Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.3738
Revised: January 8, 2014
Accepted: January 20, 2014
Published online: April 14, 2014
Processing time: 165 Days and 4 Hours
Strong evidence supports the concept of immunosurveillance and immunoediting in colorectal cancer. In particular, the density of T CD8+ and CD45+ lymphocyte infiltration was recently shown to have a better prognostic value than the classic tumor node metastasis classification factor. Other immune subsets, as macrophages, natural killer cells or unconventionnal lymphocytes, seem to play an important role. Induction of regulatory T cells (Tregs) or immunosuppressive molecules such as PD-1 or CTLA-4 and downregulation of antigen-presenting molecules are major escape mechanisms to antitumor immune response. The development of these mechanisms is a major obstacle to the establishment of an effective immune response, but also to the use of immunotherapy. Although immunotherapy is not yet routinely used in colorectal cancer, we now know that most treatments used (chemotherapy and biotherapy) have immunomodulatory effects, such as induction of immunogenic cell death by chemotherapy, inhibition of immunosuppression by antiangiogenic agents, and antibody-dependent cytotoxicity induced by cetuximab. Finally, many immunotherapy strategies are being developed and tested in phase I to III clinical trials. The most promising strategies are boosting the immune system with cytokines, inhibition of immunoregulatory checkpoints, vaccination with vectorized antigens, and adoptive cell therapy. Comprehension of antitumor immune response and combination of the different approaches of immunotherapy may allow the use of effective immunotherapy for treatment of colorectal cancer in the near future.
Core tip: Immune system is now widely accepted as a key mechanism to prevent occurrence of cancer and intratumoral T CD8+ and CD45+ lymphocytes infiltrate has shown to be a major prognosis factor in colorectal cancer. However, immunity fail in controlling tumor growth, because of strong escape mechanisms to the immune system developed by the tumor. In recent years, several immunotherapy strategies have been tested in colorectal cancer. This review provides an understanding of the mechanisms involved and identifies innovating therapeutic strategies.
- Citation: Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: What we know and perspectives. World J Gastroenterol 2014; 20(14): 3738-3750
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/3738.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.3738
With around 1 million new cases every year, colorectal cancer (CRC) is the third most frequent cancer in the world. Despite recent therapeutic advances it causes more than 500000 deaths every year. So there is a real need for therapeutic progress to reduce the risk of recurrence after surgery or prolong survival of patients with metastatic disease. Advances could be provided by understanding the role and mechanisms of the immune response in CRC and by the development of immunotherapy. Indeed there is growing evidence that the immune system may play a role in preventing the occurrence, growth and metastatic diffusion of tumors.
The aim of this review is to provide a comprehensive analysis of known mechanisms of immune response against CRC and immune escape strategies developed by tumor cells, and to present current and future perspectives in immunotherapy for CRC. In particular we will focus on the following questions: (1) What is the clinical and prognostic impact of natural immune response mechanisms (2) What are the escape mechanisms developed by the tumor which limit the efficiency of the immune system and/or immunotherapy (3) What is the impact of the immune system in the therapeutic effect of current standard treatments or (4) Can we in the future develop effective immunotherapy for CRC management
The role of immunity in cancer was suspected in 1909 by Ehrlich, who speculated that the immune system can repress the growth of carcinomas. About 50 years later, Macfarlane Burnet and Lewis Thomas elaborated the concept of immunosurveillance, as the capacity of the immune system to promote an effective immunologic reaction to tumor cell-specific neoantigens that eliminates developing cancer before clinical expression.
However, this concept of immunosurveillance has long been questioned. When Hanahan established the six criteria necessary for the development of a tumor in 2000, immunity was not cited.
In humans, the role of immune surveillance was first suspected with observation of increased occurrence of cancer in patients with immunodeficiency. Cohorts of transplanted patients and HIV-infected subjects in particular showed a strong increase in the incidence of cancers[1,2]. But in humans as in murine models the increase in occurrence of neoplasias has long been explained as a consequence of carcinogenesis related to certain infectious pathogens (EBV, HPV, HIV...). However, melanoma, renal, lung, pancreatic and colon cancer are non-pathogen-related and an increased incidence of these tumors was reported in immunocompromised patients. Registries and meta-analyses of solid organ transplant recipients have shown an increased risk of CRC[3,4] with a standardized incidence ratio of 1.2 to 1.8, this increased risk is more controversial in HIV-infected patients[5].
The anti-tumor immunosurveillance concept was finally demonstrated in animal models by Shankaran et al[6], who observed the occurrence of spontaneous neoplasias in immunocompetent or immunodeficient mice. Mice were kept under aseptic conditions for 15 to 21 mo. During this observation period immunocompetent mice did not develop any malignant tumors, while RAG2-/- mice deficient in T and B lymphocytes developed malignant colon and lung tumors (not known to be associated with an infectious agent) in about 50% of cases, and RAG2-/- STAT1-/- mice deficient in T and B lymphocytes and insensitive to IFNγ developed neoplasia in 80% of cases. Since then, many studies have shown the involvement, depending on the model, of the innate and/or adaptive immune response in the protection against the occurrence of malignant neoplasms.
The immunosurveillance concept was then completed by that of immunoediting[7], which describes the interactions between the immune system and the tumor, allowing cancer cells to escape immune surveillance. The selection pressure exerted by the immune system on tumor cells allows the emergence of resistant clones. According to the theory of immunoediting, immune escape occurs in three phases: the immunosurveillance period with the elimination of tumor cells by the immune system, the latency period, corresponding to a state of equilibrium, and the phase of escape, allowing tumor progression and clinical expression.
Natural killer (NK) cells play a crucial role in preventing recurrence, and are a prognostic factor: NK cells play a major role in the immune response to cancer. They help to prevent tumors, and control tumor growth and dissemination, as shown in murine[8,9] and human models[10,11]. NK cells have 2 types of receptors: activating receptors, including NKG2D, and killer inhibitory receptors (KIR). The NKG2D receptor can bind different activating ligands overexpressed on cancer cells. On the other hand, KIR recognize major histocompatibility (MHC) class I molecules and NK cells can thus also be activated by the decreased expression of MHC class I molecules reported on cancer cells. These two mechanisms can activate NK cells against tumor cells. In addition, NK cells may exert a cytotoxic effect against cancer cells through other mechanisms such as antibody-dependent cell-mediated cytotoxicity (ADCC), and secretion of cytokines including IFNγ[12].
In CRC, an extensive intratumoral infiltration of NK cells has been reported to be associated with a better prognosis[13]. Moreover, a direct correlation between increased outcome and NK cell infiltrates is suggested[14]. In particular, NK cells could be involved in protection against cancer-initiating cells (CICs)[15]. CICs are characterized by slow growth and resistance to drugs and radiation, and play a crucial role in tumor recurrence. Recent data suggest that CICs are more sensitive to NK cells because they strongly express activating ligands as NKP30 and NKP44 and express low levels of MHC class I molecules.
Unconventional lymphocyte T cells: Natural Killer T (NKT) cells share characteristics of both NK cells and T cells. They recognize glycolipid antigens like α-galactosylceramide presented by CD1d, an MHC class 1-like molecule. When activated, NKT cells secrete abundant pro-inflammatory cytokines and effector molecules involved in cell death (perforin, Fas-L, TRAIL). Increased tumor infiltration of NKT cells is associated in CRC with a better prognosis[16].
Human γδ T cells (γδ T cells) express a receptor to antigens combining a γ chain and a δ chain. This receptor can recognize different antigens usually in a non-MHC-restricted way, such as heat shock proteins or phosphorylated metabolites generated by tumor cells. γδ T cells been demonstrated to have a strong cytotoxic activity against tumor cells in CRC[17].
Macrophages: Tumor infiltrating macrophages (TIM) can be divided into two different subtypes with different roles in cancer[18]. M1 TIMs are intimately involved in innate immunity, as they target altered cells, produce pro-inflammatory molecules (IL-6, IL-12, IL-23 and TNFα) and promote adaptive immunity through increased expression of MHC and costimulatory molecules. They may also target tumor cells linked to antibodies because they express a receptor for immunoglobulin constant fragments (ADCC). Activated M2 TIMs are engaged in wound healing and can promote tumor progression through immunosuppressive cytokines (IL-10 and TGFβ). While infiltration by macrophages is generally a poor prognostic factor in different types of cancer, in CRC it seems to be associated with a better prognosis[19], suggesting that antitumorigenic properties dominate in vivo.
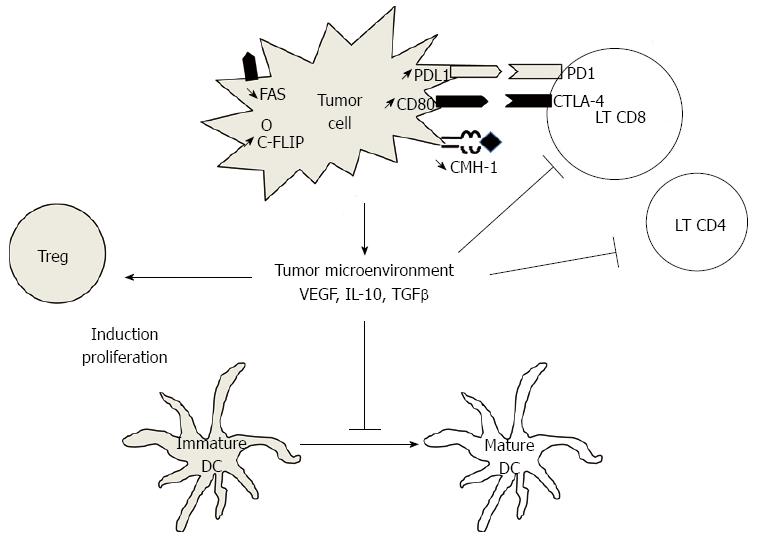
A specific antitumor response is generated by the adaptive immune system, and in particular by αβ T cells. Briefly, the antigen-presenting cells (APCs), mainly dendritic cells (DCs), capture, process and present tumor antigens to CD4 T cells through MHC class II or to CD8 T cells through MHC class I. Activation of T cells requires 3 signals: (1) recognition of antigenic peptide presented by the APCs; (2) activation of costimulatory molecules (CD80/CD28, CD40/CD40L); and (3) recruitment of cytokines (IL-1, IL-2, IL-6, IL-12, IFNγ). Activated CD8 T cells can recognize and lyse tumor cells. Activated CD4 T cells modulate the antitumor immune response. They differentiate into different cell subgroups: The Th1 response allows secretion of cytokines that promote the antitumor response, as IL-2 or IFNγ, whereas the Th2 response favors tumor growth. The Th17 subset secretes large amounts of IL-17. Its role in the immune response against cancer is controversial. Finally, a subset of CD4+ T cells called regulatory T cells (Tregs) and characterized by the expression of CD25 and Foxp3, inhibit the immune response and represent a widely described mechanism whereby the tumor can escape the immune system.
Tumor-associated antigens allow recognition of tumor cells by the immune system: Many cells and molecules are involved in immunosurveillance, they may be linked to the host or the tumor. First, tumor-associated antigens (TAAs) allow an immune response mediated by the humoral and cellular immunity. Several types of TAAs are expressed by the tumor. In CRC, the most frequent TAAs are normal self-antigens, expressed at low levels in normal cells and in embryonic tissues and at high levels in tumor cells. The most famous of them is the carcinoembryonic antigen (CEA), which is normally expressed in fetal tissue, and widely overexpressed in CRC[20]. If it has been shown initially that CEA can lead to a specific cytotoxic response[21], more recent works have shown that CEA may have an immunosuppressive role and that T cells of patients with CRC were not activated by the presentation of this antigen in vitro[22]. Other self-antigens are thought to be immunogenic in CRC, as Ep-Cam HER-2/neu[23], MUC-1 and p56. Immune responses against some neo-antigens, generated by mutations (tp53, Kras) or against antigen MAGE-3, belonging to the family of “cancer testis antigen” normally expressed by germ cells, have been less frequently identified[21].
TAAs, which likely play an important role in immunosurveillance, are also potential targets for immunotherapy in vaccination strategies.
Microsatellite instability CRC is associated to immunogenic TAAs: Microsatellite instability (MSI) is associated with CRC in patients with Lynch syndrome, but also with sporadic cancer, in particular in elderly patients, and is observed in 5% to 25% of CRC patients depending on tumor stage. MSI tumors are associated with a high density of tumor infiltrating lymphocytes (TILs)[24,25], and have a better prognosis than CRC without a microsatellite instability phenotype[26].
MSI induces frameshift somatic mutations within target genes harboring repeated sequences in their coding frame, including TGFβR2, which is mutated in 90% of cases. These mutations lead not only to the inactivation of these target genes but also to the appearance of potentially immunogenic neoantigens. Indeed, disruption of the reading frame of TGFβR2 results in a new epitope (RLSSCVPVA) and in specific T cells to this epitope in tumors and peripheral blood of patients with MSI tumors[27]. Other MSI-associated mutations, as mutations of OGT[28], MSH3[29] caspase 5, ASTE1 and PTEN, have been shown to induce production of new immunogenic TAAs. Tougeron et al[30] studied 19 frequently mutated genes in CRC with MSI. In samples of stage II or III MSI tumors, an increased number of mutated genes was correlated with a high density of TILs. Mutations of ASTE1 and PTEN were particularly associated with increased lymphocyte infiltrate. These results suggest an important role of the immune response to specific neoantigens in CRC with MSI, and its potential involvement in the better prognosis of these tumors. Nevertheless, CRC associated with MSI may develop specific mechanisms to escape the immune system as for example particularly high levels of intratumoral Treg described in these patients[31]. Frameshift mutations can also induce inactivation of beta2-microglobulin leading to HLA class I downregulation[32,33] though the association between HLA class I downregulation and MSI is still controversial. Altogether, CRC associated with MSI could lead to a more intense immune response, but also to specific immunoregulatory phenomena, making them good candidates for immunotherapy.
Tumor infiltrate of memory CD8 T cells and CD45RO memory T cells may predict recurrence: The role of cytotoxic CD8 T cells has been widely studied in CRC. Tumor-infiltrating lymphocytes (TILs) are central to the antitumor immune response. The prognostic role of the immune response has been analyzed in a large cohort of resected patients.
Pagès et al[34] showed that the absence of pathological signs of early metastatic invasion (venous, lymphatic and perineural invasion) was associated with increased infiltrates of immune cells and increased levels of messenger RNA (mRNA) for products of Th1 effector T cells.
The density of TILs, characterized by CD3 immunostaining, has been reported to be more predictive of overall survival than all the usual histopathologic prognostic factors (i.e., UICC-TNM classification)[35]. Five-year overall survivals in patients with high, intermediate or low CD3+ TILs density were of 72.6%, 49.5% and 29.9%, respectively. In multivariate analysis, the density of TILs was still an independent prognostic factor, while TNM classification was no longer an independent factor after adjustment for the density of TILs.
Regarding phenotype, TILs were increased in tumors without signs of early metastatic invasion, especially memory CD8 T cells (CD45RO+), ranging from early memory to effector memory T cells[34]. Finally, increased levels of CD45RO+ correlated with increased overall survival and increased disease-free survival. In this large cohort, patients who had tumors with a high density of CD45RO+ cells or with a low density of CD45RO+ cells had a median disease-free survival of respectively 36.5 mo and 11.1 mo, and a median overall survival of respectively 53.2 mo and 20.6 mo (P < 0.001 for all comparisons). In multivariate analysis, the density of CD45RO+ cells was still an independent prognostic factor.
Based on these results, an immune score based on immunostaining has been elaborated, considering 4 densities: density of CD8+ T infiltrates in the center of the tumor (CT), in the invasive margin (IM), and density of memory CD45RO+ cells in the CT and in the IM. This immune score was first studied in early-stage tumors (stages I and II)[36]. Patients with a high density of both CD8+ and CD45RO+ cells in both the CT and IM had a disease-free survival of 95.2%, compared with 25% in patients with a low density of both CD8+ and CD45RO+ cells in both regions. This immune score was validated in a cohort of 599 specimens of stage I to IV CRC[37]. In this study, assessment of immune score was a better predictor of tumor recurrence (HR = 0.64; P < 0.001) than TNM classification. However, the immune infiltrate is highly heterogeneous in a tumor, and quantification is observer-dependent. To simplify and harmonize the quantification of immune infiltrate, automated quantification of CD3+ cells can be used. Linear quantification of lymphocytes has been shown to be predictive of disease-free-survival in multivariate analysis with very good inter-observer reproducibility[38]. However, other teams have not confirmed these results yet and major information are lacking in this large retrospective series such as age, MSI status or the use of adjuvant therapy. Despite these promising results, there is still no immune quantification test in routine practice to use immune infiltrate to guide our therapeutic strategies. This underlines the difficulty to find a standardized and reproducible test that complies with daily practice. Such tests should be of particular interest for clinicians, especially for stage II patients for whom the indication for adjuvant treatment is more controversial.
Human leukocyte antigen class I downregulation is associated with a poor prognosis
Expression of Human Leukocyte Antigen class I (HLA-I), the human MHC, class I molecules is downregulated in more than 70% of colorectal tumors[39]. In a few cases there is complete loss of HLA-I on tumor cells. Total loss of HLA-I mainly results from beta2-microglobulin inactivation in MSI tumors and LMP7/TAP2 downregulation in MSI-negative tumors[33]. Downregulation can result from loss of HLA haplotypes due to chromosomal nondisjunction or mitotic recombination, loss of HLA locus expression, or allelic loss due to point mutations or partial deletions of HLA-I genes. The prognostic significance of HLA-I downregulation has been reported in a large cohort of CRC cases[40]. Tumors with low expression of HLA-I were associated with a significantly shorter mean disease-specific survival (41 mo, 95%CI: 26-56) compared with tumors with high expression of HLA-I (68 mo, 95%CI: 63-74). Surprisingly, patients with a tumor with complete loss of HLA-I expression had a similar prognosis to those with high expression (mean disease-specific survival 60 mo, 95%CI: 50-69). This is possibly related to the high activity of NK cells against HLA-I-negative tumor cells. Killer inhibitory receptors, which are inhibitory receptors on NK cells, are dependent on MHC class I, then NK cells are activated in the absence of MHC class I. Tumor cells with downregulation but not complete loss of HLA-I expression could therefore avoid both T-cell- and NK-cell-mediated immune surveillance, and may be associated with a poor prognosis.
Induction of immunosuppressive cells is a major mechanism in escape from the host immune system. Tregs are characterized by expression of CD4, CD25, and Foxp3. In healthy individuals, role of Tregs is to prevent autoimmune disorders. In patients with cancer, Tregs could block the immune response against tumors through cytokine-dependent or cell-cell contact mechanisms. Tregs secrete immunosuppressive cytokines as IL-10 and TGFβ and immunosuppressive metabolites such as adenosine. The role of Tregs in cancer was first suspected from the observation of increased Tregs in peripheral blood and tumor tissue.
Strong Treg infiltration of tumors is generally associated with poor clinical outcome[41]. Elevated blood and tumor Treg numbers have also been described in CRC[42]. In some studies increased density of tumor-infiltrating Tregs is associated with a better prognosis[43], although in others elevated peritumoral numbers of CD4 and CD8 Tregs are associated with advanced-stage tumors and poorer overall survival[44]. This difference may be related to the heterogeneity of methods for characterization and quantification of Tregs and the use of more reliable techniques such as flow cytometry have shown the deleterious role of Tregs. In murine models of CRC, systemic removal of Tregs using anti-CD25 antibody results in tumor rejection and in improved vaccine-induced antitumor T-cell responses[45,46]. In human models, in vitro Treg depletion from peripheral blood of patients with CRC induces CD4 and CD8 T-cell responses against tumor-associated antigens[47,48]. Altogether, there is considerable evidence that Tregs are associated with a poor outcome in CRC.
Accumulation of Treg in tumors could be explained by several mechanisms[49]. The first mechanism is the conversion of conventional CD4+ T cells into Treg in response to various signal, especially secreted or membrane TGFβ. Tumors can also induce a preferential recruitment of Treg in tumors through the production of chemokines such as CCL17, CCL22 and CCL28[50,51]. VEGF-A secreted by tumor in response to hypoxia seems also to play a crucial role in tumor-induced Treg. VEGF-A inhibits maturation of DC. Immature DC, which can express TGFβ, can favor the conversion of conventional T cells into Treg[52,53]. VEGF-A can also directly promote expansion of Treg through VEGFR-2 expressed on the cell membrane of a Treg subgroup[54]. Recent data suggest that the number of intratumoral FOXP3+/VEGR-2+ Tregs is more predictive of recurrence and survival than the number of FOXP3+ alone in CRC[55].
Other escape mechanisms are suspected in CRC (Figure 1). B7-H1, or PD-L1, is a costimulatory molecule known to regulate T cell function negatively by interaction with PD-1. B7-H1 is strongly expressed in CRC[56] and is associated with poor prognosis[57]. B7-H1 may thus play an important role in tumor cell proliferation, apoptosis, migration and invasion. Other molecules, such as CTLA-4, are involved T lymphocytes inhibition. CTLA-4 is expressed on the surface of T lymphocytes, and its ligands, CD80 and CD86, are expressed on the surface of APCs. Expression of these molecules, called “immune checkpoints”, are important mechanisms of inhibition of anti-tumor immune response. Recently some monoclonal antibodies targeting these molecules (PD1, CTLA-4) have shown more than promising efficacy results in solid neoplasia such as melanoma and others[58-60].
Myeloid-derived suppressor cells (MDSC) are immunosuppressive cells. As Tregs, they contribute to the immune tolerance by inhibiting the function of CD8+ T cells. The prognostic value of MDSC is not well known, but they are thought to be deleterious, as elimination of MDSC in mouse tumor models was shown to enhance antitumor responses, resulting in tumor regression[61].
Some cytotoxic chemotherapy are known to induce immunogenic cell death. In CRC murine models and human tissues, oxaliplatin- but not cisplatin-based chemotherapy can trigger pre-apoptotic calreticulin exposure and the post-apoptotic release of high-mobility group box 1 protein (HMGB1), two signals which are required for immunogenic cell death[62]. DCs have several receptors for HMGB1, including Toll-like receptor 4 (TLR4). In a murine model with CT26 tumor cells, oxaliplatin-treated dying cells failed to elicit an antitumor immune response in TLR4-deficient mice, while TLR4+/+ controls were protected against rechallenge with the same cancer cells. Twelve to 14% of Caucasian patients present the loss-of-function allele of TLR4. In patients from the FFCD 2000-05 randomized trial (Ducreux lancet Oncol) with stage IV CRC and treated with an oxaliplatin-based combination, the TLR4 loss-of-function allele was associated with reduced progression-free and overall survival, as compared with patients carrying the normal TLR4 allele[63]. This allele, however, was not associated with disease-free survival in another cohort of patients who underwent surgery for CRC stage II and who did not receive chemotherapy, suggesting that TLR4 is predictive of chemotherapy effectiveness, but is not a prognostic factor.
Other check-points, such as the P2X7 receptor (P2RX7), which has a high affinity for ATP released by dying tumor cells and carried by DCs, are required for the anticancer immune response induced by chemotherapy and could modulate susceptibility to treatments[64].
Others immune mechanisms could be induced by cytotoxic chemotherapy. It has been shown in murine model that 5-fluorouracil could lead to a decrease of MDSC in the spleen and tumors in vivo, combinate to a T cell-dependent antitumor responses[61], but the therapeutic impact is not well established.
All these data suggest that the immune system may participate to the therapeutic effect of chemotherapy in CRC but should be confirmed in future works prospectively dedicated to this question.
As seen above, tumors can induce immunosuppressive cell populations such as Tregs. It is now well established that antiangiogenic agents decrease Treg numbers in blood and tumors. In peripheral blood of patients with renal carcinoma and different models of tumor-bearing mice, sunitinib reduces Treg numbers, and the decrease in Tregs is associated with overall survival in patients series[65,66]. In a recent study, we investigated the immunomodulatory effect of antiangiogenic agents in a mouse model of colon cancer[54]. Tregs decrease to their physiological level after treatment with sunitinib or VEGF-A antibody. However, after masitinib treatment, a multi-target tyrosine kinase inhibitor close to sunitinib but not targeting the VEGFR, Tregs were not reduced. VEGFR-2- but not VEGFR-1-specific blockade led to the same results. These results suggest that targeting the VEGF-A/VEGFR-2 pathway is sufficient to decrease Tregs in murine models of CRC. Bevacizumab directly inhibits this pathway and has been widely used in CRC since 2004[67]. In patients with metastatic CRC, we found that bevacizumab inhibited Treg accumulation and proliferation in peripheral blood. Antiangiogenic agents could act on other immunosuppressive cells, such as myeloid-derived suppressor cells and exhausted T cells[68]. Once again it is difficult to argue that the immunomodulating effect of bevacizumab in patients with CRC has an impact on its therapeutic efficacy. But in the future Tregs monitoring could help to predict response to bevacizumab. Furthermore this immunomodulatory effect of anti-angiogenic agents could be used to potentiate immunotherapeutic strategies.
Monoclonal antibodies used in therapeutics act on specific receptors to inhibit growth pathways. Some may also induce immune phenomena related to the characteristics of natural antibodies. In particular, cetuximab (chimeric IgG1 monoclonal antibody) binds epidermal growth factor (EGFR) and is used in RAS wild type metastatic CRC. It has been suggested that cetuximab, in addition to direct inhibition of EGFR, may act via ADCC. ADCC allows the antitumor innate immune response but can also trigger the adaptive immune response[69]. In vivo, addition of CpG, a TLR9 agonist able to activate DCs, increases immune response to cetuximab and its therapeutic efficacy.
Single nucleotide polymorphisms (SNPs) in the coding region of FCγR2A or FCγR3A have been reported to correlate with responses to cetuximab. The role of FCγR2A H/H or FCγR3A V/V genotypes is especially controversial[70]. Three studies in metastatic CRC showed a beneficial effect of FCγR3A V/V polymorphisms, and two of these studies also showed a beneficial role of FCγR2A H/H polymorphism. These polymorphisms were associated with better progression-free survival or objective response rate in patients treated with cetuximab. However, three other studies reported that FCγR3A V/V polymorphism was associated with shorter survivals in patients treated with cetuximab.
As in other cancers, immunotherapy could represent a step forward in the treatment of CRC.
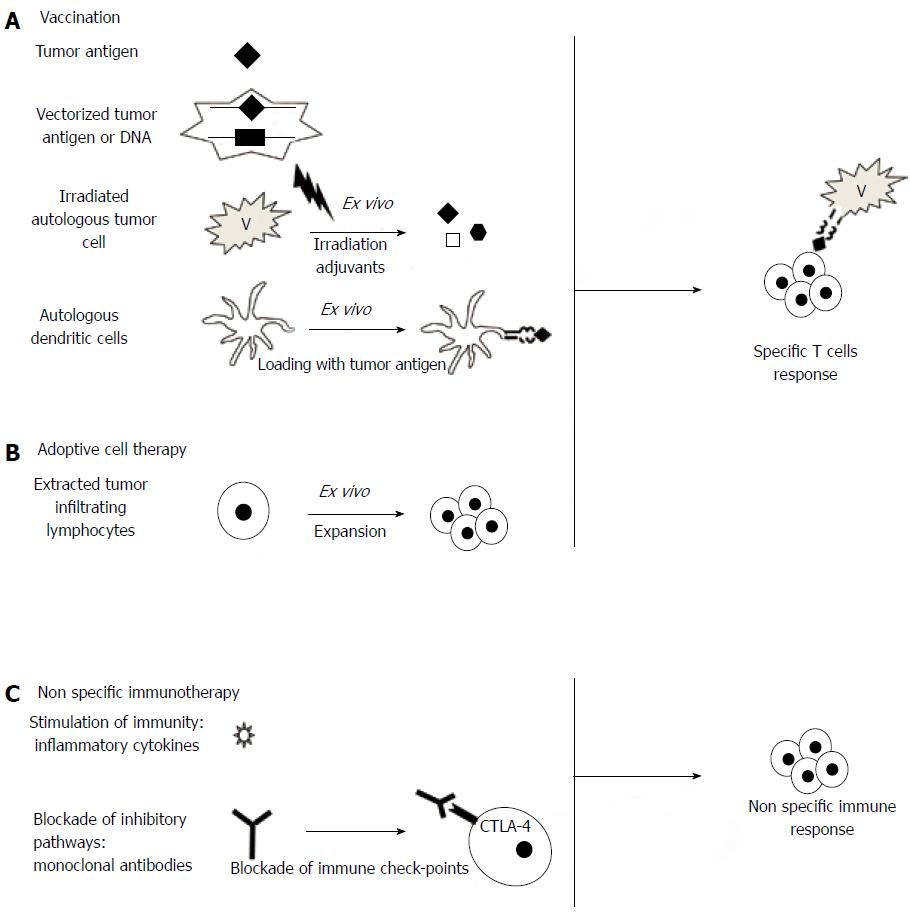
Several strategies are being investigated in the treatment of CRC. They are presented in Figure 2. Some have already been tested in clinical trials or are currently being tested in ongoing trials (Table 1).
| Principle | Phase | Specificity | Registration number |
| (A) Peptide vaccine | Targeted peptide(s): | ||
| I | Ras mutated | NCT01322815 | |
| MUC-1 | NCT01556789 | ||
| HER2/neu | NCT01730118 | ||
| I/II | Survivin | NCT00108875 | |
| Frame shift peptides (MSI) | NCT01461148 | ||
| Nor-MDP | NCT01376505 | ||
| II | MUC-1 | NCT01462513 | |
| (B) Whole cell cancer vaccine | Characteristic of cancer cells: | ||
| I/II | Allogenic cancer cell | NCT00722228 | |
| NCT00656123 | |||
| (C) DC-based therapy | Characteristic of DCs: | ||
| I/II | Autologous DCs intratumoral injection | NCT01882946 | |
| Loaded with Frame shift antigens (MSI) | NCT01885702 | ||
| CEA-pulsed DCs+ IL-2 | NCT00154713 | ||
| II | Autologous DCs | NCT01348256 | |
| NCT01413295 | |||
| (D) Inhibition of immunoregulation | Immunomodulation strategy: | ||
| I/II | Treg depletion | NCT00986518 | |
| Anti-CTLA4 + local radiation therapy | NCT01769222 | ||
| (E) Non specific immunostimulation | Immunostimulatory agent | ||
| I/II | Recombinant vaccinia virus | NCT01394939 | |
| IFN, Celecoxib + combination of chemokines | NCT01545141 | ||
| II | IL-7 | NCT01339000 | |
| Heat killed whole cell mycobacterium | NCT01539824 | ||
| III | PGG beta-glucan: binding to neutrophils | NCT01309126 | |
| (F) Cell therapy | Characteristic of cells | ||
| II | Allogenic activated lymphocytes | NCT00149006 | |
| NCT00855452 | |||
| Autologous TILS + lymphocyte depletion | NCT01174121 | ||
| Engineered autologous anti-ESO-1 lymphocytes | NCT00670748 | ||
| Engineered autologous anti-CEA lymphocytes | NCT01723306 | ||
| NS | Autologous natural killer T cells | NCT01801852 |
Nonspecific immunotherapy consists of stimulation of host immunity with cytokines such as interferon (IFN), interleukins or GM-CSF. A phase II study tested the combination of GM-CSF, gemcitabine and FOLFOX (GOLFIG regimen) in 46 patients in first- to third-line treatment[71,72]. This regimen was safe and active in pretreated patients. Prolonged survival and time to progression were associated with signs of autoimmunity and with an increase in memory T-cells and a decrease in Tregs in the peripheral blood of patients. A phase III study compared GOLFIG with FOLFOX[73]. The study was ended prematurely as an intermediate analysis showed significant superiority of GOLFIG over FOLFOX chemotherapy in terms of response rate (59.3% vs 34.4%, P = 0.0001) and progression-free survival (12.4 mo vs 7.9 mo, HR = 0.64, P = 0.0105). Autoimmunity signs, tumor infiltration by Tregs and central memory T cells were independent predictive markers of efficacy in this work.
Vaccination against tumor antigens: Few phase II trials involving antigen vaccination have been reported in the setting of CRC. Immunization with β-human chorionic gonadotropin (βHCG) peptide vaccine in mostly pretreated patients with metastatic CRC induced anti-hCG antibody in 56 of the 77 patients. High levels of antibody were associated with significantly longer survival[74]. Other adjuvant vaccinations with antigen were studied. Immunization with CEA after curative resection of hepatic metastases did not improve 2-year recurrence-free survival[75]. A pilot study of adjuvant vaccination with a mutant RAS peptide in KRAS mutated stage II and III CRC induced a specific immune response with increased IFN-γ mRNA expression in 4 out of 7 patients and was well tolerated[76]. Several ongoing phase I/II studies are studying antigen vaccines using various peptides, as mucinous glycoprotein 1 (MUC, L-BLP25), MSI, or HER2neu.
Vaccination with autologous tumor cells: Since 1992 active specific immunotherapy (ASI), consisting of immunization with irradiated autologous tumor cells as adjuvant therapy, has led to a few phase III trials. The first strategy of ASI was to use Newcastle disease virus-infected autologous tumor cell vaccine after resection of hepatic metastases with curative intent[77]. The second strategy used an autologous tumor cell BCG vaccine (OncoVax) in stage II or III CRC[78,79]. In the 3 studies no significant benefit was observed in the overall population, but some subgroups appeared to benefit from vaccination more than others, especially colon cancer (vs rectal) and stage II cancers (vs stage III). Patients with stage II CRC treated with OncoVax had a 5-year recurrence rate of 21.3% vs 37.7% in the control group, leading to a significantly better 5-year recurrence-free survival (P = 0.009), although there was no difference in stage III patients[80]. These results have not yet been confirmed and should lead to a pivotal phase III trial.
Dendritic cell-based vaccination: A significant improvement in antitumor vaccination is provided by vectorization of antigens, in particular with DCs[81]. Pilot studies have also proposed DC-based vaccination in CRC, using DCs loaded with a single antigen[82-84], two antigens[85] or multiple antigens[86-88] with a good safety profile. In some cases autologous DCs or antigens are used, making the procedure labor-intensive and costly. This promising strategy is one of the most used in ongoing immunotherapy clinical trials in CRC, but other vectorization strategies, such as synthetic vectors, could be used in the future[89] and could be more efficient and simpler than those with DCs.
Adoptive cell therapy: Adoptive cell therapy (ACT) is mostly used in melanoma. Briefly, T cells are collected from the tumor, draining lymph nodes or peripheral blood, and are activated and expanded in vitro. Autologous T cells are then administered intravenously to the patient. To optimize the activity of ACT, some authors have tried lymphodepletion of the host, optimized cytokine cocktails and selection of CD8+ T cell clones with higher affinity for tumor cells/antigens. ACT with T cells from patient lymph nodes has been tested in 16 patients with stage II to IV CRC[90]. ACT was well tolerated in all cases with no side effects, and allowed a complete response in 4 out 9 patients with metastatic disease.
Similarly, autologous genetically engineered T cells with high-avidity CEA-specific T cell receptor have been used in CRC[91]. In a phase I study, 3 patients were treated and decreased serum CEA levels were observed, but all patients developed severe colitis. Genetically engineered T cells expressing chimeric antigen receptors targeting HER2 also led to severe toxicity in a patient with CRC[92]. Similar strategies, such as allogenic lymphocytes and autologous NK therapy, are currently being tested in phase I and II studies.
The immune system plays a major role in the eradication of tumor cells, but is bypassed by the tumor at the clinical expression phase. Various antitumor immune mechanisms are inhibited by efficient escape mechanisms. The treatments currently used in CRC (cytotoxic chemotherapy, anti-EGFR antibodies, antiangiogenic molecules) are associated with immunomodulating effects shown in vitro and in vivo. However, their clinical impact has not been well evaluated. In some cases the immune escape mechanisms are associated with an aggressive phenotype. In these cases classic treatments clearly fail, and immunotherapeutic approaches is a seducing alternative to try to improve the prognosis of these patients in the future. Several approaches can be considered. First, nonspecific immunotherapy that may use immunostimulatory molecule (GM-CSF, IL-2, IL-7) or inhibit immunosuppressive mechanisms (Treg depletion, anti-PDL1, anti-CTLA4). Second, the purpose of specific immunotherapy is the induction of a specific antitumor immune response. Various vaccination strategies, with peptide, antigen, DNA combined with vectorization techniques, could lead to the development of effective vaccines, particularly in the adjuvant setting. ACT with T cells or NK cells is a labor-intensive procedure, but advances in genetic engineering raise hope for such treatments. Finally, nearly 40 phase I to III clinical trials testing immunotherapy in CRC are ongoing. This will probably lead in the near future to consider one or a combination of these different strategies in our therapeutic armamentarium to fight CRC.
| 1. | Pham SM, Kormos RL, Landreneau RJ, Kawai A, Gonzalez-Cancel I, Hardesty RL, Hattler BG, Griffith BP. Solid tumors after heart transplantation: lethality of lung cancer. Ann Thorac Surg. 1995;60:1623-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736-1745. [PubMed] |
| 3. | Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1132] [Article Influence: 75.5] [Reference Citation Analysis (1)] |
| 4. | Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 363] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 5. | Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1644] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 6. | Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2026] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 7. | Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3425] [Cited by in RCA: 3594] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 8. | Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97:2731-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 349] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 560] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 10. | Malmberg KJ, Bryceson YT, Carlsten M, Andersson S, Björklund A, Björkström NK, Baumann BC, Fauriat C, Alici E, Dilber MS. NK cell-mediated targeting of human cancer and possibilities for new means of immunotherapy. Cancer Immunol Immunother. 2008;57:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 275] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Terme M, Fridman WH, Tartour E. NK cells from pleural effusions are potent antitumor effector cells. Eur J Immunol. 2013;43:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320-2328. [PubMed] |
| 14. | Sandel MH, Speetjens FM, Menon AG, Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA, van de Velde CJ, Kuppen PJ. Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol. 2005;42:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322-7327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, Jotereau F. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175:5481-5488. [PubMed] |
| 18. | Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 3009] [Article Influence: 188.1] [Reference Citation Analysis (1)] |
| 19. | Edin S, Wikberg ML, Rutegård J, Oldenborg PA, Palmqvist R. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PLoS One. 2013;8:e74982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 932] [Article Influence: 34.5] [Reference Citation Analysis (7)] |
| 21. | Keogh E, Fikes J, Southwood S, Celis E, Chesnut R, Sette A. Identification of new epitopes from four different tumor-associated antigens: recognition of naturally processed epitopes correlates with HLA-A*0201-binding affinity. J Immunol. 2001;167:787-796. [PubMed] |
| 22. | Fauquembergue E, Toutirais O, Tougeron D, Drouet A, Le Gallo M, Desille M, Cabillic F, de La Pintière CT, Iero M, Rivoltini L. HLA-A*0201-restricted CEA-derived peptide CAP1 is not a suitable target for T-cell-based immunotherapy. J Immunother. 2010;33:402-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Nagorsen D, Keilholz U, Rivoltini L, Schmittel A, Letsch A, Asemissen AM, Berger G, Buhr HJ, Thiel E, Scheibenbogen C. Natural T-cell response against MHC class I epitopes of epithelial cell adhesion molecule, her-2/neu, and carcinoembryonic antigen in patients with colorectal cancer. Cancer Res. 2000;60:4850-4854. [PubMed] |
| 24. | Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417-2422. [PubMed] |
| 25. | Michael-Robinson JM, Biemer-Hüttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360-366. [PubMed] |
| 26. | Benatti P, Gafà R, Barana D, Marino M, Scarselli A, Pedroni M, Maestri I, Guerzoni L, Roncucci L, Menigatti M. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11:8332-8340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Saeterdal I, Gjertsen MK, Straten P, Eriksen JA, Gaudernack G. A TGF betaRII frameshift-mutation-derived CTL epitope recognised by HLA-A2-restricted CD8+ T cells. Cancer Immunol Immunother. 2001;50:469-476. [PubMed] |
| 28. | Ripberger E, Linnebacher M, Schwitalle Y, Gebert J, von Knebel Doeberitz M. Identification of an HLA-A0201-restricted CTL epitope generated by a tumor-specific frameshift mutation in a coding microsatellite of the OGT gene. J Clin Immunol. 2003;23:415-423. [PubMed] |
| 29. | Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:e26517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 30. | Tougeron D, Fauquembergue E, Rouquette A, Le Pessot F, Sesboüé R, Laurent M, Berthet P, Mauillon J, Di Fiore F, Sabourin JC. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009;22:1186-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 32. | Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. 2012;61:1359-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Cabrera CM, Jiménez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;61:211-219. [PubMed] |
| 34. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1648] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 35. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 5013] [Article Influence: 250.7] [Reference Citation Analysis (19)] |
| 36. | Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944-5951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 752] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 37. | Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 806] [Article Influence: 53.7] [Reference Citation Analysis (1)] |
| 38. | Allard MA, Bachet JB, Beauchet A, Julie C, Malafosse R, Penna C, Nordlinger B, Emile JF. Linear quantification of lymphoid infiltration of the tumor margin: a reproducible method, developed with colorectal cancer tissues, for assessing a highly variable prognostic factor. Diagn Pathol. 2012;7:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Menon AG, Morreau H, Tollenaar RA, Alphenaar E, Van Puijenbroek M, Putter H, Janssen-Van Rhijn CM, Van De Velde CJ, Fleuren GJ, Kuppen PJ. Down-regulation of HLA-A expression correlates with a better prognosis in colorectal cancer patients. Lab Invest. 2002;82:1725-1733. [PubMed] |
| 40. | Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, Durrant LG. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Pere H, Tanchot C, Bayry J, Terme M, Taieb J, Badoual C, Adotevi O, Merillon N, Marcheteau E, Quillien VR. Comprehensive analysis of current approaches to inhibit regulatory T cells in cancer. Oncoimmunology. 2012;1:326-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 42. | Sellitto A, Galizia G, De Fanis U, Lieto E, Zamboli A, Orditura M, De Vita F, Giunta R, Lucivero G, Romano C. Behavior of circulating CD4+CD25+Foxp3+ regulatory T cells in colon cancer patients undergoing surgery. J Clin Immunol. 2011;31:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 820] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 44. | Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 45. | Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borrás-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931-5939. [PubMed] |
| 46. | Taieb J, Chaput N, Schartz N, Roux S, Novault S, Ménard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jèze G. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722-2729. [PubMed] |
| 47. | Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, Taskén K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Tanchot C, Terme M, Pere H, Tran T, Benhamouda N, Strioga M, Banissi C, Galluzzi L, Kroemer G, Tartour E. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron. 2013;6:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood. 2011;118:4853-4862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 1095] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 52. | Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 53. | Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 586] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 54. | Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 541] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 55. | Suzuki H, Onishi H, Morisaki T, Tanaka M, Katano M. Intratumoral FOXP3+VEGFR2+ regulatory T cells are predictive markers for recurrence and survival in patients with colorectal cancer. Clin Immunol. 2013;146:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Hua D, Sun J, Mao Y, Chen LJ, Wu YY, Zhang XG. B7-H1 expression is associated with expansion of regulatory T cells in colorectal carcinoma. World J Gastroenterol. 2012;18:971-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 58. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 12026] [Article Influence: 751.6] [Reference Citation Analysis (0)] |
| 59. | Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3192] [Cited by in RCA: 3361] [Article Influence: 258.5] [Reference Citation Analysis (7)] |
| 60. | Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2712] [Cited by in RCA: 2755] [Article Influence: 211.9] [Reference Citation Analysis (3)] |
| 61. | Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 992] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 62. | Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1986] [Cited by in RCA: 2489] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 63. | Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 942] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 64. | Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 65. | Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 430] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 66. | Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre V. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010;33:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 67. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7792] [Article Influence: 354.2] [Reference Citation Analysis (8)] |
| 68. | Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 691] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 69. | Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 71. | Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini C. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23:8950-8958. |
| 72. | Correale P, Tagliaferri P, Fioravanti A, Del Vecchio MT, Remondo C, Montagnani F, Rotundo MS, Ginanneschi C, Martellucci I, Francini E. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial). Clin Cancer Res. 2008;14:4192-4199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Correale P, Rotundo MS, Botta C, Apollinari S, Remondo C, Tsang KY, Ciliberto D, Tassone P, Ridolfi R, Tagliaferri P. Chemo-immunotherapy with gemcitabine FOLFOX followed by granulocyte-macrophage colony stimulating factor and low dose aldesleukine (GOLFIG regimen) is a highly active frontline treatment for advanced colorectal carcinoma: Results from the GOLFIG/2 phase III trial. Proc 102nd Annual Meeting of the American Association for Cancer Research; 2011, Apr 2-6. Orlando Florida. Philadelphia: AACR, 2011. Abstract nr 5511. Available from: http://www.abstractsonline.com/Plan/ViewAbstract.aspxsKey=0752a705-7e85-4337-a2c7-02b492ae7ecc&cKey=c71f5dc7-32f2-47e8-abcc-47692b7524eb&mKey={507D311A-B6EC-436A-BD67-6D14ED39622C. |
| 74. | Moulton HM, Yoshihara PH, Mason DH, Iversen PL, Triozzi PL. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res. 2002;8:2044-2051. [PubMed] |
| 75. | Posner MC, Niedzwiecki D, Venook AP, Hollis DR, Kindler HL, Martin EW, Schilsky RL, Goldberg RM, Mayer RJ. A phase II prospective multi-institutional trial of adjuvant active specific immunotherapy following curative resection of colorectal cancer hepatic metastases: cancer and leukemia group B study 89903. Ann Surg Oncol. 2008;15:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Toubaji A, Achtar M, Provenzano M, Herrin VE, Behrens R, Hamilton M, Bernstein S, Venzon D, Gause B, Marincola F. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother. 2008;57:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, Schlag PM. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother. 2009;58:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 342] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 79. | Harris JE, Ryan L, Hoover HC, Stuart RK, Oken MM, Benson AB, Mansour E, Haller DG, Manola J, Hanna MG. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol. 2000;18:148-157. [PubMed] |
| 80. | Uyl-de Groot CA, Vermorken JB, Hanna MG, Verboom P, Groot MT, Bonsel GJ, Meijer CJ, Pinedo HM. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: a prospective study of medical and economic benefits. Vaccine. 2005;23:2379-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1600] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 82. | Morse MA, Nair SK, Mosca PJ, Hobeika AC, Clay TM, Deng Y, Boczkowski D, Proia A, Neidzwiecki D, Clavien PA. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest. 2003;21:341-349. [PubMed] |
| 83. | Liu Y, Zhang W, Zhang B, Yin X, Pang Y. DC vaccine therapy combined concurrently with oral capecitabine in metastatic colorectal cancer patients. Hepatogastroenterology. 2013;60:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Lesterhuis WJ, de Vries IJ, Aarntzen EA, de Boer A, Scharenborg NM, van de Rakt M, van Spronsen DJ, Preijers FW, Figdor CG, Adema GJ. A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients. Br J Cancer. 2010;103:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Morse MA, Niedzwiecki D, Marshall JL, Garrett C, Chang DZ, Aklilu M, Crocenzi TS, Cole DJ, Dessureault S, Hobeika AC. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg. 2013;258:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007;30:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Barth RJ, Fisher DA, Wallace PK, Channon JY, Noelle RJ, Gui J, Ernstoff MS. A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res. 2010;16:5548-5556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca MB, Claesson MH, Rosenberg J. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep. 2008;20:1305-1311. [PubMed] |
| 89. | Tartour E, Benchetrit F, Haicheur N, Adotevi O, Fridman WH. Synthetic and natural non-live vectors: rationale for their clinical development in cancer vaccine protocols. Vaccine. 2002;20 Suppl 4:A32-A39. [PubMed] |
| 90. | Karlsson M, Marits P, Dahl K, Dagöö T, Enerbäck S, Thörn M, Winqvist O. Pilot study of sentinel-node-based adoptive immunotherapy in advanced colorectal cancer. Ann Surg Oncol. 2010;17:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 823] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 92. | Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 2044] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
P- Reviewers: Srinivasa S, Vigano L S- Editor: Wen LL L- Editor: A E- Editor: Liu XM