Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.3976
Revised: January 9, 2014
Accepted: February 17, 2014
Published online: April 14, 2014
Processing time: 144 Days and 2.3 Hours
Irritable bowel syndrome (IBS) is a commonly encountered chronic functional gastrointestinal (GI) disorder. Approximately 10% of IBS patients can trace the onset of their symptoms to a previous a bout of infectious dysentery. The appearance of new IBS symptoms following an infectious event is defined as post-infectious-IBS. Indeed, with the World Health Organization estimating between 2 and 4 billion cases annually, infectious diarrheal disease represents an incredible international healthcare burden. Additionally, compounding evidence suggests many commonly encountered enteropathogens as unique triggers behind IBS symptom generation and underlying pathophysiological features. A growing body of work provides evidence supporting a role for pathogen-mediated modifications in the resident intestinal microbiota, epithelial barrier integrity, effector cell functions, and innate and adaptive immune features, all proposed physiological manifestations that can underlie GI abnormalities in IBS. Enteric pathogens must employ a vast array of machinery to evade host protective immune mechanisms, and illicit successful infections. Consequently, the impact of infectious events on host physiology can be multidimensional in terms of anatomical location, functional scope, and duration. This review offers a unique discussion of the mechanisms employed by many commonly encountered enteric pathogens that cause acute disease, but may also lead to the establishment of chronic GI dysfunction compatible with IBS.
Core tip: This review discusses the long-term consequences of acute enteric infections that may serve to trigger post-infectious irritable bowel syndrome, a routinely diagnosed disorder. This unique discussion elucidates novel initiation mechanisms, underlying pathophysiological features of post-infectious irritable bowel syndrome, employed by commonly encountered enteric pathogens.
- Citation: Beatty JK, Bhargava A, Buret AG. Post-infectious irritable bowel syndrome: Mechanistic insights into chronic disturbances following enteric infection. World J Gastroenterol 2014; 20(14): 3976-3985
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/3976.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.3976
Irritable bowel syndrome (IBS) is among the most commonly encountered chronic functional gastrointestinal (GI) disorders afflicting individuals in westernized nations. Based on the Rome III criteria abdominal pain accompanied by sustained changes in bowel habit constitute IBS, whose diagnosis is achieved in the absence of biochemical markers of disease[1]. Clinical presentation of constipation, diarrhea, or a combination, constitutes the different subtypes of IBS: IBS with constipation (IBS-C), diarrheal IBS subtype (IBS-D), mixed IBS (IBS-M), respectively[2]. Often perceived as a female-dominant disorder, IBS is thought to afflict between 5%-10% of the population[3], especially in westernized nations. Elucidating the mechanisms underlying the typical multifaceted clinical presentation of IBS is a topic of considerable research efforts in the medical community[4]. A growing body of evidence implicates numerous triggering events in contributing to IBS pathophysiology, including an initiating bout of infectious enteritis, low grade inflammation, altered functionalities in GI cell types, increases in epithelial permeability, and alterations in the GI microbiota, although the precise mechanisms of underlying each remain obscure[2,5-8]. Approximately 10% of IBS patients believe that their symptoms began following a bout of infectious dysentery[6], leading to the coinage of the term; Post infectious (Pi)-IBS. While many enteric pathogens cause self-limiting, acute diarrheal disease, subsequent chronic physiological consequences may persist in some individuals[9]. Many commonly encountered enteric pathogens can produce physiological changes that may provide important initiation mechanisms underlying chronic GI conditions, such as Pi-IBS. This article critically reviews the evidence supporting a role for key physiological changes initiated during enteric infection, that may in turn be responsible for IBS symptom.
Based on the Rome criteria for diagnosis, any onset of new IBS symptoms subsequently following an infectious event is defined as Pi-IBS[6]. Pi-IBS cases often exhibit characteristics of the IBS-D, and can occur in 4%-31% of patients following acute gastroenteritis[6,10-12]. A large body of work provides evidence supporting a role for pathogen-mediated modifications in the resident intestinal microbiota, epithelial barrier integrity, enterochromaffin cell function, and innate immune features[5,13,14] in Pi-IBS manifestation. Any number of these pathogenic consequences have been reported following enteric infection incited by an array of pathogens such as Shigella spp., pathogenic Escherichia coli, Salmonella, Campylobacter jejuni, and Giardia duodenalis[14-18]. Enteric pathogens must employ a vast array of machinery to evade the host protective immune mechanisms, and illicit successful infections. Recent work identifying genetic mutations, namely in genes responsible for epithelial and innate immune functionalities, in patients experiencing both the post-infectious, and traditional forms of IBS, point to defects in innate immunity and epithelial homeostasis as an important risk factor for IBS susceptibility[19,20]. The impact of infectious events on host physiology can be multidimensional in terms of anatomical location, functional scope, and duration. Indeed, anatomical, immunological, and neurological dysfunctions, or combinations of such, have all been shown as risk factors determining Pi-IBS manifestation. This review will provide an in-depth discussion surrounding the potential roles in which a variety of commonly encountered enteric pathogens may play in initiating important pathophysiological features of Pi-IBS.
Abnormal bowel habits and abdominal hypersensitivity, or reduced threshold of pain, are the hallmark clinical signs of IBS. The classification of IBS as a functional disorder stems from a lack of determinant histopathological, or structural biomarkers in afflicted patients. The Rome criteria requires the incidence of abdominal pain, accompanied by alterations in bowel habit for complete IBS diagnosis[21].
Abnormal GI motility is commonly associated with altered bowel habits producing diarrheal, constipation, and mixed IBS subtypes[22]. The potential for dysfunctional intestinal motility in contributing to altered bowel habits in IBS is supported by studies looking at intestinal transit rates between healthy and IBS individuals, with IBS-D subtypes exhibiting enhanced rates of SI transit, and the opposite trend observed for IBS-C patients[22,23]. Moreover, a recent report demonstrated that the normal colorectal reflex (normal increase in rectal tone in response to phasic colonic distention) was largely abolished in IBS patients, regardless of bowel habit, providing some evidence for altered colonic motility in these individuals[24]. Interestingly, muscle hypercontractility and abnormal motility patterns are observed subsequent to Trichinella spiralis infection in a commonly used murine model of PI-IBS[13,25-27], suggesting that persistent dysfunctional intestinal motility can be incited following an acute infection.
Lower thresholds for pain tolerance in IBS patients have been documented along the entire length of the GI tract[22], an effect that is thought to occur in upwards of 60% of afflicted individuals[7]. Hypersensitivity often occurs locally in response to colonic distention[7]. Furthermore, overall visceral hypersensitivity, even upon brief stimuli such as the ingestion of food, is well documented in IBS patients, and may contribute to additional bloating, nausea, and urgency symptoms[8,28].
Stressful events can drastically affect the processing of visceral stimuli[29,30] and result in dysfunctional central neural processes culminating into heightened pain perception. Injury to visceral afferents, for example, is a common cause underlying visceral hypersensitivity[7]. Studies using a rat model of TNBS-induced transient colonic inflammation have highlighted that persistent tissue injury may directly produce heightened visceral pain perception[31]. Importantly, chemically induced colonic inflammation models have stark parallels to many of the physiological events accompanying enteric infections. Initial processes of inflammation, for instance, may act to first sensitize effector, neuronal, and immune cells within the GI tract.
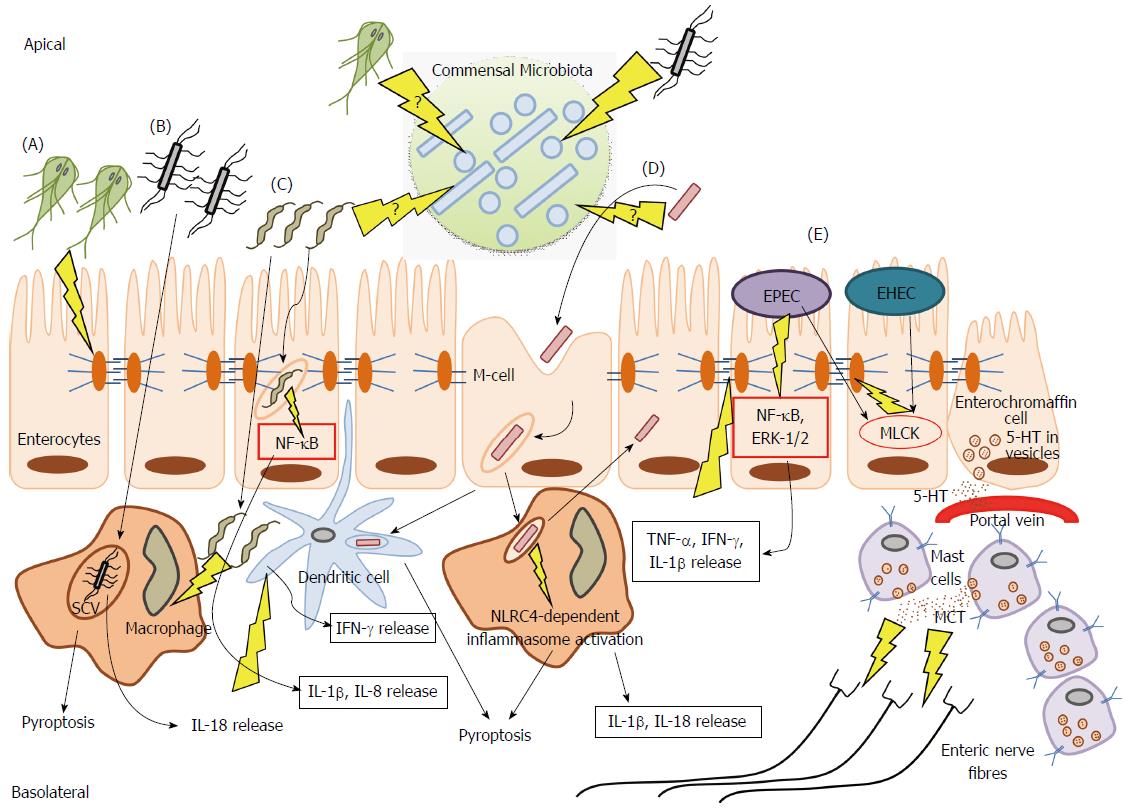
Interestingly, many of the physiological consequences that can result from infectious events within the GI tract have also been proposed as determinants capable of contributing to abnormal motility and hypersensitivity symptoms seen in IBS patients. The major mechanisms currently thought to underlie IBS pathogenesis, and the evidence surrounding possible contributions made to each by distinct enteric pathogens, will be discussed in the following sections (Figure 1).
Accumulating evidence suggests subtle alterations in the immune system in both the gut, and peripheral circulation of PI-IBS patients[32]. Pathogen-mediated disruptions of the mucosal barrier have the ability to allow for persistent immune activation within the intestine, largely due to increased exposure to luminal antigens. Likewise, the host inflammatory response towards perceived pathogens, while meant to be protective, may result in detrimental, perpetuated activation of effector cells and inflammatory mediators. The incidence of PI-IBS symptoms in many patients following enteric infection has fuelled interest in looking at persistent immune infiltrate, and/or altered immune functionalities, as plausible driving forces in the generation of IBS symptoms[33].
Mast cells/macrophages/dendritic cells: Certain enteric pathogens have been shown to promote mast cell accumulation. A recent study found that a large proportion of patients experiencing Shigellosis, caused by invasive Shigella spp., go on to develop PI-IBS, and that this effect is accompanied by augmented mast cell numbers[34]. Under normal conditions, mucosal mast cells are highly involved in wound-healing, and defense against pathogens[5]. However, multiple reports document heightened numbers of mast cells within the small[35,36], and large intestines[37-39] of IBS patients. One study, which observed increased mast cells specifically within the duodenum of IBS patients suggested that infiltration of these cells may provide some explanation behind the observation that symptoms differ depending upon the affected site along the GI tract[36]. Also, mast cells can secrete serotonin, therefore increased populations of these cells may provide a link between cellular infiltrate and altered serotonin signaling leading to changes along the brain-gut axis, and dysmotility, characteristic of either IBS-D or IBS-C[36]. Furthermore, augmented numbers of mast cells, and particularly those closely associated with nerve fibers, have been reported in both IBS and Pi-IBS[38] (Figure 1), an effect which may be correlated with enhanced bloating and pain perception symptoms[2,40-42].
The T. spiralis mouse model of Pi-IBS has provided important insight into many pathophysiological changes following acute enteric infection. A recent study, for instance, documented numerical and phenotypic alterations in lamina propria dendritic cells (LPDC), following acute T. spiralis infection[43]. In what the authors defined as the “Pi-IBS stage” of infection, i.e., no recovery of nematode in the stool, LPDCs exhibited enhanced expression of co-stimulatory molecules, and greater ability to migrate to and drive CD4+ T cell proliferation[43]. Furthermore, the altered LPDC phenotype was proposed to underlie enhanced levels of pro-inflammatory interferon (IFN)-γ, IL-23 and tumor necrosis factor (TNF)-α production in the Pi-IBS stage[43]. The important role that these cells play in directing T-cell responses may have implications in promoting a low-grade inflammatory milieu, and requires further investigation in relation to IBS pathogenesis.
Monocytes and macrophages are at the forefront of initiating an inflammatory response to pathogens, in addition to providing essential directives to the adaptive immune system[5]. In Pi-IBS cases confirmed following C. jejuni infection the numbers of resident CD68+ macrophages are diminished, perhaps owing to the cytotoxic nature of the pathogen inside host cells[9]. Likewise, Shigella spp.[15,16] and Salmonella infections have been implicated in causing Pi-IBS, and both are obligate intracellular pathogens, which preferentially exploit phagocytic machinery of the macrophage. Specifically, Shigella is transported into the lamina propria through M cells in the epithelium, and presented to resident macrophages and dendritic cells (DCs) for phagocytosis upon which activation of the nucleotide-binding oligomerization domain (NOD)-like receptor protein (NLRC4) inflammasome occurs[44,45] (Figure 1). Consequently, the resulting activation of pro-inflammatory cytokines, interleukin (IL)-18 and IL-1β, are thought to be major determinants of the high inflammatory conditions characteristic of early Shigella infection[45]. Inflammasome activation can also produce heightened rates of macrophage cell death via pyroptosis, which acts as an “inflammatory” form of programmed cell death (Figure 1). Thus, Shigella infection promotes a high status of inflammation, while simultaneously resulting in the detrimental loss of lamina propria (LP) macrophages. LP macrophages have an important regulatory, and anti-inflammatory role in maintaining intestinal homeostasis[45]. Furthermore, as a consequence of resident LP macrophage depletion, additional circulating monocytes may be recruited to the site of infection, and often differentiate into macrophages possessing a more pro-inflammatory capacity[45]. Considering ample reports documenting low-grade inflammation IBS patients[46,47], pathogen-mediated inflammatory conditions, in addition to the promotion of pro-inflammatory cell phenotypes, may be especially relevant triggers underlying Pi-IBS development.
In contrast to Shigella, Salmonella is seemingly less cytotoxic to macrophages[48], yet Pi-IBS symptoms have been reported following anywhere between 6%-32% of confirmed infections[2,19]. Following phagocytosis, Salmonella forms the characteristic Salmonella Containing Vacuole (SCV) in macrophages, in which it replicates while effectively evading host immune machinery, and pyroptosis[48] (Figure 1). While capable of avoiding certain immune parameters, Salmonella still evokes a strong IL-18 response[48] which has important implications in exerting paracrine effects on surrounding immune cells to induce IFN-γ expression, and also result in increased levels of activated T cells in the infected intestine, accumulation of which has been documented in many examinations of IBS[9,32,33,42,49].
Cytokine profiles: Substantial regulation exists within the GI tract in order to maintain a functional balance between pro- and anti-inflammatory mediators under homeostatic conditions. Engagement of the Toll-like receptors (TLRs), NOD-like receptors (NLRs), and other host pathogen-recognition-receptors (PRRs) occurs through ligation by various pathogen-associated-molecular-patterns (PAMPs). Shigella, for instance, is known to stimulate excess production of IL-1β from immune cells during infection via the NLRC4 inflammasome[44,45] (Figure 1). Also, excessive IL-8 secretion is a hallmark of Campylobacter pathogenesis[50], and is initiated upon host recognition of the pathogen-associated lipooligosaccharide[51]. Interestingly, a recent report demonstrated a disruption in TLR9 expression on epithelial cells to be implicated in the enhanced susceptibility to mild pro-inflammatory stimuli post-campylobacteriosis in mice[52]. C. jejuni is also know to promote the translocation of non-invasive commensal bacteria via paracellular and transcellular pathways[53,54]. Campylobacter has also been shown to activate copious amounts of nuclear factor (NF)-κB and IL-1β from immune cells, in vitro[51]. Likewise, recognition of EPEC flagellin and endotoxin results in NF-κB and extracellular signal regulated kinase (ERK)-1/2 –driven IL-8 release, and enhanced TNF-α, IFN-γ and IL-1β in the infected mucosa[55,56] (Figure 1). Interestingly, at least some of the pro-inflammatory cytokines, including TNF-α, IL-1β, and IFN-γ may themselves disrupt the epithelial barrier through alterations of the tight junctions (TJs), and promote increased permeability[57-59]. Thus, residual pro-inflammatory infiltrate following enteric infection combined with the sub-epithelial penetration of commensal bacteria, can create extensive damage to surrounding intestinal tissues, and likely promote chronic pathophysiological consequences. Consequently, many reports have drawn links between altered cytokine profiles and IBS generation[60], and findings include increased levels of pro-inflammatory IL-6, IL-8, and TNF-α in plasma and circulating blood mononuclear secretions from IBS patients[47,61]. Lower detection of typical anti-inflammatory cytokines, IL-10 and transforming growth factor (TGF)-β, at the level of mRNA has also been reported[62]. Also, evidence from the T. spiralis Pi-IBS murine infection model has shown greater levels of IFN-γ, IL-23 and TNF-α produced by DCs in the Pi-IBS stage[43]. Additionally, sustained levels of pro-inflammatory mediators have been documented in a 21-d Citrobacter rodentium model of murine E. coli pathogenesis[63]. Regardless of these promising observations, the implications of pathogen-mediated alterations in normal cytokine profiles in providing sufficient trigger for IBS symptom establishment requires further investigation.
The intestinal epithelium provides an interface between the luminal space and the dynamic environment of the underlying subepithelial compartment. This physical barrier is intricately involved in regulating the controlled passage of vital nutrients, molecules, and water, via a semipermeable function maintained by TJs. TJs actively maintain the polarized characteristic of the epithelial barrier, and are composed of over 40 proteins consisting of occludin, junctional adhesion molecule (JAM), and claudins[64]. Patients with a history of infectious events experiencing Pi-IBS show drastic increases in permeability[65,66]. A prospective study, however, following a large waterborne outbreak of bacterial gastroenteritis, incited by mixed infection of EHEC O157:H7 and C. jejuni, documented increased permeability to be associated with IBS, regardless of whether symptoms were post-infectiously initiated[65]. Enterohemorrhagic E. coli (EHEC) is known to have deleterious impacts on the epithelial barrier through number of mechanisms, including TJ disruptions, and abnormal rates of intestinal epithelial cell (IEC) apoptosis[67,68]. These effects can be mediated directly via physical interaction through EHEC formation of characteristic attaching and effacing lesions (A/E lesions), and/or diffusely through toxin release[64,69]. EHEC, and its close relative: Enteropathogenic E. coli (EPEC), are known to hijack various pathways regulating the semi-permeable profile of TJs, and both have been shown to activate myosin light chain kinase (MLCK) to produce abnormally leaky barrier functionalities[70-72] (Figure 1). Additionally, Giardia duodenalis, a protozoan pathogen recently implicated in promoting Pi-IBS development[18,73], is well-known to disturb homeostatic barrier function through alterations in key TJ elements[74]. Specifically, Giardia has been shown to disrupt zonula occludins protein (ZO)-1, numerous transmembrane claudin proteins, and alter F-actin and α-actinin in order to disrupt paracellular flow[75,76] (Figure 1), which may have important implications in providing a mechanistic link between initial giardiasis, and subsequent development of IBS symptoms. Indeed, recent analysis of colonic biopsies from IBS patients indicated decreased expression of ZO-1, which was associated with increased permeability[77]. Moreover, an earlier report examining fecal extracts indicated higher levels of serine proteases in samples from IBS-D patients. When these extracts were applied to healthy colonic mucosa, they could elicit a proteinase activated receptor (PAR)-2 dependent increase in paracellular permeability in mice via increased myosin light chain (MLC) phosphorylation and delayed redistribution of ZO-1[78]. Numerous pathogens, including both EPEC and EHEC, produce potentially cytotoxic serine proteases[79], suggesting another possible link between enteric infection and IBS pathogenesis. Proteases are known to be involved in the infectious processes of pathogens such as EHEC and EPEC where they can prove detrimental to the epithelial barrier via modifications of the extracellular matrix[80], and or by activating protease-activated receptors, which have been shown to stimulate sensory neurons to produce hypersensitivity reactions[81]. Consequently, the possibility of residual pathogen mediators, such as inherent proteases, contributing to persistent changes in GI function requires further examination.
Enterochromaffin cells: Enterochromaffin cells (ECs) lining the GI mucosa are primary sources of Serotonin (5-HT) within the body. Alterations in the biosynthesis of 5-HT, in its release from ECs and degradation, and/or in its re-uptake, may have severe ramifications and perturb normal GI function[82]. Multiple studies have shown significantly higher 5-HT levels in the plasma of Pi-IBS patients compared with that of healthy controls, even in comparison to patients of the sporadic IBS-C subtype[83]. Recent studies have observed such significant alterations in EC counts and 5-HT levels, that the authors declared Pi-IBS as a distinct IBS subtype[10,11]. Augmented numbers of 5-HT-contatining ECs have been observed in colonic biopsies from patients following C. jejuni infection[9]. Up to 25% of C. jejuni infections are known to result in IBS[9], and the resulting implications on EC hyperplasia and excessive 5-HT bioavailability suggest a possible mechanism whereby enteric infection may provide sufficient trigger for IBS symptom generation. Additionally, numerous reports have suggested a defect in the serotonin reuptake transporter (SERT) expression, and function in IBS patients[82,84,85], which may dictate inadequacies in homeostatic serotonin turnover. Interestingly, in the T. spiralis model of Pi-IBS, mice develop chronic abnormal motility patterns subsequent to infection, an effect that is accompanied by EC hyperplasia and 5-HT release[6,13], and blocked upon administration of a 5-HT antagonist[86]. In contrast, patients with persisting abdominal symptoms after acute Giardia infection have lower duodenal 5-HT-containing ECs, and lower plasma 5-HT postprandially, compared to controls[87], further underscoring the complexity of IBS pathophysiology.
Intestinal microbiota disruptions: The intestinal microbiota have extensive protective capacities[88] that are maintained by a diverse species profile. The characteristic high fat, high protein diets employed by the majority of people living in westernized countries facilitates the establishment of distinct microbiota species profile, as compared to that of those living in rural areas of developing countries, with a polysaccharide-rich diet[89]. Particular bacterial groups, mainly Bacteroidetes are known to harbor significant genetic capabilities to hydrolyse xyloses, making it an important constituent of the microbiota of people subsisting on carbohydrate-dominant food sources. The relative sensitivity of these distinct microbiota to enteropathogens, and how in turn disruptions in their respective flora may differentially regulate post-infectious disorders, is unknown.
Interestingly, changes in the relative Firmicutes to Bacteroidetes ratio[90,91], loss of Bifidobacteria spp. and Faecalibacterium[91], and overall diminished diversity[92], are all apparent in the microbiota profile of IBS patients. Additionally, numerous studies have demonstrated small intestinal bacterial overgrowth in IBS patients, where excessive colonization of the small intestine occurs with colonic flora[33,93]. There is the possibility that enteropathogens may disrupt the indigenous microbiota, either directly through pathogen-microbiota interactions, indirectly via the host mucosal immune response to the pathogen, or by a combination of the two[94]. For example, S. enterica serovar Typhimurium induced the loss of 95% of total bacterial numbers throughout the murine intestinal tract, 7 d following infection[94]. Findings from ongoing research also indicate that G. duodenalis and C. jejuni are able to directly alter species distribution of human commensal microbiota[95]. Pathogenic effects, however, may only provide a suitable trigger, and ultimately require the accompaniment of a host inflammatory response in order to markedly alter the microbiota ecosystem. The necessity of these compounding factors is exemplified in contrasting C. rodentium and C. jejuni murine infection models, where the former induces overt host inflammation, while the later can successfully colonize without producing inflammatory reactions[96]. It appears then, that both enteropathogen assault, combined with pathogen-mediated intestinal inflammation, can elicit dramatic changes in the total abundance of the intestinal microbiota, and shift in anaerobic:aerobic species[96].
Many studies that classify patients as experiencing Pi-IBS do so based upon questionnaires, highlighting the fact that they rely exclusively on a patient’s recall of past medical events, including infections and/or prescription drug use. Some antibiotics, for example, have established causality in disturbing the overall fecal microbial composition through drastic reduction of Firmicutes and Bacteroidetes, and a corresponding promotion of Proteobacteria spp.[97].
Also, the classification of IBS as biopsychosocial disorder challenges the mantra of body and mind being distinct entities, and suggests an equal consideration of both when examining disease manifestation. The risk of developing IBS symptoms following enteric infection may also differ in individuals depending on psychological parameters such as stress level, emotional status, and upbringing. High stress and anxiety levels, for instance, are associated with IBS development following Campylobacter infection[98]. Anxiety, as well as depression, is also correlated with altered pain perception in IBS patients[30]. Additionally, anxiety and depressive states in IBS patients were recently shown to lead to changes in serum levels of gastrointestinal hormones. Indeed, the authors suggest increased secretion of somatostatin and vasoactive intestinal peptide seen in IBS patients exhibiting anxiety-depression emotional state ratings, may contribute to altered gastrointestinal motility and function[99]. An important mediator in the endocrine arm of the stress response, corticotropin-releasing factor, may also contribute to Pi-IBS development through direct local action on specific cellular targets, namely mast cells, and consequently lead to the modification the intestinal inflammatory process[100].
Additionally, as Pi-IBS is defined based upon the development of exclusively new IBS symptom presentation, researchers must be certain that no preceding presentation of IBS occurred. Indeed, clear cause-to effect relationship studies need to establish mechanistic causalities in Pi-IBS.
Unfortunately, the link between physiological consequences of enteric infection and altered gut function (sensitivity and motility) seen in IBS remains largely circumstantial. As many as 30%-40% of patients experiencing enteritis can go on to develop chronic GI abnormalities compatible with IBS; however, this means that a greater percentage of patients make a full recovery. Susceptibility, in turn, to developing IBS is determined by a number of factors, with enteric pathogens constituting only one possible route of initiation. Regardless of the heterogeneous initiation mechanisms culminating into disease, the pathophysiological implications of enteric infection provide important clues towards elucidating the mechanics underlying IBS manifestation. Animal models are becoming increasingly appreciated as divergent means in which IBS triggering mechanisms may be elucidated. Indeed, the maternal separation stress model in rodents is well documented in mimicking early life stress that can result in lifelong dysfunctions in the brain-gut axis, and is implicated in predisposing to IBS development[101]. Furthermore, animal models of post-infectious, or post-inflammatory conditions, such as those using T. spiralis or TNBS, are proving useful in examining the mechanisms underlying motility and pain perception changes subsequent to diverse stimuli, without the challenges associated with patient recall, or the need for complex psychological status analyses.
This is especially relevant in terms of developing treatment technologies to combat IBS, most of which currently target overt symptomology. Many of the physiological consequences of GI infections represent parallels with fundamental triggering mechanisms currently though to contribute to IBS. Understanding the similarities between remnants of enteric infections, and the detrimental outcomes, can lead to the development of prevention strategies and therapeutic techniques to target IBS generation; before it can even start.
| 1. | Bixquert Jiménez M. Treatment of irritable bowel syndrome with probiotics. An etiopathogenic approach at last. Rev Esp Enferm Dig. 2009;101:553-564. [PubMed] |
| 2. | Spiller R, Campbell E. Post-infectious irritable bowel syndrome. Curr Opin Gastroenterol. 2006;22:13-17. [PubMed] |
| 3. | Chang FY, Lu CL. Irritable bowel syndrome and migraine: bystanders or partners. J Neurogastroenterol Motil. 2013;19:301-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Ford AC, Talley NJ. IBS in 2010: advances in pathophysiology, diagnosis and treatment. Nat Rev Gastroenterol Hepatol. 2011;8:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 453] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 6. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 484] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 7. | Zhou Q, Verne GN. New insights into visceral hypersensitivity--clinical implications in IBS. Nat Rev Gastroenterol Hepatol. 2011;8:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Zhou Q, Fillingim RB, Riley JL, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 2010;148:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [PubMed] |
| 10. | Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651-1659. [PubMed] |
| 11. | Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Zanini B, Ricci C, Bandera F, Caselani F, Magni A, Laronga AM, Lanzini A. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Bercík P, Wang L, Verdú EF, Mao YK, Blennerhassett P, Khan WI, Kean I, Tougas G, Collins SM. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology. 2004;127:179-187. [PubMed] |
| 14. | Parry SD, Stansfield R, Jelley D, Gregory W, Phillips E, Barton JR, Welfare MR. Does bacterial gastroenteritis predispose people to functional gastrointestinal disorders A prospective, community-based, case-control study. Am J Gastroenterol. 2003;98:1970-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Ji S, Park H, Lee D, Song YK, Choi JP, Lee SI. Post-infectious irritable bowel syndrome in patients with Shigella infection. J Gastroenterol Hepatol. 2005;20:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Kim HS, Kim MS, Ji SW, Park H. [The development of irritable bowel syndrome after Shigella infection: 3 year follow-up study]. Korean J Gastroenterol. 2006;47:300-305. [PubMed] |
| 17. | Wang H, Chang L. The Walkerton outbreak revisited at year 8: predictors, prevalence, and prognosis of postinfectious irritable bowel syndrome. Gastroenterology. 2011;140:726-78; discussion 726-78;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Hanevik K, Dizdar V, Langeland N, Hausken T. Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol. 2009;9:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Thabane M, Marshall JK. Post-infectious irritable bowel syndrome. World J Gastroenterol. 2009;15:3591-3596. [PubMed] |
| 20. | Villani AC, Lemire M, Thabane M, Belisle A, Geneau G, Garg AX, Clark WF, Moayyedi P, Collins SM, Franchimont D. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138:1502-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 22. | Posserud I, Ersryd A, Simrén M. Functional findings in irritable bowel syndrome. World J Gastroenterol. 2006;12:2830-2838. [PubMed] |
| 23. | Small PK, Loudon MA, Hau CM, Noor N, Campbell FC. Large-scale ambulatory study of postprandial jejunal motility in irritable bowel syndrome. Scand J Gastroenterol. 1997;32:39-47. [PubMed] |
| 24. | Ng C, Danta M, Kellow J, Badcock CA, Hansen R, Malcolm A. Attenuation of the colorectal tonic reflex in female patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2005;289:G489-G494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology. 2005;129:131-141. [PubMed] |
| 26. | Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226-G232. [PubMed] |
| 27. | Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX, Bian ZX. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J Gastroenterol. 2011;46:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7-14. [PubMed] |
| 29. | Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 30. | Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [PubMed] |
| 33. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 357] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 34. | Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 36. | Walker MM, Talley NJ, Prabhakar M, Pennaneac’h CJ, Aro P, Ronkainen J, Storskrubb T, Harmsen WS, Zinsmeister AR, Agreus L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] |
| 38. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 597] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 39. | O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O’Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449-457. [PubMed] |
| 40. | Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116:207-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Park JH, Rhee PL, Kim HS, Lee JH, Kim YH, Kim JJ, Rhee JC. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | Long Y, Wang W, Wang H, Hao L, Qian W, Hou X. Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome. J Gastroenterol Hepatol. 2012;27:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nuñez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 45. | Sasakawa C. A new paradigm of bacteria-gut interplay brought through the study of Shigella. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:229-243. [PubMed] |
| 46. | Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 500] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 48. | Miao EA, Rajan JV. Salmonella and Caspase-1: A complex Interplay of Detection and Evasion. Front Microbiol. 2011;2:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | Hickey TE, McVeigh AL, Scott DA, Michielutti RE, Bixby A, Carroll SA, Bourgeois AL, Guerry P. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun. 2000;68:6535-6541. [PubMed] |
| 51. | Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 546] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 52. | O'Hara JR, Feener TD, Fischer CD, Buret AG. Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodium-induced colitis in mice. Infect Immun. 2012;80:1563-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Kalischuk LD, Leggett F, Inglis GD. Campylobacter jejuni induces transcytosis of commensal bacteria across the intestinal epithelium through M-like cells. Gut Pathog. 2010;2:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009;1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Sharma R, Tesfay S, Tomson FL, Kanteti RP, Viswanathan VK, Hecht G. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G685-G694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Savkovic SD, Koutsouris A, Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160-C1167. [PubMed] |
| 57. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [PubMed] |
| 58. | Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 59. | Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641-4649. [PubMed] |
| 60. | Bashashati M, Rezaei N, Andrews CN, Chen CQ, Daryani NE, Sharkey KA, Storr MA. Cytokines and irritable bowel syndrome: where do we stand. Cytokine. 2012;57:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, Quigley EM. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 62. | Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Guttman JA, Samji FN, Li Y, Vogl AW, Finlay BB. Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect Immun. 2006;74:6075-6084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Lapointe TK, O’Connor PM, Buret AG. The role of epithelial malfunction in the pathogenesis of enteropathogenic E. coli-induced diarrhea. Lab Invest. 2009;89:964-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 66. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 67. | Roxas JL, Koutsouris A, Bellmeyer A, Tesfay S, Royan S, Falzari K, Harris A, Cheng H, Rhee KJ, Hecht G. Enterohemorrhagic E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in a Shiga toxin independent manner. Lab Invest. 2010;90:1152-1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Flynn AN, Wang A, McKay DM, Buret AG. Apoptosis-inducing factor contributes to epithelial cell apoptosis induced by enteropathogenic Escherichia coli. Can J Physiol Pharmacol. 2011;89:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | O'Brien AO, Lively TA, Chen ME, Rothman SW, Formal SB. Escherichia coli O157: H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet. 1983;1:702. [PubMed] |
| 70. | Manjarrez-Hernandez HA, Baldwin TJ, Williams PH, Haigh R, Knutton S, Aitken A. Phosphorylation of myosin light chain at distinct sites and its association with the cytoskeleton during enteropathogenic Escherichia coli infection. Infect Immun. 1996;64:2368-2370. [PubMed] |
| 71. | Philpott DJ, McKay DM, Mak W, Perdue MH, Sherman PM. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect Immun. 1998;66:1680-1687. [PubMed] |
| 72. | Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873-1882. [PubMed] |
| 73. | Wensaas KA, Langeland N, Hanevik K, Mørch K, Eide GE, Rortveit G. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut. 2012;61:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 74. | Cotton JA, Beatty JK, Buret AG. Host parasite interactions and pathophysiology in Giardia infections. Int J Parasitol. 2011;41:925-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 75. | Teoh DA, Kamieniecki D, Pang G, Buret AG. Giardia lamblia rearranges F-actin and alpha-actinin in human colonic and duodenal monolayers and reduces transepithelial electrical resistance. J Parasitol. 2000;86:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Buret AG, Mitchell K, Muench DG, Scott KG. Giardia lamblia disrupts tight junctional ZO-1 and increases permeability in non-transformed human small intestinal epithelial monolayers: effects of epidermal growth factor. Parasitology. 2002;125:11-19. [PubMed] |
| 77. | Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 78. | Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 79. | Navarro-García F, Canizalez-Roman A, Sui BQ, Nataro JP, Azamar Y. The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli. Infect Immun. 2004;72:3609-3621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, Mair K, Krueger D, Pruteanu M, Shanahan F. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology. 2011;141:959-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 81. | Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 466] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 82. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [PubMed] |
| 83. | Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349-357. [PubMed] |
| 84. | Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 85. | Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 86. | Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 87. | Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 1036] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 89. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691-14696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3584] [Cited by in RCA: 4135] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 90. | Salonen A, de Vos WM, Palva A. Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology. 2010;156:3205-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 91. | Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 790] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 92. | Carroll IM, Ringel-Kulka T, Keku TO, Chang YH, Packey CD, Sartor RB, Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799-G807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 93. | Pimentel M, Chatterjee S, Chang C, Low K, Song Y, Liu C, Morales W, Ali L, Lezcano S, Conklin J. A new rat model links two contemporary theories in irritable bowel syndrome. Dig Dis Sci. 2008;53:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 95. | Andre G, Buret SVA, Feener T, McKnight G, Wallace J, Rioux K, Beatty JK. Campylobacter Jejuni- or Giardia duodenalis-Mediated Disruptions of Human Intestinal Microbiota Biofilms: Novel Mechanisms Producing Post-Infectious Intestinal Inflammatory Disorders. Gastroenterology. 2013;144:s-309. |
| 96. | Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. [PubMed] |
| 97. | Cotter PD, Stanton C, Ross RP, Hill C. The impact of antibiotics on the gut microbiota as revealed by high throughput DNA sequencing. Discov Med. 2012;13:193-199. [PubMed] |
| 98. | Spence MJ, Moss-Morris R. The cognitive behavioural model of irritable bowel syndrome: a prospective investigation of patients with gastroenteritis. Gut. 2007;56:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 99. | Han B. Correlation between gastrointestinal hormones and anxiety-depressive states in irritable bowel syndrome. Exp Ther Med. 2013;6:715-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 101. | O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl). 2011;214:71-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 301] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
P- Reviewers: Jadallah KA, Kohen R, Sinagra E S- Editor: Qi Y L- Editor: A E- Editor: Ma S