, medians are shown. To determine the association between the relevant measures and the study endpoints, possible predictor variables were dichotomized, and the sensitivities, specificities, positive and negative predictive values and odds ratios were estimated. To test for associations, continuity-corrected chi-squared tests were performed. Multiple logistic regression models, including all relevant measures as the independent variables, were fit to the data. Goodness of fit was assessed by a receiver operating characteristics (ROC) analysis investigating the relationship between the predicted probabilities and the true value of the dependent variable of the logistic regression model. To illustrate the additional information obtained from the multiple regression model compared with the univariate results, sensitivities and specificities for all relevant measures were drawn in the ROC plot. All statistical tests were performed based on a two-sided level of significance (α = 5%). The statistical software programs SPSS version 20 and R version 2.15.1 were used for the analyses.
Six hundred eighty-three (329 male) patients who fulfilled the inclusion criteria were identified retrospectively by analysis of our pH/MII database [median age, 54.8 years; median body mass index (BMI), 24.7]. During the pH/MII, 460 patients were off (group A) and 223 patients were on (group B) PPI therapy.
First, the therapeutic response to standard PPI therapy was analyzed (Table 1). In group A, the positive predicting parameters for the therapeutic response were SI, pathological results from the pH-metry, heartburn, regurgitation, hoarseness, fullness and pathological results from the MII measurement. In group B, alcohol consumption was associated with the therapeutic response.
| Parameters | Sensitivity | Specificity | PPV | NPV | OR (univ) | Pval (univ) | OR (mult) | Pval (mult) |
| Group A | ||||||||
| SI | 0.57 | 0.67 | 0.58 | 0.66 | 2.66 | < 0.001 | 1.67 | 0.021 |
| pH-metry | 0.37 | 0.79 | 0.58 | 0.61 | 2.15 | < 0.001 | 1.63 | 0.055 |
| Gender | 0.53 | 0.49 | 0.45 | 0.56 | 1.07 | 0.772 | 0.97 | 0.9 |
| BMI | 0.46 | 0.57 | 0.46 | 0.57 | 1.13 | 0.567 | 0.85 | 0.465 |
| Heartburn | 0.74 | 0.56 | 0.57 | 0.73 | 3.6 | < 0.001 | 2.31 | 0.001 |
| Regurgitation | 0.68 | 0.59 | 0.57 | 0.7 | 3.08 | < 0.001 | 1.64 | 0.059 |
| Globus sensation | 0.44 | 0.56 | 0.45 | 0.55 | 1 | 1 | 0.8 | 0.315 |
| Burning tongue | 0.22 | 0.77 | 0.44 | 0.55 | 0.98 | 1 | 0.67 | 0.129 |
| Coughing | 0.46 | 0.6 | 0.48 | 0.58 | 1.25 | 0.279 | 1.05 | 0.849 |
| Hoarseness | 0.45 | 0.67 | 0.53 | 0.6 | 1.69 | 0.009 | 1.59 | 0.054 |
| Fullness | 0.58 | 0.58 | 0.53 | 0.63 | 1.95 | 0.001 | 1.52 | 0.067 |
| Nausea | 0.3 | 0.77 | 0.52 | 0.58 | 1.47 | 0.087 | 0.83 | 0.505 |
| Vomiting | 0.09 | 0.91 | 0.43 | 0.55 | 0.93 | 0.944 | 0.65 | 0.275 |
| Halitosis | 0.29 | 0.77 | 0.5 | 0.57 | 1.37 | 0.166 | 1.13 | 0.619 |
| Bile taste | 0.28 | 0.77 | 0.5 | 0.57 | 1.31 | 0.255 | 0.9 | 0.67 |
| Imp path | 0.34 | 0.85 | 0.64 | 0.61 | 2.81 | < 0.001 | 1.99 | 0.009 |
| Smoking | 0.06 | 0.91 | 0.35 | 0.55 | 0.66 | 0.342 | 0.65 | 0.905 |
| Alcohol | 0.2 | 0.73 | 0.38 | 0.53 | 0.71 | 0.152 | 0.77 | 0.313 |
| Group B | ||||||||
| SI | 0.41 | 0.66 | 0.67 | 0.39 | 1.35 | 0.373 | 1.18 | 0.627 |
| Ph-metry | 0.19 | 0.84 | 0.67 | 0.38 | 1.26 | 0.662 | 1.09 | 0.848 |
| Gender | 0.52 | 0.51 | 0.65 | 0.38 | 1.16 | 0.693 | 0.88 | 0.689 |
| BMI | 0.43 | 0.54 | 0.62 | 0.35 | 0.88 | 0.759 | 0.84 | 0.569 |
| Heartburn | 0.66 | 0.45 | 0.67 | 0.43 | 1.59 | 0.134 | 1.88 | 0.086 |
| Regurgitation | 0.6 | 0.43 | 0.64 | 0.38 | 1.13 | 0.769 | 0.71 | 0.345 |
| Globus sensation | 0.45 | 0.65 | 0.68 | 0.4 | 1.48 | 0.222 | 1.33 | 0.367 |
| Burning tongue | 0.22 | 0.78 | 0.63 | 0.37 | 1 | 1 | 0.84 | 0.636 |
| Coughing | 0.45 | 0.6 | 0.66 | 0.39 | 1.2 | 0.614 | 1.21 | 0.575 |
| Hoarseness | 0.31 | 0.68 | 0.63 | 0.37 | 0.98 | 1 | 0.75 | 0.406 |
| Fullness | 0.52 | 0.61 | 0.7 | 0.42 | 1.68 | 0.089 | 2 | 0.033 |
| Nausea | 0.3 | 0.68 | 0.62 | 0.36 | 0.95 | 0.969 | 0.62 | 0.205 |
| Vomiting | 0.1 | 0.89 | 0.61 | 0.36 | 0.89 | 0.984 | 0.88 | 0.8 |
| Halitosis | 0.28 | 0.76 | 0.66 | 0.38 | 1.19 | 0.707 | 1.21 | 0.595 |
| Bile taste | 0.3 | 0.71 | 0.64 | 0.37 | 1.03 | 1 | 0.88 | 0.717 |
| Imp path | 0.32 | 0.76 | 0.69 | 0.39 | 1.45 | 0.299 | 1.38 | 0.381 |
| Smoking | 0.07 | 0.9 | 0.56 | 0.36 | 0.71 | 0.653 | 0.75 | 0.595 |
| Alcohol | 0.16 | 0.71 | 0.49 | 0.33 | 0.47 | 0.034 | 0.43 | 0.03 |
Second, the findings associated with pathological parameters in the pH/MII were analyzed (Table 2). In group A, the positive predicting parameters that correlated with pathological results from the pH/MII were SI, male gender, increased BMI index, heartburn, regurgitation, nausea and alcohol consumption. In group B, SI and vomiting were associated with pathological results from the pH/MII.
| Parameters | Sensitivity | Specificity | PPC | NPV | OR (univ) | Pval (univ) | OR (mult) | Pval (mult) |
| Group A | ||||||||
| SI | 0.59 | 0.66 | 0.54 | 0.71 | 2.92 | < 0.001 | 2.74 | < 0.001 |
| Gender | 0.44 | 0.42 | 0.34 | 0.53 | 0.59 | 0.008 | 0.63 | 0.045 |
| BMI | 0.58 | 0.65 | 0.53 | 0.7 | 2.56 | < 0.001 | 2.36 | < 0.001 |
| Heartburn | 0.68 | 0.49 | 0.47 | 0.69 | 2.01 | 0.001 | 1.88 | 0.017 |
| Regurgitation | 0.6 | 0.51 | 0.45 | 0.66 | 1.58 | 0.023 | 1.06 | 0.816 |
| Globus sensation | 0.39 | 0.52 | 0.36 | 0.56 | 0.72 | 0.102 | 0.66 | 0.064 |
| Burning tongue | 0.2 | 0.76 | 0.36 | 0.58 | 0.78 | 0.325 | 0.73 | 0.228 |
| Coughing | 0.43 | 0.57 | 0.41 | 0.6 | 1.03 | 0.958 | 0.99 | 0.978 |
| Hoarseness | 0.39 | 0.63 | 0.42 | 0.61 | 1.1 | 0.678 | 1.21 | 0.432 |
| Fullness | 0.49 | 0.51 | 0.4 | 0.6 | 1.02 | 0.998 | 0.83 | 0.424 |
| Nausea | 0.32 | 0.78 | 0.49 | 0.63 | 1.64 | 0.027 | 1.63 | 0.078 |
| Vomiting | 0.11 | 0.92 | 0.48 | 0.6 | 1.39 | 0.389 | 1.06 | 0.876 |
| Halitosis | 0.25 | 0.74 | 0.39 | 0.59 | 0.96 | 0.938 | 0.93 | 0.783 |
| Bile taste | 0.23 | 0.73 | 0.36 | 0.59 | 0.81 | 0.41 | 0.69 | 0.154 |
| Smoking | 0.07 | 0.92 | 0.38 | 0.6 | 0.91 | 0.95 | 0.81 | 0.606 |
| Alcohol | 0.3 | 0.8 | 0.51 | 0.63 | 1.78 | 0.012 | 1.9 | 0.012 |
| Group B | ||||||||
| SI | 0.61 | 0.75 | 0.59 | 0.77 | 4.78 | < 0.001 | 4.091 | < 0.001 |
| Gender | 0.45 | 0.45 | 0.32 | 0.58 | 0.66 | 0.172 | 0.84 | 0.625 |
| BMI | 0.51 | 0.59 | 0.42 | 0.67 | 1.49 | 0.195 | 1.73 | 0.097 |
| Heartburn | 0.63 | 0.39 | 0.38 | 0.63 | 1.05 | 0.969 | 0.95 | 0.906 |
| Regurgitation | 0.61 | 0.42 | 0.39 | 0.65 | 1.16 | 0.699 | 1.1 | 0.795 |
| Globus sensation | 0.43 | 0.6 | 0.39 | 0.64 | 1.15 | 0.723 | 1.31 | 0.428 |
| Burning tongue | 0.17 | 0.75 | 0.29 | 0.6 | 0.61 | 0.211 | 0.55 | 0.163 |
| Coughing | 0.46 | 0.59 | 0.4 | 0.65 | 1.19 | 0.621 | 1.2 | 0.608 |
| Hoarseness | 0.29 | 0.67 | 0.34 | 0.61 | 0.83 | 0.643 | 0.7 | 0.334 |
| Fullness | 0.48 | 0.54 | 0.38 | 0.64 | 1.07 | 0.907 | 1 | 0.998 |
| Nausea | 0.3 | 0.69 | 0.36 | 0.62 | 0.94 | 0.957 | 0.83 | 0.65 |
| Vomiting | 0.17 | 0.94 | 0.61 | 0.65 | 2.95 | 0.024 | 2.81 | 0.052 |
| Halitosis | 0.25 | 0.73 | 0.36 | 0.62 | 0.91 | 0.885 | 0.92 | 0.836 |
| Bile taste | 0.29 | 0.7 | 0.36 | 0.62 | 0.95 | 0.984 | 0.91 | 0.816 |
| Smoking | 0.11 | 0.94 | 0.5 | 0.64 | 1.77 | 0.36 | 1.25 | 0.682 |
| Alcohol | 0.28 | 0.83 | 0.49 | 0.66 | 1.85 | 0.089 | 1.47 | 0.336 |
Third, parameters associated with a pathological SI were analyzed (Table 3). In group A, the positive predicting parameters for a pathological SI were pathological results from the pH measurement, heartburn, regurgitation, fullness, nausea, vomiting and pathological results from the impedance measurement. In group B, pathological results from the pH and MII measurements were significantly associated with a positive SI. Furthermore, receiver operating characteristics (ROC) of the respective symptoms in association with PPI response were calculated (Figure 1).
| Parameters | Sensitivity | Specificity | PPC | NPV | OR (univ) | Pval (univ) | OR (mult) | Pval (mult) |
| Group A | ||||||||
| pH-metry | 0.39 | 0.81 | 0.61 | 0.63 | 2.67 | < 0.001 | 2.04 | 0.004 |
| Gender | 0.54 | 0.5 | 0.46 | 0.58 | 1.18 | 0.44 | 1.1 | 0.681 |
| Bmi | 0.49 | 0.6 | 0.49 | 0.6 | 1.42 | 0.077 | 1.14 | 0.547 |
| Heartburn | 0.7 | 0.52 | 0.54 | 0.69 | 2.6 | < 0.001 | 1.28 | 0.34 |
| Regurgitation | 0.7 | 0.6 | 0.58 | 0.72 | 3.42 | < 0.001 | 2.73 | < 0.001 |
| Globus sensation | 0.49 | 0.59 | 0.48 | 0.6 | 1.4 | 0.092 | 1.35 | 0.174 |
| Burning tongue | 0.22 | 0.77 | 0.43 | 0.56 | 0.97 | 0.97 | 0.66 | 0.113 |
| Coughing | 0.47 | 0.6 | 0.48 | 0.59 | 1.36 | 0.129 | 1.18 | 0.49 |
| Hoarseness | 0.43 | 0.66 | 0.5 | 0.6 | 1.46 | 0.062 | 1.15 | 0.553 |
| Fullness | 0.58 | 0.58 | 0.52 | 0.64 | 1.91 | 0.001 | 1.34 | 0.206 |
| Nausea | 0.34 | 0.8 | 0.57 | 0.61 | 2.11 | 0.001 | 1.13 | 0.665 |
| Vomiting | 0.12 | 0.93 | 0.59 | 0.58 | 2 | 0.048 | 1.56 | 0.252 |
| Halitosis | 0.26 | 0.74 | 0.44 | 0.56 | 1.03 | 0.958 | 0.65 | 0.085 |
| Bile taste | 0.29 | 0.78 | 0.5 | 0.58 | 1.42 | 0.129 | 1.01 | 0.952 |
| Imp path | 0.34 | 0.84 | 0.63 | 0.62 | 2.77 | < 0.001 | 2.26 | 0.002 |
| Smoking | 0.07 | 0.93 | 0.44 | 0.56 | 1.01 | 1 | 1.1 | 0.808 |
| Alcohol | 0.22 | 0.74 | 0.4 | 0.55 | 0.81 | 0.402 | 0.85 | 0.537 |
| Group B | ||||||||
| pH-metry | 0.33 | 0.91 | 0.7 | 0.68 | 5.03 | < 0.001 | 3.28 | 0.006 |
| Gender | 0.49 | 0.47 | 0.37 | 0.6 | 0.86 | 0.687 | 0.86 | 0.678 |
| BMI | 0.42 | 0.54 | 0.36 | 0.6 | 0.85 | 0.642 | 0.59 | 0.115 |
| Heartburn | 0.66 | 0.41 | 0.41 | 0.66 | 1.36 | 0.353 | 1.32 | 0.492 |
| Regurgitation | 0.65 | 0.44 | 0.42 | 0.67 | 1.5 | 0.198 | 1.19 | 0.664 |
| Globus sensation | 0.43 | 0.6 | 0.4 | 0.63 | 1.13 | 0.776 | 0.84 | 0.62 |
| Burning tongue | 0.21 | 0.77 | 0.37 | 0.61 | 0.9 | 0.895 | 1.08 | 0.841 |
| Coughing | 0.5 | 0.61 | 0.45 | 0.66 | 1.58 | 0.128 | 1.56 | 0.22 |
| Hoarseness | 0.36 | 0.71 | 0.44 | 0.64 | 1.42 | 0.299 | 1.34 | 0.43 |
| Fullness | 0.51 | 0.55 | 0.42 | 0.64 | 1.3 | 0.407 | 1.21 | 0.588 |
| Nausea | 0.33 | 0.7 | 0.41 | 0.62 | 1.13 | 0.791 | 1 | 0.995 |
| Vomiting | 0.15 | 0.93 | 0.56 | 0.63 | 2.26 | 0.101 | 1.67 | 0.347 |
| Halitosis | 0.27 | 0.74 | 0.39 | 0.62 | 1.02 | 1 | 1.05 | 0.907 |
| Bile taste | 0.3 | 0.71 | 0.39 | 0.62 | 1.05 | 0.989 | 0.84 | 0.653 |
| Imp path | 0.5 | 0.84 | 0.66 | 0.73 | 5.23 | < 0.001 | 4.84 | < 0.001 |
| Smoking | 0.1 | 0.93 | 0.5 | 0.62 | 1.66 | 0.431 | 2.06 | 0.21 |
| Alcohol | 0.23 | 0.8 | 0.43 | 0.62 | 1.24 | 0.643 | 1.03 | 0.941 |
The aim of this study was to identify objective and subjective predictors for the reliable diagnosis of GERD and the reported therapeutic response to PPIs to facilitate a more focused therapeutic approach in the future. Predicting the success of PPI therapy in symptomatic patients suspected of GERD would be helpful for preventing futile trials of empiric PPI medication and repeated reflux measurements and for reducing health care costs. In particular, the therapeutic response rates in patients with non-erosive reflux disease (NERD) and atypical/extraesophageal symptoms are not satisfactory[17]. To solve this problem, an effort was made to discriminate NERD from functional disorders (FD) with special pH/MII patterns. However, despite the known overlap between FD and reflux symptoms, approximately 38% of FD patients also report symptom relief upon PPI therapy[12]. Therefore, the ultimate clinical implication is to specifically detect patients responding to PPIs, regardless of NERD, atypical/extraesophageal symptoms or FD.
The focus was based on patient characteristics and anamnestic parameters (gender, BMI, smoking habits, alcohol). As expected, patients with both typical reflux symptoms, such as heartburn, regurgitation or positive SI, and fullness and hoarseness sufficiently respond to PPIs. These anamnestic parameters are good predictors for PPI therapeutic success. According to our data, empirical PPI trials are warrantable in patients with the respective anamnestic data. Male gender, obesity and alcohol consumption are also associated with positive therapeutic responses to PPIs, which might be of high interest in the primary care setting. Interestingly, smoking habits were not significant predictors for the PPI response. Patients with objective pathological results from pH/MII also respond to PPIs sufficiently.
In nonspecific anamnesis, the pH/MII and the SI are comparable options for guiding PPI therapy. As shown in previous trials, pH/MII can potentially facilitate a more tailored therapeutic approach in patients with PPI-resistant GERD symptoms and ensures the success of further escalating PPI therapy[23]. In this retrospective analysis, we used the same objective parameters because the number of reflux episodes can be easily assessed in a standardized manner and because reference values are available. As a subjective parameter, the SI was evaluated. All parameters used in the pH/MII were able to predict the PPI response with a comparable odds ratio to that of typical GERD symptoms. Because of the strong correlation of pH/MII with GERD, we analyzed the anamnestic parameters associated with pathological parameters in pH/MII. Again, a positive SI and regurgitation were associated, but there were also positive associations with increased BMI and male gender. Analyzing the SI did not reveal any new aspects.
Hence, an index empiric PPI trial is a warrantable option in patients with typical reflux symptoms (heartburn and regurgitation), male gender, obesity or atypical GERD symptoms (fullness, hoarseness). Furthermore, in accordance with our data, pH/MII is a reliable tool for guiding therapy if the anamnesis is inconclusive. Anamnestic parameters, including gender, obesity and drinking habits, also predict therapy response.
More conflicting are our results in patients who were assessed while their PPI therapies were ongoing. The indications for pH/MII were persistent symptoms despite PPI or therapy monitoring. Neither anamnestic nor pH/MII parameters were evaluable for predicting the PPI response. One might argue that the number of reflux episodes of 73 fluid and/or mixed reflux episodes within 22-24 h used in this study is too high because these values were generated in patients who were off PPI therapy. However, we also analyzed the SI as a subjective parameter. Nonetheless, it was not possible to predict the PPI response. On the one hand, it may therefore be assumed that the number of FD patients is most likely higher in the “non-responding group”. This assumption is supported by the high number of normal pH/MII results in the “on therapy” group. On the other hand, it is known that patients who are unresponsive to standard PPI therapy respond to escalating PPI therapy in up to 90% of cases[23]. However, the effect of an escalating PPI dose was not analyzed in this trial. In clinical practice, patients with persistent symptoms despite standard therapy should undergo pH/MII testing. If the results are pathological, then escalating PPI therapy is a promising treatment[23]. In PPI-unresponsive patients, extra-esophageal signs and symptoms are more likely due to causes other than GERD. Continued PPI therapy in this group is not recommended[24].
From previous reports and in accordance with our data, increased BMI is a risk factor for GERD[23], and pH/MII monitoring reveals pathologic findings particularly in these patients. It is also known that persistent gastroesophageal reflux despite standard PPI-therapy is a common problem in patients with increased BMIs (> 25). Therefore, a possible explanation is increased intra-abdominal pressure due to adipose tissue, leading to increased gastric pressure, decreased gastric emptying and consecutive relaxation of the lower esophageal sphincter[25,26]. In accordance with previous trials, we detected good clinical responses to standard PPI therapy in obese patients[27].
To the best of our knowledge, the present study provides the largest series of pH/MII data. The limitation of our study is its single-center setting. Furthermore, it is noteworthy that certain methodological problems existed due to the retrospective approach. First, the patients were not subject to a previously created study protocol that involved the use of different PPI agents. Second, it was not possible to precisely monitor PPI intake prior to the pH/MII. Notwithstanding, we believe that the high number of patients suffices as a robust database.
In conclusion, in patients who are off PPIs and have typical reflux symptoms (heartburn and regurgitation), male gender, obesity or atypical GERD symptoms (fullness, hoarseness), empiric PPI therapy is most likely to be successful. In non-obese, female patients with non-typical reflux symptoms, pH/MII (including evaluation of the SI) should be considered instead of empiric PPI therapy. Anamnestic parameters, including gender, obesity and drinking habits, also predict the therapeutic response. With respect to predicting the therapeutic response, pH/MII during ongoing PPI therapy is not useful. Thus, particularly in primary care settings, a more focused therapeutic approach should be conducted instead of treating patients empirically, thereby avoiding ineffective, long-term PPI trials in the future.
Gastroesophageal reflux disease (GERD) is one of the most prevalent gastrointestinal disorders. The general therapeutic aim is the relief of symptoms and the prevention of associated complications. Standard empiric proton pump inhibitor (PPI) therapy poses a substantial economic burden and yields varying success.
pH/impedance monitoring (pH/MII) provides a reliable pattern for discriminating reflux disease and functional disorders. Reliable objective parameters or specific anamnestic findings for predicting the therapeutic response to PPIs in high-volume studies are absent.
The present study provides the largest series of pH/MII data to identify objective and subjective predictors for the reliable diagnosis of GERD and the response to PPI therapy.
In patients off PPIs and with typical reflux symptoms, male gender, obesity or atypical GERD symptoms, empiric PPI therapy is most likely to be successful. In non-obese, female patients with non-typical reflux symptoms, pH/MII should be applied instead of empiric PPI therapy. With respect to predicting the therapeutic response, pH/MII during ongoing PPI therapy is not useful.
Up to 40% of the adult population suffers occasionally from reflux symptoms. Symptoms are subdivided into typical (heartburn, regurgitation) and atypical symptoms (chronic cough, hoarseness, recurrent sinusitis, globus sensations in the throat, a burning feeling on the tongue, dental erosions, fullness). The most common therapeutic approaches are PPI trials, which have variable success.
This manuscript provides systematic theoretical analyses and valuable conclusions.
| 1. | Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1043] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 2. | Dent J. Pathogenesis of gastro-oesophageal reflux disease and novel options for its therapy. Neurogastroenterol Motil. 2008;20 Suppl 1:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Pandolfino JE, Kwiatek MA, Kahrilas PJ. The pathophysiologic basis for epidemiologic trends in gastroesophageal reflux disease. Gastroenterol Clin North Am. 2008;37:827-843, viii. [PubMed] |
| 4. | Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 5. | Fedorak RN, Veldhuyzen van Zanten S, Bridges R. Canadian Digestive Health Foundation Public Impact Series: gastroesophageal reflux disease in Canada: incidence, prevalence, and direct and indirect economic impact. Can J Gastroenterol. 2010;24:431-434. [PubMed] |
| 6. | Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1384] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 7. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2519] [Article Influence: 126.0] [Reference Citation Analysis (2)] |
| 8. | Mearin F, Ponce J, Ponce M, Balboa A, Gónzalez MA, Zapardiel J. Frequency and clinical implications of supraesophageal and dyspeptic symptoms in gastroesophageal reflux disease. Eur J Gastroenterol Hepatol. 2012;24:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 553] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 10. | John M. Eisenberg Center for Clinical Decisions and Communications Science. Managing Chronic Gastroesophageal Reflux Disease. Comparative Effectiveness Review Summary Guides for Clinicians[Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007; AHRQ Comparative Effectiveness Reviews. 2011 Sep 23. |
| 11. | Weijenborg PW, Cremonini F, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012;24:747-757, e350. [PubMed] |
| 12. | Taghavi SA, Ghasedi M, Saberi-Firoozi M, Alizadeh-Naeeni M, Bagheri-Lankarani K, Kaviani MJ, Hamidpour L. Symptom association probability and symptom sensitivity index: preferable but still suboptimal predictors of response to high dose omeprazole. Gut. 2005;54:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Kushnir VM, Sayuk GS, Gyawali CP. Abnormal GERD parameters on ambulatory pH monitoring predict therapeutic success in noncardiac chest pain. Am J Gastroenterol. 2010;105:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Zerbib F, Roman S, Ropert A, des Varannes SB, Pouderoux P, Chaput U, Mion F, Vérin E, Galmiche JP, Sifrim D. Esophageal pH-impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol. 2006;101:1956-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 319] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Savarino E, Zentilin P, Tutuian R, Pohl D, Gemignani L, Malesci A, Savarino V. Impedance-pH reflux patterns can differentiate non-erosive reflux disease from functional heartburn patients. J Gastroenterol. 2012;47:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Kushnir VM, Sathyamurthy A, Drapekin J, Gaddam S, Sayuk GS, Gyawali CP. Assessment of concordance of symptom reflux association tests in ambulatory pH monitoring. Aliment Pharmacol Ther. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Talley NJ, Meineche-Schmidt V, Paré P, Duckworth M, Räisänen P, Pap A, Kordecki H, Schmid V. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies). Aliment Pharmacol Ther. 1998;12:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 262] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Bytzer P, Jones R, Vakil N, Junghard O, Lind T, Wernersson B, Dent J. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:1360-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Francis DO, Rymer JA, Slaughter JC, Choksi Y, Jiramongkolchai P, Ogbeide E, Tran C, Goutte M, Garrett CG, Hagaman D. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Cho YK. How to Interpret Esophageal Impedance pH Monitoring. J Neurogastroenterol Motil. 2010;16:327-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 613] [Article Influence: 27.9] [Reference Citation Analysis (4)] |
| 22. | Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, Zhang X, Adhami T, Murray J, Peters J. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 386] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther. 2007;26:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Naik RD, Vaezi MF. Extra-esophageal manifestations of GERD: who responds to GERD therapy. Curr Gastroenterol Rep. 2013;15:318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Mercer CD, Rue C, Hanelin L, Hill LD. Effect of obesity on esophageal transit. Am J Surg. 1985;149:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Mercer CD, Wren SF, DaCosta LR, Beck IT. Lower esophageal sphincter pressure and gastroesophageal pressure gradients in excessively obese patients. J Med. 1987;18:135-146. [PubMed] |
| 27. | Sharma P, Vakil N, Monyak JT, Silberg DG. Obesity does not affect treatment outcomes with proton pump inhibitors. J Clin Gastroenterol. 2013;47:672-677. [PubMed] |
P- Reviewers: Onyekwere CAA, Niu CY S- Editor: Wen LL L- Editor: A E- Editor: Liu XM