Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.3889
Revised: November 23, 2013
Accepted: March 5, 2014
Published online: April 14, 2014
Processing time: 172 Days and 17.6 Hours
Gastric cancer is one of the leading causes of death for cancer worldwide, although geographical variations in incidence exist. Over the last decades, its incidence and mortality have gradually decreased in Western countries, while these have increased, or remained stable, in the other world regions. Gastric cancer is often diagnosed at an advanced stage, with the only notable exception of Japan, where nationwide screening programs are enforced, due to local high incidence. Curative- intent surgery (i.e., gastrectomy, total or partial, and lymphadenectomy) remains the cornerstone of treatment of gastric cancer. Much has been debated about the extent of lymph node dissection and, although it is a valuable contribution to staging and cure, operative treatment only represents one aspect of overall effective management, as the risk of both locoregional and distant recurrences are high, and bear a poor prognosis. As a matter of fact, surgery, as a single modality treatment, has probably achieved its maximum efficacy for local control and survival, while other accompanying nonsurgical treatment modalities have to be taken into account, although their role is still the subject of considerable debate. The authors in this review present an update on the outcome of treatment of gastric cancer in relation to the extent of lymphadenectomy and of various nonsurgical preoperative, intraoperative, and postoperative strategies.
Core tip: The authors in this review present an update on treatment of gastric cancer in relation to the role of extent of lymphadenectomy and of new nonoperative strategies, to employ preoperatively, intraoperatively, and postoperatively. The above therapeutical options are assessed by reviewing the most authoritative, large, and referenced randomised controlled trials and meta-analyses published in the English literature.
- Citation: Giuliani A, Miccini M, Basso L. Extent of lymphadenectomy and perioperative therapies: Two open issues in gastric cancer. World J Gastroenterol 2014; 20(14): 3889-3904
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/3889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.3889
Gastric cancer is one of the most common malignancies in the world. Over the last decades, its incidence and mortality have gradually decreased in Western countries, while these have increased, or remained stable, in the other world regions. In countries with a higher incidence, nationwide endoscopic screening programs lead to earlier identification of a large number of gastric cancers, while, in Western countries, the lack of similar screening programs often causes later diagnoses.
Surgery with lymphadenectomy is the best treatment option for resectable gastric cancer, as it provides better results both in terms of progression-free survival and prognosis. Long-term survival after radical surgery strongly depends on the stage of the tumor. The resection of tumors limited to the mucosa shows a survival rate of 90%-95%[1-3]. On the other hand, progression of the disease through the gastric wall and/or through regional lymph nodes shows higher recurrence rates of the disease, with five-year survival rates lower than 30% in Western countries[3]. Over the last decades, this has led to the development of further treatment options, such as extension of lymphadenectomy, and the use of pre-, intra-, and postoperative chemoradiotherapy. Indication, timing, and effectiveness of these options, however, are still controversial. The aim of this paper is to report on the current views on treatment of gastric cancer, and to possibly clarify the topics introduced above. Data from randomized controlled trials (RCT) and meta-analysis are reported. RCTs are considered the gold standard of all research design, while meta-analysis provide a comprehensive up-to-date summary of the average effect of all the relevant RCTs and are a reliable guidance for clinical practice and future research.
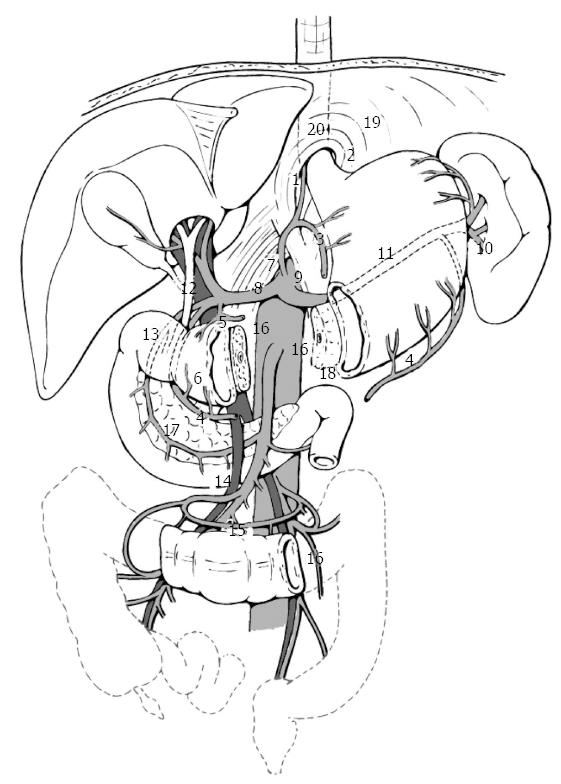
Gastric carcinoma shows a high tendency to lymph node metastasis. The risk of regional nodal involvement increases with deep penetration through the gastric wall[4], and the nodal extension of the cancer takes place gradually, radiating from primary location via the lymphatic system[5,6]. Nodal metastases are observed in 3%-5% of gastric carcinomas which are limited to the mucosa, in 11%-25% of those which extend to the submucosa, in 50% of those which reach the muscularis (T2), and in 83% of those which extend to the serosa (T3)[7,8]. After curative radical resection, local recurrence is represented, in 87.5% of cases, by nodal metastases to local or regional lymph node stations[9]. The Japanese Classification of Gastric Carcinoma (Japanese Gastric Cancer Association, JGCA, 1998)[10] has defined 16 different lymph node stations (n) which drain the stomach. These are subdivided in three levels, according to their distance from the tumor (Figure 1, Table 1), thus entailing three types of lymph node dissection (D) that can be associated to total or partial gastrectomy: D1, in which perigastric lymph nodes from n1 to n6 are removed (N1 level); D2, in which perigastric lymph nodes are removed as well as those located along the main arterial vessels from n7 to n12 (N2 level); D3, in which stations n13 to n16 are removed, as well as those mentioned before (N3 level) (Table 2). During the ‘60s, the Japanese authors first introduced D2 lymphadenectomy[11], which they still consider as the standard procedure to associate with curative gastric resection, as it yields the best results in terms of local recurrence and of long term survival. In Western countries, D2 lymph node dissection is not as common as Japan, not only due to lower incidence of gastric cancer (and, consequently, to the fact that surgeons have less experience with this technique), but mainly to high morbidity and mortality linked to this type of lymph node dissection. Indeed, D2-D3 lymphadenectomy is a challenging surgical procedure, which implies a thorough surgical training conducted under the supervision of experienced gastric surgeons[12]. A significant difference between Japanese clinical outcomes and those of other countries has been observed in short- and long-term results and in loco-regional control, with better results for Japanese clinical records. The systematic lymph node dissection practiced since the ‘60s-‘70s by Japanese surgeons may have contributed to achieve better results[13]. The International Union Against Cancer (UICC)[14] adopted in 1997 a new classification system for lymph node metastases, which, unlike the Japanese system, was not based on the anatomic location of positive nodes[15], but on their number. It was recommended that at least 15 lymph nodes should be removed and examined for proper staging. Secondaries affecting 1 to 6 lymph nodes were classified as pN1, from 7 to 15 as pN2, more than 15 as pN3. It has been shown[16] that lymph node removal was much higher with D2 resection (more than 25 lymph nodes) than with D1 (less than 25 lymph nodes), and that survival is related to the number of lymph nodes with metastasis[17-19]. The extent of lymphadenectomy, therefore, has always been controversial. Most Western surgeons criticize D2 dissection because some benefits have only been confirmed in retrospective observations[20]. RCTs, mainly conducted by Western authors, since the ‘80s compare short-term and long-term results in D1 and D2 resections. Dent performed a RCT on 43 cases[21], and the group who received D2 resection (21 patients), showed worse results compared to the D1 lymphadenectomy group (22 patients) in terms of duration of surgery, blood transfusion requirement, postoperative morbidity, and duration of hospital stay. Four patients in D2 group required reoperation; in both groups there were no postoperative deaths. There was no difference in the probability of survival at a median follow-up of 3 years. In the mid-‘90s, the Dutch Gastric Cancer Trial[22] and the Medical Research Council (MRC) Gastric Cancer Surgical Trial (STO1)[23] published the morbidity and mortality results of two multicentric RCTs. The studies, conducted, respectively, on 711 and 400 patients receiving curative D1 or D2 resection for advanced gastric carcinoma, showed a higher incidence of postoperative complications and mortality and longer duration of hospitalization in patients treated with more extended lymph node dissection. Both studies also showed that the higher number of postoperative complications and mortality observed in the D2 group were not linked to the extent of the lymphadenectomy as such, rather to associated pancreatectomy and/or splenectomy. The protocol of D2 lymph node dissection, in case of total gastrectomy, included these resections[10,24] in order to remove all lymph nodes in stations 10 and 11 (Table 1). In the late ‘90s[25,26], the same authors reported the long-term survival data. Both trials showed that, 5 years after surgery, the survival rate, risk of relapse, risk of death for cancer and duration of disease free survival were not significantly different in D1 and D2 groups. However, in the MRC STO1 trial[26], a better long-term survival was observed in patients receiving D2 gastrectomy without pancreatectomy and/or splenectomy. On the basis of these data, the British National Health Service Cancer Guidance in 2001 discouraged the use of D2 resection in routine clinical practice[27]. On the other hand, lymphadenectomies even more extended than D2 have been performed since the ‘80s in several specialized Japanese centers, on the grounds that lymph node para-aortic metastases (N3) were frequently observed (20%-30% of cases)[28,29]. Some Japanese authors (JCOG 9501)[30] published in 2004 a multicentric RCT on 523 patients receiving surgical treatment for gastric cancer, comparing short-term results in D2 standard and in D2 extended to para-aortic lymph nodes. In the extended para-aortic lymphadenectomy group, duration of surgery and intraoperative blood loss were significantly higher (P = 0.0001), while mortality and reoperation rates were not statistically different compared to standard D2 group. However, morbidity in the more extended surgery group was slightly higher than in the standard group (P = 0.067). Uni- and multivariate analysis later conducted by the same authors[31], showed that key factors for complications were: age > 56 (P = 0.026), associated pancreatectomy (P = 0.004), duration of surgery > 297 min (P = 0.045) and a body mass index (BMI) ≥ 25 (P = 0.002). The long-term results of the same trial[32] were published in 2008, showing no significant differences between standard and extended para-aortic lymphadenectomy in terms both of 5-year overall survival (69.2% vs 70.3%) and of 5-year disease-free survival (62.6% vs 61.7%). Interestingly, extended D2 resection only showed better results in terms of 5-year survival in patients without lymph node metastasis (HR for death 0.39; P = 0.009), while it resulted pointless in those with lymph node metastases (HR for death 1.39; P = 0.04). Based on these data, standard D2 resection was judged as adequate by the authors for patients with potentially curable advanced gastric carcinoma. Short-[33] and long-term[34] results of a comparative RCT between D1 and D3 (the D3 definition reported in[34] did not include para-aortic lymph nodes), conducted on 221 patients who received curative surgery in a single-institution (Taipei Veterans General Hospital, Taiwan) were reported in 2004 and 2006. No cases of operative mortality were observed in the two groups. Duration of surgery, blood loss, blood transfusion required, volume of abdominal drainage, duration of postoperative stay (P = 0.001), and number of surgical complications (P < 0.001) resulted higher in D3 group. The incidence of complications was higher (P = 0.017) in patients receiving resection with distal pancreatectomy and splenectomy. Overall 5-year survival was higher in D3 group (59.5% vs 53.6%, P = 0.041), while 5-year recurrences after R0 (radical resection) showed no difference (50.6% in D1 group and 40.3% in D3 group, P = 0.197). The authors concluded that D3 dissection improves survival rates, and suggested that it should be performed in specialized centers in order to limit the chance of postoperative complications. A RCT conducted by the East Asia Surgical Oncology Group in 2008[35] compared data of 135 patients treated with D2 gastrectomy with those of 134 patients receiving D4 gastrectomy (in D4 dissection inter-, pre-, and latero-aortic lymph nodes of abdominal aorta as far as bifurcation are removed). No significant advantages were observed in terms of 5-year survival in patients who received extended lymphadenectomy (P = 0.80). Twelve patients of D4 group with metastases to para-aortic lymph-nodes had a median survival of 2.8 years, and a 5-year survival rate of 25%. The authors maintained that D4 dissection is not the best treatment option for patients with gastric carcinoma, whereas D2 dissection is recommended if performed by experienced surgeons. The Dutch Gastric Cancer Group Trial[36], published in 2004, updated data on survival of 711 patients previously enrolled in published RCTs[22,25]. At a median follow-up of 11 years, survival rates were 30% in D1 group and 35% in D2 group (P = 0.53), the risk of recurrence was 70% and 65%, respectively (P = 0.43). The authors concluded that D2 lymph node dissection can be recommended only if operative morbidity and mortality can be reduced. A further update of these data was published in 2010[37], with a median follow-up of 15.2 years. The overall 15-year survival was 21% after D1 resection and 29% after D2 resection (P = 0.34). Gastric cancer-related mortality rates resulted significantly higher in D1 than in D2 (41% vs 37%; P = 0.01). The incidence of local recurrence (D1 = 22% vs D2 = 12%) and distant recurrence (D1 = 19% vs D2 = 13%) were different, albeit not significantly. Patients who received splenectomy and pancreatectomy had significantly lower overall survival rates in both D2 and D1 groups. On the other hand, patients who received D2 resection without pancreatico-splenectomy had a significantly higher overall 15-year survival compared to patients receiving D1 resection (35% vs 22%, P = 0.006). The authors concluded that D2 resection should be considered the standard procedure to treat resectable gastric carcinoma. The Italian Gastric Cancer Study Group[38] in 2010 published a multicentric RCT on 267 patients, comparing the short-term results of D1 and D2 gastrectomy for curable gastric cancer. Pancreatico-splenectomy was not considered as a routine part of the D2 gastrectomy and spleen and pancreas were removed only when indicated by the surgeon. The study did not show significant differences in terms of operative mortality, morbidity and duration of postoperative hospital stay. The authors concluded that D2 gastrectomy is a safe option to treat gastric carcinoma of Western patients as well, if it is performed in specialized centers. Three meta-analyses of RCTs evaluating D1 vs D2 vs D3 lymphadenectomy for operable gastric carcinoma were conducted in 2009[39], 2011[40], and 2012[41]. These three studies examined 14, 6 and 5 RCTs, totaling 3432, 1876 and 1642 patients, respectively. The 2009 meta-analysis[39], conducted on Western and Asiatic trials, compared D1 and D2 dissections and D2 and D3 dissections. In the first comparison, duration of surgery, operative mortality and postoperative complications resulted significantly lower in D1 dissection than in D2 (P = 0.00001, P < 0.001, P < 0.001), while 3- and 5-year survival rates did not show significant differences. No substantial differences were found between D2 and D3 dissections in relation to operative mortality, postoperative morbidity, operative time, and hospital stay. The meta-analysis of 2011[40] also showed better results for D1 dissections, with shorter duration of postoperative hospitalization (P = 0.0036), lower incidence of mortality (P = 0.0054), complications (P = 0.0002), anastomotic dehiscence (P = 0.0001), and reoperations (P = 0.006). However, no differences were observed in 5-year survival (P = 0.76). The meta-analysis of 2012[41] confirmed a higher incidence of mortality and reoperations after D2 resection compared to D1 (P = 0.02 and P < 0.0001 respectively). The hospital mortality was significantly higher for D2 resections performed before 1995 (P = 0.0003), while after that date hospital mortality was no longer different between groups (P = 0.70). A further analysis showed that the difference in hospital mortality was related to associated distal pancreas and/or spleen removal. Patients in D2 group with spleen preservation had significantly lower hospital mortality than those who had their spleen resected (P < 0.0001). Overall 5-year survival was not significantly different in the two types of lymph node dissection (P = 0.58). Main data regarding the extent of nodal dissection are shown in Table 3.
| No. 1 Right paracardial LN |
| No. 2 Left paracardial LN |
| No. 3 LN along the lesser curvature |
| No. 4 LN along greater curve (short gastric vessels, left and right gastroepiploic vessels) |
| No. 5 Suprapyloric LN |
| No. 6 Infrapyloric LN |
| No. 7 LN along the left gastric artery |
| No. 8 LN along the common hepatic artery (anterosuperior and posterior group) |
| No. 9 LN around the celiac artery |
| No. 10 LN at the splenic hilum |
| No. 11 LN along the splenic artery (proximal and distal tract) |
| No. 12 LN in the hepatoduodenal ligament (along hepatic artery, bile duct and portal vein) |
| No. 13 LN retropancreatic |
| No. 14 LN along superior mesenteric vessels (vein and artery) |
| No. 15 LN along the middle colic vessels |
| No. 16 LN paraaortic (of upper, middle and lower abdominal aorta, in relation to the intragastric tumor site) |
| The classification includes also the following lymph node compartments: |
| No. 17 LN on the anterior surface of the pancreatic head |
| No. 18 LN along the inferior margin of the pancreas |
| No. 19 Infradiaphragmatic LN1 |
| No. 20 LN in the esophageal hiatus of the diaphragm1 |
| No. 110 Paraesophageal LN in the lower thorax1 |
| No. 111 Supradiaphragmatic LN1 |
| No. 112 Posterior mediastinal LN1 |
| Tumor site | LN D1 dissection | LN D2 dissection | LN D3 dissection |
| Upper stomach | 1, 2, 3, 4 | 4, 7, 8, 9, 10, 11 | 5, 6, 8, 12, 16 |
| Middle stomach | 1, 3, 4, 5, 6 | 7, 8, 9, 11, 12 | 4, 8, 10, 11, 12, 13, 14, 16 |
| Lower stomach | 3, 4, 5, 6 | 1, 7, 8, 9, 11, 12, 14 | 4, 8, 12, 13, 16 |
| Ref. | Study design | No. of patients | Post-operative mortality (P value) | Post-operative morbidity (P value) | Survival (P value) | Recurrence (P value) |
| Dent et al[21], 1988 | RCT D1 vs D2 | D1: 22; D2: 21 | None | D1 < D2 (nv) | At 3 yr (NS) | - |
| Bonenkamp et al[22], 1995 Dutch D1D2 trial | RCT D1 vs D2 | D1: 380; D2: 331 | D1 < D2 (0.004) | D1 < D2 (0.001) | - | - |
| Cuschieri et al[23], 1996 | RCT D1 vs D2 | D1: 200; D2: 200 | D1 < D2 (0.04) | D1 < D2 (0.001) | - | - |
| MRC ST01 | ||||||
| Bonenkamp et al[25], 1999 | RCT D1 vs D2 | D1: 380; D2: 331 | - | - | At 5 yr D1 vs D2 (NS) | At 5 yr D1 vs D2 (NS) |
| Dutch D1D2 trial | ||||||
| Cuschieri et al[26], 1999 | RCT D1 vs D2 | D1: 200; D2: 200 | - | - | At 5 yr D1 vs D2 (NS) | At 5 yr D1 vs D2 (NS) |
| MRC ST01 | ||||||
| Sano et al[30], 2004 | RCT D2 vs D3 | D2: 263; D3: 260 | D2 vs D3 (NS) | D2 < D3 (NS) | - | - |
| JCOG Study 9501 | ||||||
| Sasako et al[32], 2008 | RCT D2 vs D3 | D2: 263; D3: 260 | - | - | At 5 yr D2 vs D3 (NS) | At 5 yr D2 vs D3 (NS) |
| JCOG Study 9501 | ||||||
| Wu et al[33], 2004 | RCT D1 vs D3 | D1: 110; D3: 111 | None | D1 < D3 (0.012) | - | - |
| Wu et al[34], 2006 | RCT D1 vs D3 | D1: 110; D3: 111 | - | - | At 5 yr D1 < D3 (0.041) | At 5 yr D1 vs D2 (NS) |
| Hartgrink et al[36], 2004 | RCT D1 vs D2 | D1: 380; D2: 331 | - | - | At 11 yr D1 vs D2 (NS) | At 11 yr D1 vs D2 (NS) |
| Dutch D1D2 trial | ||||||
| Songun et al[37], 2010 | RCT D1 vs D2 | D1: 380; D2: 331 | - | - | At 15 yr D1 vs D2 (NS) | At 15 yr D1 > D2 (0.005) |
| Dutch D1D2 trial | ||||||
| Degiuli et al[38], 2010 | RCT D1 vs D2 | D1: 133; D2: 134 | D1 vs D2 (NS) | D1 vs D2 (NS) | - | - |
| IGCSG | ||||||
| Yang et al[39], 2009 | Meta-analysis | D1: 907; D2: 875 | D1 < D2 (0.001) | D1 < D2 (0.0001) | At 3 and 5 yr D1 vs D2 (NS) | - |
| D1 vs D2 | ||||||
| Meta-analysis D2 vs D3 | D2: 599; D3: 588 | D2 vs D3 (NS) | D2 vs D3 (NS) | - | - | |
| Memon et al[40], 2011 | Meta-analysis D1 vs D2 | D1: 946; D2: 930 | D1 < D2 (0.005) | D1 < D2 (0.0002) | At 5 yr D1 vs D2 (NS) | - |
| Seevaratnam et al[41], 2012 | Meta-analysis | D1: 845; D2 797 | D1 < D2 (0.002) | D1 < D2 (0.0001) | At 5 yr D1 vs D2 (NS) | - |
| D1 vs D2 |
In conclusion, in Western countries the prognostic value of D2 lymphadenectomy is still controversial, while in Eastern countries it is considered a standard procedure, likely to be further extended. Japanese authors do not even conduct RCT comparing D1 and D2 lymphadenectomies, on the grounds that they consider D1 dissection unethical. Data indicate that D2 dissection is an adequate and potentially beneficial staging and treatment approach if operative mortality is avoided. Dissections extended to para-aortic lymph nodes does not show significant advantages in terms of survival. Splenectomy and distal pancreatectomy increase operative morbidity and mortality. D2 dissection is considered a difficult procedure, and should be performed by experienced surgeons in specialized centers. Authors suggest that a surgeon should perform at least 200 gastrectomies under the supervision of an experienced surgeon before he can perform D2 lymph node dissections with acceptable morbidity and mortality rates[12]. In Western countries, due to the lower incidence of gastric carcinoma, a surgeon is very unlikely to achieve such an experience.
In Western countries, the 5-year survival rates for advanced gastric carcinoma treated with potentially curative surgery range between 25% and 30%[16]. Recurrences occur in the abdomen in 40%-60% of the cases, both as the only site and as part of a systemic diffusion of disease[9,42,43]. The most frequent abdominal sites of recurrence are the area previously occupied by the tumor, the anastomosis and the non-resected regional lymph nodes. These data show that surgery, as a single modality treatment, cannot detect and remove the satellite micrometastases around the primary tumor, nor the tumor cells disseminated during the operative maneuvers. Pre-, intra- and post-operative treatments have been developed in the last decades, in order to improve loco-regional control of disease and long term survival.
Adjuvant chemoradiotherapy: Adjuvant treatments for gastric carcinoma have been employed since the ‘70s[44-46], on the assumption that if surgery alone could not cure the disease, as shown by the high incidence of local and distant recurrences, adjuvant treatments could improve the outcomes by acting on the remaining tumor. Theoretically, adjuvant treatments should eradicate cancer cells already metastasized prior to surgery or accidentally disseminated during surgery. Therefore, the level of surgical radicality or the residual tumor after surgery can affect the results of adjuvant therapies. Furthermore, these treatments do not show significant benefits compared to surgery alone for early stage tumors (T1, N0)[10], in which surgery is likely to achieve the cure[47]. The results of one of the first RCTs on this issue were published in 1984[44]. The study was conducted on 62 patients receiving resective surgery for poor-prognosis cardia and gastric adenocarcinoma. The studied population was randomized to either surgery alone (23 patients) or to surgery with adjuvant treatment [intravenous (iv) 5-fluorouracil plus radiotherapy for 4-5 wk: 39 patients]. The 5 year survival rate was 23% in the experimental group, while it only reached 4% in controls (P < 0.052). Patients of the experimental group had a significantly longer postoperative disease-free period (P = 0.02) and lived longer than controls (P = 0.012). A lower incidence of loco-regional recurrence in experimental group was observed (39% vs 54%), but it did not reach statistical significance. The British Stomach Cancer Group published preliminary and final data of a RCT, in 1989[45] and in 1994[46], respectively, which included 436 patients resected for gastric carcinoma, randomized to either receive surgery alone (145 patients), surgery with radiotherapy (4500-5000 Gy in 25 fractions, 153 patients), or surgery followed by chemotherapy (iv 5-fluorouracil, adriamicin, mitomycin C given intravenously, for eight cycles: 138 patients). The tumors were at stage II-IV, in stage IV were included also local non-radical resections. The median time to randomization was 13 d, with a range of 1-81 d. The chemotherapy protocol was completed by 42% of patients of that group, while the radiotherapy protocol was completed by 102 of the 117 patients (87.2%) who had been randomized for adjuvant radiotherapy. The protocols were not completed for the following reasons: worsening postoperative health status, withdrawal of consent, gastrointestinal, hematological and biochemical alterations due to the adjuvant treatments. The median overall survival was 15 mo, significantly influenced by the stage of primary lesion (P < 0.0001). The overall survival did not differ significantly in the three randomized groups (P = 0.07), and the 5-yearsurvival of the two experimental groups was not significantly different from that of the control group. Clinical recurrence in the area of the stomach or in regional lymph nodes was significantly lower (P < 0.01) in the two experimental groups. The milestone of adjuvant chemoradiotherapy treatment, however, is the multicentric RCT of Intergroup 0116 (SWOG 9008)[42], whose data were published in 2001. This study examined 556 patients who received curative surgery [only R0 resections were considered, while macroscopic (R2) and microscopic (R1) residual disease in relation to surgical treatment or to resulting pathology report of the removed specimen were excluded] for gastric carcinoma at stage IB-IVM0[48]. AD2 lymphadenectomy was recommended, but it was performed in 10% of the cases, 36 % had a D1 dissection, and 54% had a D0 lymphadenectomy (not all perigastric lymph nodes were removed). After gastrectomy 281 patients were randomized for experimental adjuvant treatment with chemotherapy (iv 5-fluorouracil and leucovorin) and loco-regional radiotherapy (area of the stomach bed and regional lymph nodes, 4500 cGy), while the other 275 patients received surgery alone. Of the 281 patients assigned to the experimental group, 64% completed the protocol treatment, while 17% did not, because of hematological and gastrointestinal side effects. Other reasons for suspending treatment were disease progression and withdrawal of consent. Before and after radiotherapy deviations from protocol were observed in 40% of cases. After a median follow-up period of 5 years, the median survival period in the experimental group was 36 mo, compared to 27 mo in controls. The 3-year survival rate was higher in the experimental group (50% vs 41%). The HR for death in controls, compared to the experimental group, was 1.35 (P = 0.005); the HR for recurrence was also higher in the control group than in the experimental group: 1.52 (P < 0.001). The median duration of recurrence-free survival was 30 mo in the experimental group and 19 mo in controls. The 3-year recurrence-free survival rate was 48% in the experimental group and 31% in controls. Recurrence was observed in 64% of patients in the control group and in 43% of patients in the experimental group. The incidence of both local and regional recurrence was lower in the experimental group (19% vs 29% and 65% vs 72%, respectively). The study showed that postoperative loco-regional radiotherapy and systemic chemotherapy significantly improved both overall and recurrence-free survival. In relation to these results, adjuvant chemoradiotherapy for gastric carcinoma has become common, although the Intergroup 0116 trial was criticized as the adjuvant treatment was considered a form of “compensation” for the type of employed lymphadenectomy. This criticism seems justified, considering the results of a retrospective study published in 2010[49] which examined survival and recurrence data of 91 patients receiving macroscopic radical gastrectomy with at least D1 lymphadenectomy (perigastric lymph nodes), for gastric carcinoma at stage Ib-IV (AJCC)[50] followed by radiotherapy (on gastric area, anastomosis, and regional lymph nodes) combined with different chemotherapy schedules (fluorouracil and leucovorin, capecitabine alone or capecitabine and cisplatin). The control group included 694 patients from the Dutch Gastric Cancer Group Trial[25,36] who were randomized between D1 (369 patients) and D2 lymphadenectomy (325 patients). The characteristics of the studied population differed significantly in sex, age, parietal extension of the tumor, lymph node involvement, histological type of tumor, radicality of surgery, and extension of lymphadenectomy. At the time of analysis, the median follow-up for the experimental group was 19 mo, while that of controls was 51 mo. Over a 24-mo period, local recurrence was significantly lower in the experimental group (HR = 3.23; P = 0.0015). This was especially due to the high incidence of local recurrence after D1 resections in controls, compared to D1 resections of the experimental group (HR = 11.1; P = 0.001). The D2 resections in the experimental group and in the controls showed no significant differences in terms of local recurrence. Survival at 24-mo was not significantly different in the experimental group and in the controls, both overall and in D1 and D2 resections. The outcomes were significantly better in the experimental group than in controls in relation to overall survival at 24 mo after R1 resection (HR = 2.91; P = 0.002) and to local recurrence after R1 (HR = 5.36; P = 0.02) and after R0 (HR = 2.53; P = 0.03).
The results of an observational study on the efficacy of adjuvant chemoradiotherapy after D2 gastrectomy for operable gastric carcinoma were published in 2005[51]. The experimental group included 544 patients radically resected (R0), receiving the same adjuvant treatment performed in Intergroup 0116 trial (SWOG-9008)[42]. The controls comprised 446 patients who received the same surgery as the experimental group. In the experimental group there was a higher incidence of undifferentiated carcinomas (P = 0.0021), and of stage IIIA (P = 0.005) and stage IV (P = 0.0011) (AJCC)[50] carcinomas than in controls. The treatment protocol was completed in 75.2% of the experimental cases. Forty three percent of patients in the experimental group and 49.8% in the control group had died at a median follow-up of 66 mo. The median duration of overall survival in the experimental group was significantly longer than in controls (95.3 mo vs 62.6 mo), with a HR for death of 0.80 (P = 0.02) in the experimental group, which entails a 20% reduction of the death risk. Five-year survival was significantly higher in the experimental group than in controls (57.1% vs 51%; P = 0.01). The survival benefit of adjuvant treatment was observed for all stages of gastric carcinoma and the average duration of recurrence-free survival was higher in the experimental group (75.6 mo vs 52.7 mo; HR for recurrence 0.80; P = 0.016). The probability of recurrence-free survival at 5 years was 54.5% in the experimental group and 47.9% in controls (P = 0.01). The HR for recurrence was better in the experimental group for all stages of gastric carcinoma, and in both groups distant recurrences prevailed (37.7%). The incidence of loco-regional recurrence within the radiation field was lower in the experimental group than in the surgery-alone group (14.9% vs 21.7%; P = 0.005). The Intergroup 0116 (SWOG 9008) in 2009[52] and in 2012[53] published the updated results of the earlier 2001 RCT[42] at a median follow-up of more than 10 years. The HR for overall survival and the HR for relapse-free survival were still better in the experimental group (HR = 1.32, P = 0.004; HR = 1.51, P = 0.001 respectively) than in controls. Recurrence rates were significantly lower (P < 0.001) in the experimental group. Furthermore, diffuse histotype tumors, which are more frequent in women, had lower response to adjuvant treatment. A meta-analysis published in 2007[54] was aimed to determine if there was any benefit of employing adjuvant chemoradioterapy, compared to surgery alone. The five RCTs analyzed included 868 patients, 444 in the experimental group and 424 in the controls. The adjuvant treatment protocol was not completed in 26.7% of patients due to hematological and gastrointestinal toxicity. The experimental group showed a 5-year OR for mortality significantly lower than the control group (0.45; P = 0.00001). The authors comment that the benefits of adjuvant chemoradiotherapy treatment may outweigh the risks in patients with a high probability of local and distant recurrence, while the risks outweigh the benefits in patients with low probability of local and distant failure. The results of a multicentric trial of the Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST)[55] were published in 2011. The study included 458 patients who received curative D2 gastrectomy for cancer and randomized to receive two types of adjuvant treatment after surgery. One group (226 patients) was assigned to receive chemotherapy (capecitabine and cisplatin for 6 cycles), while the other group (230 patients), was assigned to receive chemoradiotherapy (capecitabine and cisplatin for 2 cycles, then radiotherapy with capecitabine for 5 wk and capecitabine and cisplatin for 2 cycles). The adjuvant protocol was completed by 75.4% of patients in the chemotherapy group and by 81.7% of patients in the chemoradiotherapy group. Gastrointestinal and hematological toxicity of grade 3-4 was observed in both groups with similar rates, while neutropenia was more frequent in the chemioradiotherapy group (43.6% vs 35%). After a median follow-up of 53.2 mo, these authors observed 72 cases of recurrence in the chemotherapy group and 55 cases in the chemoradiotherapy group (P = NS). No significant statistical differences were observed in loco-regional and distant recurrence rates within the two populations. The 3-year disease-free survival rates were 78.2% in the chemoradiotherapy group and 74.2 in the chemotherapy group (P = 0.086).
In relation to patients with lymph node metastases, disease-free survival was longer in the chemoradiotherapy than in the chemotherapy group (77.5% vs 72.3%, P = 0.035). At multivariate analysis, the duration of stage-adjusted disease-free survival was positively influenced by chemoradiotherapy in cases with lymph node metastasis (HR = 0.68, 95%CI: 0.47-0.99; P = 0.04). Main data regarding adjuvant chemoradiotherapy for gastric cancer are shown in Table 4.
| Ref. | Study design | No. of patients | Survival (P value) | Disease free survival (P value) | Recurrence (P value) | Loco-regional recurrence (P value) |
| Moertel et al[44], 1984 | RCT surgery alone (S) vs surgery + chemoradiotherapy (SCRT) | S: 39 | At 5 yr | At 5 yr | - | S vs SCRT |
| SCRT: 23 | S < SCRT | S < SCRT | (NS) | |||
| (0.05) | (0.02) | |||||
| Allum et al[45], 1989 BSCG | RCT surgery alone (S) vs surgery + chemoradiotherapy (SCRT) vs surgery + chemotherapy (SCT) | S: 145 | At 5 yr | - | - | S vs SCRT (NS) |
| SCRT: 153 | S vs SCRT | S vs SCT (NS) | ||||
| SCT: 138 | vs CT (NS) | |||||
| Hallissey et al[46], 1994 BSCG | RCT surgery alone (S) vs surgery + chemoradiotherapy (SCRT) vs surgery + chemotherapy (SCT) | S: 145 | At 5 yr | - | - | - |
| SCRT: 153 | S vs SCRT | |||||
| SCT: 138 | vs CT (NS) | |||||
| Macdonald et al[42], 2001INT-0116 | RCT surgery alone (S) vs surgery + chemoradiotherapy (SCRT) | S: 275 | At 3 yr | At 3 yr | At 3 yr | At 3 yr |
| SCRT: 281 | S < SCRT | S < SCRT | S > SCRT | S > SCRT | ||
| (0.005) | (0.001) | (0.001) | (0.0001) | |||
| Dikken et al[49], 2010 | Retrospective surgery alone (S) vs surgery + chemoradiotherapy (SCRT) | S: 694 | At 24 mo S vs SCRT (NS) | At 24 mo | - | At 24 mo |
| SCRT: 91 | S vs SCRT (NS) | S > SCRT (0.0015) | ||||
| Kim et al[51], 2005 | Observational surgery alone (S) vs surgery + chemoradiotherapy (SCRT) | S: 446 | At 5 yr | At 5 yr | - | At 5 yr |
| SCRT: 544 | S < SCRT | S < SCRT | S > SCRT | |||
| (0.01) | (0.01) | (0.005) | ||||
| Smalley et al[53], 2012 | RCT surgery alone (S) vs surgery + chemoradiotherapy (SCRT) | S: 227 | At 10 yr | At 10 yr | At 10 yr | Ar 10 yr |
| INT-0116 | SCRT: 282 | S < SCRT | S < SCRT | S > SCRT | S > SCRT | |
| (0.0046) | (0.001) | (0.006) | (0.0001) | |||
| Fiorica et al[54], 2007 | Meta-analysis surgery alone (S) vs surgery + chemoradiotherapy (SCRT) | S: 424 | At 5 yr | - | - | - |
| SCRT: 444 | S < SCRt | |||||
| (0.00001) | ||||||
| Lee et al[55], 2011 | RCT surgery + chemoradiotherapy (SCRT) vs surgery + chemotherapy (SC) | SCRT: 230 | - | At 3 yr | SCRT vs SC (NS) | SCRT vs SC (NS) |
| SC: 228 | SCRT vs SC (NS) |
Adjuvant chemotherapy: The clinical trials of adjuvant chemotherapy for gastric carcinoma have a long history, therefore many drug regimens have been studied, but few report evidenced survival benefit[56-58]. One of the main large-scale RCTs (more than 500 patients), in which adjuvant chemotherapy was tested in patients receiving curative surgery for gastric cancer (stage II, IIIA, IIIB[10]) was examined, was published in 2007 by ACTS-GS group[59]. In this multicentric study 529 patients were randomized to receive adjuvant chemotherapy (S-1, an oral fluoropyrimidine, 6-wk cycles for one year), while 530 patients were treated by surgery alone. Patients of both groups were followed up for 5 years after surgery. The first interim analysis, conducted one year after enrollment of the last patient, at a median follow-up of 3 years, showed that the chances of overall and relapse-free survival of the experimental group might be significantly higher than those of controls and close to the predetermined threshold value (P < 0.001). Therefore the trial was closed. Adverse events of grade 3-4 were observed in both groups, although with higher rates in the experimental group. Sixty five percent of patients of the experimental group completed the treatment, but for 46.5% of them the chemotherapy dosage was reduced. Reasons for the interrupting adjuvant treatment included withdrawal of consent, complications, disease progression. The HR for death and for recurrence in the experimental group compared to those of controls were 0.68 (P = 0.003) and 0.62 (P = 0.001), respectively. The 3-year overall survival rates were 80.1% in the experimental group and 70.1% in controls (P = 0.003). The 3-year recurrence-free survival rates were 72.2% in the chemotherapy group and 59.6% in the surgery-alone group (P < 0.001). The rates of nodal and peritoneal recurrence were higher in the control group (P = 0.006). The 5-year update of this trial[60] confirmed the benefits of adjuvant chemotherapy compared to surgery alone in terms of 5-year survival (71.7% vs 61.1%), HR for death (HR = 0.66, 33.1% reduction of death risk in the experimental group), 5-year recurrence-free survival rate (65.4% vs 53.1%), HR for recurrence (HR = 0.65, 34.7% reduction of recurrence risk in the experimental group). The percentages of five-year overall and recurrence-free survival were analyzed according to the tumor stage (II, IIIA, IIIB),and proved to be better in the experimental group than in controls (HR between 0.50 and 0.79). An Asiatic multicentric RCT, whose results were published in 2012[61], examined the effects of adjuvant chemotherapy on disease-free survival (oral capecitabine and iv oxaliplatin, for 8 cycles in 6 mo) compared to surgery alone. Of the 1035 patients of the study, all receiving radical surgery and D2 lymph node dissection for gastric cancer at stage II-IIIB[10], 515 were randomized to receive surgery alone, 520 to receive adjuvant chemotherapy. Sixty seven percent of patients in the experimental group completed treatment. Adverse events of grade 3-4 were nine times more common in the experimental group and required reduction of dosage, delay in administration, or interruption of chemotherapy. Also this trial showed the benefits of adjuvant chemotherapy compared to surgery alone in terms of 3-year disease-free (74% vs 59%, HR = 0.56; P < 0.0001), overall survival (83% vs 78%, HR = 0.72; P = 0.049), recurrence or new occurrences of gastric cancer (18% vs 30%), loco-regional recurrence (21% vs 44%). The benefits of adjuvant chemotherapy on 3-year disease-free survival were clear even when analyzed according to stage of the tumor, but not for patients without lymph node metastases. The results of two meta-analyses assessing if patients with gastric cancer could benefit from chemotherapy after curative resection were published in 2009[62] and in 2010[63]. These researches considered 12 RCTs, with a total of 3809 patients[62], and 17 RCTs, with a total of 3838 patients[63], respectively. Over 60% of the patients in the experimental group completed the treatment protocol[62]. The overall survival rates at 5 and 10 years were higher in the adjuvant chemotherapy groups than in controls, even if the differences were not significant in all the RCTs. In the two meta-analyses the HR for death was significantly better (between 0.78 and 0.82) (P < 0.001), with a reduction of the death risk ranging from 18% to 22% in the experimental group. Estimated median overall survival was 4.9 years in the surgery-alone group and 7.8 years in the adjuvant chemotherapy group. The rates of absolute benefits in the experimental group were 5.8% (55.3% vs 49.6%) at 5 years, and 7.4% (44.9% vs 37.5%) at 10 years,compared to the surgery-alone group[63]. Adjuvant chemotherapy better influenced disease-free survival than surgery alone, with a HR of 0.82 (P < 0.001). The rate of absolute benefit for five-year disease-free survival after adjuvant chemotherapy was 5.3 (54.0% vs 48.7%) compared to surgery alone[62]. With regard to the various chemotherapy regimens (mono chemotherapy and polychemotherapy) used in the RCTs examined in the two meta-analyses, 5-fluorouracil was used in all the studies, and anthracycline and mitomycin C were used along with it in various trials. Adjuvant monochemotherapy showed a significant benefit in 5-year survival compared to surgery alone (71.4% vs 53.9%, HR = 0.6; P = 0.03), with better results compared to adjuvant polychemotherapy[63]. The data of these two meta-analyses confirm those of another meta-analysis dated 2008 in terms of survival rate and disease-free survival[64]. In addition, the latter study proved a positive influence of adjuvant therapy in relation to loco-regional and distant recurrence rate (RR = 0.78).
The main disadvantage of adjuvant treatments, both chemotherapy and chemoradiotherapy, is that these cannot be performed before post-surgery healing is complete and the general conditions of the patients are satisfactory. Therefore, postoperative complications or slow recovery after surgery can delay the beginning of the treatment. Due to the anatomic conditions created by surgery, radiotherapy can affect other organs and possibly cause irradiation damage. Chemotherapy can often cause negative effects or toxicity in the gastrointestinal system of patients whose new anatomical gastrointestinal conditions created by surgery often cause functional alterations. Main data regarding adjuvant chemotherapy for gastric cancer are shown in Table 5.
| Ref. | Study design | n | Survival | Disease free | Recurrence (P value) | Loco-regional |
| (P value, HR, RR) | Survival (P value, HR, RR) | Recurrence (P value) | ||||
| Sakuramoto et al[59], 2007 | RCT | S: 530 | At 3 yr | At 3 yr | At 3 yr | At 3 yr |
| Surgery alone (S) vs surgery + chemoterapy (SC) | SC: 529 | S < SC (0.003) | S < SC (0.001) | S > SC (0.001) | S > SC (0.006) | |
| Sasako et al[60], 2011 | RCT | S: 530 | At 5 yr | At 5 yr | At 5 yr | At 5 yr |
| Surgery alone (S) vs surgery + chemoterapy (SC) | SC: 529 | SC benefit vs S | SC benefit vs S | S > SC (0.008) | S > SC (0.005) | |
| HR = 0.66 | HR = 0.65 | |||||
| Bang et al[61], 2012 | RCT | S: 515 | At 3 yr | At 3 yr | At 3 yr | At 3 yr |
| Surgery alone (S) vs surgery + chemoterapy (SC) | SC: 520 | SC benefit vs S | SC benefit vs S | S > SC (0.0006) | S > SC (0.0003) | |
| HR = 0.72 (0.049) | HR = 0.56 (0.0001) | |||||
| Liu et al[64], 2008 | Meta-analysis | S: 2313 | At median 5 yr | At median 5 yr | At median 5 yr | At median 5 yr |
| Surgery alone (S) vs | SC: 2286 | SC benefit vs S | SC benefit vs S | SC benefit vs S | SC benefit vs S | |
| surgery + chemoterapy (SC) | RR = 0.85 (0.00001) | RR = 0.85 (0.04) | RR = 0.78 | RR between 0.62-0.65 | ||
| Sun et al[62], 2009 | Meta-analysis | S: 1914 | At 5 yr | |||
| Surgery alone (S) vs surgery + chemoterapy (SC) | SC: 1931 | SC benefit vs S | ||||
| HR = 0.78 (0.001) | ||||||
| Paoletti et al[63], 2010 | Meta-analysis | S: 1885 | At 10 yr | At 10 yr | ||
| Surgery alone (S) vs surgery + chemoterapy (SC) | SC: 1953 | SC benefit vs S | SC benefit vs S | |||
| HR = 0.82 (0.001) | HR = 0.82 (0.001) |
The aim of neoadjuvant treatments is to reduce the biological potential of tumor cells, to increase surgical radicality, and to eradicate subclinical micrometastases. The advantages of neoadjuvant treatments lie in the fact that patients who receive these are in good health condition, the treatment can start immediately after diagnosis and clinical staging are conducted. If radiotherapy is performed, the treatment is aimed at the target organ that, anyway, will be resected, while if chemotherapy is performed, the possible gastrointestinal side effects are not worsened by the anatomical and functional alterations that may occur after surgery. Also, the downsizing of the neoplasm after neoadjuvant treatment can facilitate surgery. For these reasons, the neoadjuvant treatment seems attractive and can be administered to more patients than adjuvant treatment. The disadvantage of neoadjuvant treatment is that it delays surgery, with the possibility that the tumor will extend beyond the stage of resectability or that the health conditions after the neoadjuvant treatment will not allow for surgery[65]. For these reasons, the studies which have been examined report a limited number of patients because of difficulty of enrollment or early closure of the trials, with consequences on the quality of the studies.
Neoadjuvant chemotherapy: The Dutch Gastric Cancer Group in 2004 published the long-term results of the RCT FAMTX[66], which examined the effect of pre-operative chemotherapy (methotrexate, 5-fluorouracil, leucovorin and doxorubicin every four weeks for 4 cycles) in patients with gastric carcinoma, in terms of resectability and survival. After randomization, the analysis was conducted on 27 patients in the experimental group (neoadjuvant chemotherapy followed by surgery) and on 29 controls (surgery alone). The interval between randomization and surgery in the experimental group was significantly longer than in controls (P < 0.001). Forty four percent of patients in the experimental group interrupted chemotherapy because of toxicity. Lymphadenectomy was limited to perigastric lymph nodes (D1) in both groups. The rate of curative resections (R0) was similar in both groups. The median postoperative follow-up for the two groups was 83 mo. The median survival after randomization was 18.2 mo in the experimental group and 30.3 mo in controls. The 5-year survival rate was 21% in the experimental group and 34% in controls (P = 0.17). The last data confirmed a trend to adverse effects due to preoperative chemotherapy, although not significant. The survival rates of patients receiving curative surgery (R0) were 32% in the experimental group and 53% in controls. The trial was closed after the enrollment of 59 patients and after an interim analysis showed inadequate rates of curative resections in the experimental group. The results of a RCT of the European Organization for Research and Treatment of Cancer were published in 2010[67]. This study compared neoadjuvant chemotherapy and surgery alone in patients with gastric and cardia adenocarcinoma, at clinical stage UICC III and IV, cM0. Patients were randomized in experimental group (72 patients) treated with neoadjuvant chemotherapy (iv cisplatin, folinic acid, fluorouracil, 2 cycles of 48 d), and controls (72 patients), only receiving surgery. Sixty two percent of patients in the experimental group completed neoadjuvant treatment. Reasons for interruption of treatment were: toxicity, withdrawal of consent and progression of the disease. Surgical resection was performed within 14 d from randomization in controls (68 patients), and within four weeks from last day of chemotherapy in the experimental group (70 patients). D2 gastrectomy was performed in the majority of patients. In the experimental group, the tumor had smaller dimensions than in controls, and in both groups a complete resection was possible in 87.5% of cases. Postoperative morbidity was more frequent in the experimental group (P = 0.09), operative mortality was observed in one patient of the controls and in two patients of the experimental group. In the experimental group, a complete clinical response was obtained in 5.8% of patients, while a partial clinical response was obtained in 30.4%. Following pathological assessment of the operative specimen, 81.9% of patients in the experimental group had received a radical resection (R0), compared to 66.7% of controls (P = 0.036). In the experimental group, complete pathological response was observed in 7.1% of the patients, and 65.7% of tumors in the experimental group were T0-1-2, which compares to 50% in controls. Lymph node metastases and lymphatic invasion were significantly higher in controls (P = 0.018 and P = 0.01 respectively). At a median follow-up of 4.4 years, however, no significant survival benefit was observed in the experimental group (HR for overall survival 0.84; P = 0.46). In a multicentric RCT, published in 2010[68], the short-term effects of neoadjuvant chemotherapy (docetaxel, cisplatin, 5-fluorouracil for 4 cycles of 21 days) in 34 patients with gastric carcinoma (T3-4 any N M0 or any T N1-3 M0 TNM 1997) were examined, comparing them with the same chemotherapy in adjuvant treatment (in 35 patients). Patients in the neoadjuvant group received surgery 3-4 wk after beginning the last cycle of therapy. Neoadjuvant therapy was completed in 74% of patients of that group. D1 lymphadenectomy was always performed, and it was sometimes extended to some stations of the main local vessels[10]. Although it is not possible to compare the two groups, because of the characteristics of the study, radical R0 resection was performed in 85% of cases in the neoadjuvant group and in 91% of the adjuvant group. Complete pathological response was observed in 12% of cases in the neoadjuvant group. Postoperative complications and operative mortality did not differ significantly (P = 0.86) in the two groups. Complete adjuvant therapy was administered to 34% of patients in the adjuvant group. Thirty four percent of patients in this group did not receive adjuvant treatment. Severe adverse events were more frequent in the adjuvant group (P = 0.07).
D’Ugo et al[69], based on a study with a single treatment group of 34 patients with resectable gastric carcinoma, state that the postponement of resection in favour of a systemic treatment does not exclude patients from the benefits of a potentially curative delayed resection and does not worsen surgical outcomes. However, they also admit that tumor progression affects some patients. This statement alone would be enough to suggest an accurate selection of those patients who can benefit from preoperative chemotherapy.
Pre- and post-operative chemotherapy (perioperative chemotherapy): This approach presents the disadvantages of both modalities. Besides, a percentage of patients does not start postoperative treatment due to progression of the disease, toxicity during preoperative treatment, withdrawal of consent, or postoperative complications.
The results of an international multicentric RCT were published in 2006[43] by Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC), and it has become a landmark for all the following studies on neoadjuvant and adjuvant treatments for gastric carcinoma. The study examined 503 patients with gastric, esophagogastric junction and distal esophageal adenocarcinoma, amenable to curative surgery. Seventy four percent of the examined cases were gastric carcinomas. Patients were randomized to receive perioperative chemotherapy (250 patients, three preoperative cycles and three postoperative cycles of epirubicin, cisplatin and fluorouracil for 21 d) or surgery alone (253 patients). Eighty six percent of patients in the experimental group (215 patients) completed neoadjuvant treatment, and toxic effects of the therapy were the main reasons for interruption. Two hundred and twenty nine patients of the experimental group were resected. One hundred and four of them completed the three cycles of pre- and post-operative chemotherapy. The main reasons for not performing postoperative therapy were: progression of the disease, death, withdrawal of consent. The median interval between randomization and surgery was 99 d in the experimental group and 14 d in controls. Smaller maximum diameter of the tumor, higher number of T1-T2 tumor and smaller lymph node involvement were observed in the experimental group, with significant differences as compared to controls (P = 0.001, P = 0.002, P = 0.01, respectively). The percentages of death, death for progression of the disease, local and distant recurrence were higher in controls. The HR for progression risk and for death in the experimental group was better than in the control group (HR = 0.66; P = 0.001 and HR = 0.75; P = 0.009 respectively). Even after stratification, HR for death was better in the experimental group (0.74; P = 0.008). The percentages of survival at 5 years were 36.3% in the experimental group and 23% in controls.
The results of the French multicentric RCT FLNCC ACCORD07-FFCD 9703 were published in 2011[70]. This research compared pre- and postoperative chemotherapy in patients with resectable gastric, esophagogastric junction and distal esophagus adenocarcinoma (cisplatin, fluorouracil; 2-3 preoperative cycles and 3-4 postoperative cycles of 28 d) with surgery alone. One hundred and thirteen patients were randomized in the experimental group, 111 in the controls. Seventy five percent of the adenocarcinomas were in the distal esophagus or in the esophagogastric junction. Eighty seven percent of patients in the experimental group received at least 2 cycles of preoperative chemotherapy. Toxicity of grade 3-4 developed during preoperative therapy in 38% of patients in the experimental group. Surgery was performed in 96.5% of cases in the experimental group, at a median interval of 78 d after randomization, and in 99% of controls, at a median interval of 13 d after randomization. R0 resections and a lower incidence of lymph node metastases were found in the experimental group (P = 0.04 and P = 0.054 respectively). Fifty percent of patients in the experimental group received postoperative chemotherapy. At a median follow-up of 5.7 years, the experimental group showed a significant benefit in terms of overall (HR for death 0.69; P = 0.02) and disease-free survival (HR = 0.65; P = 0.003) compared to controls. The 5-year survival rates were 38% in the experimental group vs, 24% in controls. The disease-free survival rates were 34% and 19%, respectively. At multivariate analysis, preoperative chemotherapy was a significant prognostic factor (P = 0.01). The results of this trial, in relation to gastric carcinoma, need to be cautiously assessed, considering that gastric adenocarcinomas were only 25% of all the adenocarcinomas.The study protocol of an international multicentric RCT, CRITICS (ChemoRadiotherapy after induction Chemotherapy in Cancer of the Stomach, NCT00407186), was published in 2011[71]. According to this study, patients with a resectable gastric cancer (stage IB-IVa AJCC 6th edition) should be treated with three cycles of preoperative chemotherapy (epirubicin, cisplatin, capecitabine) followed by surgery with D2 lymphadenectomy, then, following these, three more cycles of the same chemotherapy or chemoradiotherapy. The number of patients to be enrolled is high, 788 patients. These should be randomized after diagnosis and before beginning neoadjuvant treatment. The primary endpoint is to improve overall survival compared to surgery alone. It is expected that the results of this trial will be useful to treat patients with resectable gastric cancer.
The aim of neoadjuvant and intraoperative radiotherapy is to reduce the biological potential of tumor cells, to increase surgical radicality by reducing the extension of the tumor, and to eliminate residual subclinical local metastases after surgery. The surgeons, however, are particularly concerned about the technical difficulties of operating in a pretreated field, because of the possibility of wound anastomotic healing problems, damage to nearby abdominal organs, and postoperative pulmonary complications. Also for these reasons, these methods of treatment are not common, and there are few trials examining their efficacy. In addition, these techniques also bear logistic difficulties, especially in relation to intraoperative radiotherapy.
The short- and long-term results of a RCT were published in 1998[72] examining the role of neoadjuvant radiotherapy compared to surgery alone, in the treatment of gastric cardia adenocarcinoma. After randomization, the experimental group included 171 patients, compared to 199 controls. The radiation dose was 40 Gy, and it was administered in 4 wk. Surgery was performed 2-4 wk after completion of radiotherapy. The rates of overall, radical and palliative resectability were significantly higher in the experimental group (P = 0.01, 0.001, 0.025 respectively). The experimental group presented a smaller local extension of the tumor, a lower number of patients with lymph node metastases and a lower number of lymph nodes affected by metastases (P < 0.01, P < 0.001 and P < 0.0001 respectively). Preoperative radiotherapy obtained better overall 5- and 10-year survival rates, both in resected and in non-resectable patients, while the difference compared to surgery alone was only significant (P = 0.009) in the latter. Non-resectable patients in the experimental group had significantly longer mean and median survivals (P = 0.008) than non-resectable patients in controls. After histological examination, it was observed that a higher effect of radiotherapy on tumor cells corresponded to better 5-year survival rates (P = 0.05). Local and lymph node recurrences were lower in the experimental group (P < 0.025 and P < 0.005 respectively) than in controls. It should be considered that the examined cases were adenocarcinoma of gastric cardia, which can account for the positive results. The data of a RCT of Russian MRRC RAMS were published in 2000[73]. It randomized 40 patients with gastric carcinoma to receive concentrated preoperative radiotherapy (20 Gy in 5 d), gastrectomy and intraoperative radiotherapy (IORT, 20 Gy) and 38 patients to receive gastrectomy alone. The use of IORT allows for a higher administration of radiations directly on a target area and, together with concentrated adjuvant radiotherapy, it has the theoretical potential to reduce local and regional recurrence and to improve survival. D1 gastrectomy was prevalently performed in the two groups. In 25% of cases neoadjuvant radiotherapy caused toxicity in the experimental group, but the preoperative radiotherapy treatment was always completed. Postoperative complications were observed in 35% of cases in the experimental group and in 50% in the control group. Overall survival was not significantly different in the two groups (P = 0.31). However, in the experimental group a benefit in survival was observed, when lymph nodes were metastasized (P = 0.04), when tumors were T3-4 (P = 0.04), and for stage II and IIIA tumors (P = 0.04). This trend was also seen in T3-4 N1-2 cases: median survival time was 21.4 mo in the experimental group, 9.0 mo in controls. These data confirm that, for T1-2 N0 stage tumors, neoadjuvant and adjuvant therapies do not improve the results of surgery, which, in these particular instances, is ideal and sufficient. The data of another RCT of the MRRC RAMS were published in 2002[74]. This research examined the short- and long-term outcomes of neoadjuvant radiotherapy employed in resectable gastric carcinomas. Fifty one patients were randomized in the experimental group and treated with concentrated preoperative radiotherapy (20 Gy administered in 5 consecutive days) followed by surgery within 4-5 d. The 51 controls received surgery within 7 d from randomization. Radiotherapy, completed by all patients in the experimental group, was generally well tolerated. D1 gastrectomy was always performed, and postoperative (surgical and non-surgical) complications were more frequent in the experimental group,while operative mortality did not differ in the two groups. Long-term survival in the two groups was not significantly different (39% in the experimental group vs 30% in controls after 5 years; 32% in the experimental group vs 18% in the control group after 10 years) (P = 0.55), although median duration of survival of tumors T3-4 and of tumors with positive lymph nodes was longer in the experimental group. The survival curves in the experimental group became better than in controls three years after surgery. The authors comment that the long-term aim of radiotherapy is to reduce the development of loco-regional recurrence, and the trend of the curves confirmed that this result had been achieved. A meta-analysis was conducted in 2007[54], examining four RCTs for a total of 405 patients treated with adjuvant radiotherapy for resectable gastric carcinoma, and it showed a benefit of radiotherapy after 3 and 5 years compared to surgery alone (OR = 0.57; P = 0.0001 and OR = 0.62; P = 0.002, respectively). One other meta-analysis, in 2009[75], also considered the role of neoadjuvant and adjuvant intraoperative radiotherapy in gastric carcinoma. The modalities of the analysis, however, do not allow to understand which results refer to the various types of radiotherapy.
Negative outcomes after surgery for gastric carcinoma are related, in 50% of the cases, to dissemination of the tumor in the peritoneal cavity[9,76]. The peritoneum is involved either as a result of trans-parietal invasion by tumor cells, or of intraperitoneal seeding caused by surgical maneuvers such as manipulation of the tumor or section of lymphatic and blood vessels. Chemotherapy medications injected during neoadjuvant or adjuvant therapies do not reach effective concentration at peritoneal level. Intraperitoneal chemotherapy aims at eradicating tumor cells after surgery by using high concentrations of drugs in the peritoneum, thus reducing systemic toxicity. The use of intraperitoneal chemotherapy can be considered a procedure with prophylactic and therapeutic aim, but, to date, it is not accepted as a standard therapy[77]. It can be given in several ways: as hypertermic intraoperative intraperitoneal chemotherapy (HICC), as normothermic intraoperative intraperitoneal chemotherapy (NIIC), as early postoperative chemotherapy (EPIC) or as delayed postoperative intraperitoneal chemotherapy (DPIC). Some problems are still unsolved: the short- and long-term outcomes of intraperitoneal chemotherapy, the correct timing, the role of hyperthermia, the drugs to be employed, and, more importantly, the selection of patients to be treated. Since the ‘90s, several RCT have been conducted, especially by Eastern authors, in order to examine the role of intraperitoneal chemotherapy in gastric carcinoma, with the use of cisplatin, mitomycin C, 5-fluorouracil alone or in combination, comparing intraperitoneal chemotherapy to surgery alone[78-80]. The results of these studies are ambiguous. A RCT in 2001[80] showed a survival benefit in patients with resection of stage IV and stage III gastric cancer followed by intraperitoneal chemotherapy (mitomycin C and 5-fluorouracil), compared to surgery-alone controls, while there was no significant benefit in patients with stage I or II. The 5-year survival for patients with stage III and stage IV disease were 57% and 28% in the treated group, and 23% and 5% in the surgery-alone controls, respectively (P = 0.0024 and P = 0.0098). The benefits of intraperitoneal chemotherapy on survival were significant as compared to controls in tumors with gross serosal invasion (P = 0.0004), lymph node metastases (P = 0.0027), in tumors > 5 cm in diameter (P = 0.0029), and in poorly differentiated tumors (P = 0.0004). Furthermore, the incidence of peritoneal dissemination in the treated group was 15%, compared to 30% after surgery alone (P = 0.03). A significant benefit in terms of long-term survival (P = 0.03) and peritoneal recurrence (P = 0.00008) of patients who received gastrectomy for gastric carcinoma with macroscopic serosal invasion had been observed in a previous RCT[81]. A meta-analysis published in 2003[82] examined the use of intraperitoneal chemotherapy in patients with gastric cancers resected with curative aim. Eight RCTs were examined, 495 patients were randomized in the experimental group, while 500 patients only received surgery. The analysis showed that intraperitoneal chemotherapy, administered during or immediately after surgery, obtained some benefit compared to surgery alone. A more recent meta-analysis, published in 2007[83] examined 13 RCTs in which patients with advanced gastric or gastroesophageal junction adenocarcinoma who received curative resection were randomized to receive surgery with associated intraperitoneal chemotherapy (873 patients), or surgery alone (775 patients). HR of the overall 3-year survival was better in the intraperitoneal chemotherapy group in all the modalities of administration, but the benefit was particularly significant in cases treated with HICC alone or with associated EPIC (HR = 0.60; P = 0.002 and HR = 0.45; P = 0.0002 respectively). The relative risk (RR) of locoregional recurrence, examined for HICC, NIIC and EPIC, did not show significant differences between experimental group and controls. The incidence of perioperative mortality of the procedures of intraperitoneal chemotherapy was not significantly different than in controls (RR = 1.03; P = 0.96). The most common postoperative complications in the experimental group were intra-abdominal abscess (RR = 2.37; P = 0.003) and neutropenia (RR = 4.33; P = 0.007).
Gastric carcinoma still represents one of the main causes of death for cancer in the world, although it presently has a lower incidence in Western countries. The adoption of programs of primary and secondary prophylaxis and the use of effective treatments are the only ways to tackle this condition. The optimal treatment for advanced gastric carcinoma is still controversial. Surgical gastric resection (partial or total) associated with lymphadenectomy of perigastric and regional lymph node stations (D2) represents the treatment of choice. However, surgery alone cannot guarantee satisfactory results for patients with advanced disease. The use of multimodal therapies, chemotherapy and radiotherapy, alone or combined, used as neoadjuvant, intraoperative or adjuvant treatments is supported by a series of trials substantiating their effectiveness on recurrence and survival. Timing, type of therapy, dosing of administration and possible combination between different modalities still have to be assessed and tailored in order to achieve the best outcome in the individual patient.
| 1. | Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Is D2 lymph node dissection necessary for early gastric cancer. Ann Surg Oncol. 2002;9:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Nitti D, Marchet A, Mammano E, Ambrosi A, Belluco C, Mencarelli R, Maino M, Marconato G, Farinati F, Lise M. Extended lymphadenectomy (D2) in patients with early gastric cancer. Eur J Surg Oncol. 2005;31:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531-546. [PubMed] |
| 4. | Lawson JD, Sicklick JK, Fanta PT. Gastric cancer. Curr Probl Cancer. 2011;35:97-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Peeters KC, Hundahl SA, Kranenbarg EK, Hartgrink H, van de Velde CJ. Low Maruyama index surgery for gastric cancer: blinded reanalysis of the Dutch D1-D2 trial. World J Surg. 2005;29:1576-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | McCulloch P, Nita ME, Kazi H, Gama-Rodrigues JJ. WITHDRAWN: Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2012;1:CD001964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Oñate-Ocaña LF, Aiello-Crocifoglio V, Mondragón-Sánchez R, Ruiz-Molina JM. Survival benefit of D2 lympadenectomy in patients with gastric adenocarcinoma. Ann Surg Oncol. 2000;7:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | de Gara CJ, Hanson J, Hamilton S. A population-based study of tumor-node relationship, resection margins, and surgeon volume on gastric cancer survival. Am J Surg. 2003;186:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 320] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 10. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 1947] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 11. | Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 878] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Wu CW, Hsieh MC, Lo SS, Wang LS, Hsu WH, Lui WY, Huang MH, P’eng FK. Morbidity and mortality after radical gastrectomy for patients with carcinoma of the stomach. J Am Coll Surg. 1995;181:26-32. [PubMed] |
| 13. | Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 443] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Sobin LH, Wittekind CH, editors . TNM classification of malignant tumours. International Union Against cancer. 5th ed. New York: John Wiley & Sons 1997; . |
| 15. | Nishi M, Omori Y, Miwa K, editors . Japanese classification of gastric carcinoma. Japanese Research Society for Gastric Cancer. 1st ed. Tokio: Kanehara & Co 1995; . |
| 16. | Siewert JR, Böttcher K, Roder JD, Busch R, Hermanek P, Meyer HJ. Prognostic relevance of systematic lymph node dissection in gastric carcinoma. German Gastric Carcinoma Study Group. Br J Surg. 1993;80:1015-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 294] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Volpe CM, Driscoll DL, Douglass HO. Outcome of patients with proximal gastric cancer depends on extent of resection and number of resected lymph nodes. Ann Surg Oncol. 2000;7:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Katai H, Yoshimura K, Maruyama K, Sasako M, Sano T. Evaluation of the New International Union Against Cancer TNM staging for gastric carcinoma. Cancer. 2000;88:1796-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Seevaratnam R, Bocicariu A, Cardoso R, Yohanathan L, Dixon M, Law C, Helyer L, Coburn NG. How many lymph nodes should be assessed in patients with gastric cancer A systematic review. Gastric Cancer. 2012;15 Suppl 1:S70-S88. [PubMed] |
| 20. | McCulloch P, Niita ME, Kazi H, Gama-Rodrigues JJ. Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer. Br J Surg. 2005;92:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 278] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 737] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 23. | Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 696] [Article Influence: 23.2] [Reference Citation Analysis (1)] |
| 24. | Aiko T, Sasako M. The new Japanese Classification of Gastric Carcinoma: Points to be revised. Gastric Cancer. 1998;1:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1072] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 26. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 1001] [Article Influence: 37.1] [Reference Citation Analysis (2)] |
| 27. | Department of Health. Guidance on Commissioning Cancer Service: Improving outcomes in upper gastrointestinal cancers. The research evidence. London: NHS Executive 2001; . |
| 28. | Isozaki H, Okajima K, Fujii K, Nomura E, Izumi N, Mabuchi H, Nakamura M, Hara H. Effectiveness of paraaortic lymph node dissection for advanced gastric cancer. Hepatogastroenterology. 1999;46:549-554. [PubMed] |
| 29. | Baba M, Hokita S, Natsugoe S, Miyazono T, Shimada M, Nakano S, Takao S, Aikou T. Paraaortic lymphadenectomy in patients with advanced carcinoma of the upper-third of the stomach. Hepatogastroenterology. 2000;47:893-896. [PubMed] |
| 30. | Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 31. | Kodera Y, Sasako M, Yamamoto S, Sano T, Nashimoto A, Kurita A. Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. Br J Surg. 2005;92:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 764] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 33. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg. 2004;91:283-287. [PubMed] |
| 34. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 35. | Yonemura Y, Wu CC, Fukushima N, Honda I, Bandou E, Kawamura T, Kamata T, Kim BS, Matsuki N, Sawa T. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008;13:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT. Extended lymph node dissection for gastric cancer: who may benefit Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 658] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 37. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1337] [Article Influence: 83.6] [Reference Citation Analysis (1)] |
| 38. | Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Yang SH, Zhang YC, Yang KH, Li YP, He XD, Tian JH, Lv TH, Hui YH, Sharma N. An evidence-based medicine review of lymphadenectomy extent for gastric cancer. Am J Surg. 2009;197:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Memon MA, Subramanya MS, Khan S, Hossain MB, Osland E, Memon B. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg. 2011;253:900-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, Law C, Coburn N. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012;15 Suppl 1:S60-S69. [PubMed] |
| 42. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2461] [Article Influence: 98.4] [Reference Citation Analysis (1)] |
| 43. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4736] [Article Influence: 236.8] [Reference Citation Analysis (7)] |
| 44. | Moertel CG, Childs DS, O’Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249-1254. [PubMed] |
| 45. | Allum WH, Hallissey MT, Ward LC, Hockey MS. A controlled, prospective, randomised trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer: interim report. British Stomach Cancer Group. Br J Cancer. 1989;60:739-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Hallissey MT, Dunn JA, Ward LC, Allum WH. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 187] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, Fujiya T, Inada T, Sasako M, Ohashi Y. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007;94:1468-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 48. | Beahrs OH, Henson DE, Hutter RVP, Myers MH, editors . Manual for staging of cancer. 3rd ed. Philadelphia: JB Lippincot 1988; . |
| 49. | Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, Boot H, Putter H, Peeters KC, van de Velde CJ. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Greene FL. AJCC Cancer Staging Manual (ed. 6). New York, NY: Springer-Verlag 2002; . [DOI] [Full Text] |
| 51. | Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, Park SH, Lee SH, Kim K, Park JO. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 52. | Macdonald JS, Benedetti J, Smalley S, Haller D, Hundahl S, Jessup J, Ajani J, Gunderson L, Goldman B, Martenson J. Chemoradiation of resected gastric cancer: a 10-year follow-up of the phase III trial INT0116 (SWOG 9008). J Clin Oncol. 2009;27:4515. |
| 53. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 636] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 54. | Fiorica F, Cartei F, Enea M, Licata A, Cabibbo G, Carau B, Liboni A, Ursino S, Cammà C. The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev. 2007;33:729-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [PubMed] |
| 56. | Nakajima T, Nashimoto A, Kitamura M, Kito T, Iwanaga T, Okabayashi K, Goto M. Adjuvant mitomycin and fluorouracil followed by oral uracil plus tegafur in serosa-negative gastric cancer: a randomised trial. Gastric Cancer Surgical Study Group. Lancet. 1999;354:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Nitti D, Wils J, Dos Santos JG, Fountzilas G, Conte PF, Sava C, Tres A, Coombes RC, Crivellari D, Marchet A. Randomized phase III trials of adjuvant FAMTX or FEMTX compared with surgery alone in resected gastric cancer. A combined analysis of the EORTC GI Group and the ICCG. Ann Oncol. 2006;17:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Oba K, Morita S, Tsuburaya A, Kodera Y, Kobayashi M, Sakamoto J. Efficacy of adjuvant chemotherapy using oral fluorinated pyrimidines for curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials in Japan. J Chemother. 2006;18:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1979] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 60. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamagichi T, Nashinoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [PubMed] |
| 61. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1332] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 62. | Sun P, Xiang JB, Chen ZY. Meta-analysis of adjuvant chemotherapy after radical surgery for advanced gastric cancer. Br J Surg. 2009;96:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [PubMed] [DOI] [Full Text] |
| 64. | Liu TS, Wang Y, Chen SY, Sun YH. An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur J Surg Oncol. 2008;34:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Ajani JA, Mayer RJ, Ota DM, Steele GD, Evans D, Roh M, Sugarbaker DJ, Dumas P, Gray C, Vena DA. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst. 1993;85:1839-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 67. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol. 2010;16:868-874. [PubMed] |
| 69. | D’Ugo D, Rausei S, Biondi A, Persiani R. Preoperative treatment and surgery in gastric cancer: friends or foes. Lancet Oncol. 2009;10:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Dikken JL, van Sandick JW, Maurits Swellengrebel HA, Lind PA, Putter H, Jansen EP, Boot H, van Grieken NC, van de Velde CJ, Verheij M. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer. 2011;11:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Zhang ZX, Gu XZ, Yin WB, Huang GJ, Zhang DW, Zhang RG. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)--report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 73. | Skoropad VY, Berdov BA, Mardynski YS, Titova LN. A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. Eur J Surg Oncol. 2000;26:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Skoropad V, Berdov B, Zagrebin V. Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. J Surg Oncol. 2002;80:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Valentini V, Cellini F, Minsky BD, Mattiucci GC, Balducci M, D’Agostino G, D’Angelo E, Dinapoli N, Nicolotti N, Valentini C. Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol. 2009;92:176-183. [PubMed] [DOI] [Full Text] |
| 76. | Landry J, Tepper JE, Wood WC, Moulton EO, Koerner F, Sullinger J. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1357-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 171] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Dikken JL, van de Velde CJ, Coit DG, Shah MA, Verheij M, Cats A. Treatment of resectable gastric cancer. Therap Adv Gastroenterol. 2012;5:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Fujimura T, Yonemura Y, Muraoka K, Takamura H, Hirono Y, Sahara H, Ninomiya I, Matsumoto H, Tsugawa K, Nishimura G. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg. 1994;18:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Rosen HR, Jatzko G, Repse S, Potrc S, Neudorfer H, Sandbichler P, Zacherl J, Rabl H, Holzberger P, Lisborg P. Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol. 1998;16:2733-2738. [PubMed] |
| 80. | Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg. 2001;25:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 82. | Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol. 2003;21:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, Morris DL. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
P- Reviewers: Chai J, Cullen JJ, Chen YP, Masui T S- Editor: Ma YJ L- Editor: A E- Editor: Ma S